Abstract
Introduction
Intravenous immunoglobulin (IVIG) is an important but costly blood product. In 2003, Canadian Blood Services distributed 1965 million grams of IVIG, which is one of the highest per capita use of all industrial countries. The price per gram for IVIG ranges from USD50-USD64. Moreover, in the last 5 years, there was 6.8–20% annual growth of IVIG utilization.
Method
To study the dosing pattern of IVIG, we prospectively collected IVIG utilization data (demographic, clinical and product) from 5 community and 2 teaching hospitals from 01 May 2003 to 31 July 2003. The indications and the dosing of IVIG therapy were compared with previous published guidelines. Excessive dose was defined as the difference between the prescribing dose and the calculated dose based on the patient’s body weight with the nearest rounding to 2.5 gm. A sub-optimal dose was defined when the prescribing dose was less than 20% of the dose suggested by the guidelines.
Results
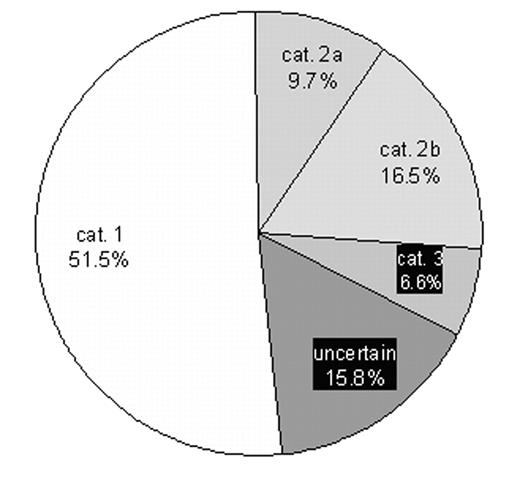
There were 1265 administrations of IVIG of which 221 (17.5%) contained complete information about the indications of IVIG therapy; patients’ body weight; and the prescribing doses. These 221 data entries captured 9028.1 gm (30.1%) of the IVIG utilized in the region within the 3 months interval. Among the 9028.1 gm IVIG prescribed, 4653.5 gm (51.5%) were given to the patients with medical conditions for which there is convincing evidence of benefit (category I); 875 gm (9.7%) were given to patients with medical conditions for which there is inconclusive but high level of evidence of benefit (category IIa); 1495 gm (16.5%) were given to the patients with medical conditions for which there is inconclusive and low level of evidence of benefit (category IIb); and 593 gm (6.6%) were given to the patients with medical conditions for which there is no convincing evidence of benefit (category III) (Table 1 and Figure 1). Thirty one data entries using 1426.6 gm IVIG (15.8%) had insufficient clinical data for categorization. 495.5 gm of IVIG (10.6% of total use in category I) exceed the recommended doses based on the guidelines. In category IIb, 450 gm of IVIG were classified as sub-optimal. Excessive dosing mainly occurs in category I; whereas sub-optimal dosing occurs in category IIb. No analysis is possible for category III because there are no universally acceptable doses for those medical conditions.
Conclusion
33.8% of IVIG utilization was inappropriate. Opportunities for optimizing IVIG use include: (1) decreasing IVIG use in medical conditions without convincing evidences of benefit; (2) inappropriate rounding of doses result in either excessive dosing or suboptimal dosing.
Summary of IVIG utilization in various categories
| . | . | Excessive Dosing . | Sub-optimal Dosing . | |
|---|---|---|---|---|
| Clinical Indications . | Amount gm (%) . | Amount gm (%) . | Amount gm (%) . | Degree of Deviation (%) . |
| Category 1 | ||||
| # of prescription | 112 | Median: 28.6% | ||
| 4653.5 (51.5%) | 495.5 (10.6%) | 250.0 (5.4%) | Average: 40.5% | |
| Cetegory 2a | ||||
| # of prescription | 27 | --- | ||
| 875.0 (9.7%) | −75 (−8.6%) | 0.0 (0%) | --- | |
| Category 2b | ||||
| # of prescription | 33 | Median: 42.9% | ||
| 1495.0 (16.5%) | −46.4 (−3.1%) | 450.0 (30.1%) | Average: 44.5% | |
| Category 3 | ||||
| # of prescription | 15 | |||
| 593.0 (6.6%) | ||||
| Category 0 | ||||
| # of prescription | 31 | |||
| 1426.6 (15.8%) | ||||
| . | . | Excessive Dosing . | Sub-optimal Dosing . | |
|---|---|---|---|---|
| Clinical Indications . | Amount gm (%) . | Amount gm (%) . | Amount gm (%) . | Degree of Deviation (%) . |
| Category 1 | ||||
| # of prescription | 112 | Median: 28.6% | ||
| 4653.5 (51.5%) | 495.5 (10.6%) | 250.0 (5.4%) | Average: 40.5% | |
| Cetegory 2a | ||||
| # of prescription | 27 | --- | ||
| 875.0 (9.7%) | −75 (−8.6%) | 0.0 (0%) | --- | |
| Category 2b | ||||
| # of prescription | 33 | Median: 42.9% | ||
| 1495.0 (16.5%) | −46.4 (−3.1%) | 450.0 (30.1%) | Average: 44.5% | |
| Category 3 | ||||
| # of prescription | 15 | |||
| 593.0 (6.6%) | ||||
| Category 0 | ||||
| # of prescription | 31 | |||
| 1426.6 (15.8%) | ||||

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal