Abstract
Background: Systemic mastocytosis (SM) is characterized by infiltration of extracutanous tissues by neoplastic mast cells. Primary target organs are liver, spleen and bone marrow. In some instances, SM progresses to aggressive systemic mastocytosis (ASM) or mast cell leukemia (MCL), which are associated with extensive mast cell infiltration into various organs and their failure. Almost all cases of SM exhibit point mutations at codon 816 of Kit, a receptor tyrosine kinase. These mutations (most commonly D816V) lead to constitutive activation of the kinase and are the causative lesion of SM. Here, we describe a novel murine model of SM/ASM that shares many characteristics with the human disease and may be useful for in vivo drug testing, including targeted therapy of D816 mutant Kit with small molecule inhibitors.
Materials and methods: P815 cells, a cell line expressing D814Y Kit (homologous to human D816V kit) that was established in DBA2 mice (
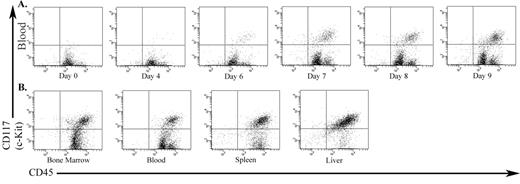
Results: Both cell doses induced an aggressive disease, with all animals reaching a moribund stage on day 9 (5x104 cells) and 16 (1x102 cells). A significant (p<0.001, student’s t test) drop in the platelet count regularly accompanied the appearance of mast cells in the peripheral blood (PB) (figure 1A). Subsequently, the animals developed marked granulocytosis. Autopsy demonstrated gross enlargement of liver and spleen, while lungs and kidneys were unaffected. Histopathology and FACS showed extensive infiltration of spleen, liver and bone marrow by Kit-positive cells (figure 1B). Immunoblotting revealed high levels of tyrosine phosphorylated Kit protein in whole cell lysates from PB, BM and spleen.
Conclusion: We have established a highly reproducible model of SM/ASM that resembles the human disease. A particular advantage of this model is that the onset of disease can conveniently be monitored by serial PB counts. In addition, the latency of the disease can be modified by the size of the initial inoculum. Its extremely predictable course together with the parameters it provides for monitoring disease progression should make this model useful for the study of small molecules that target D816 mutant Kit.
The infiltration of Kit-positive P815 cells into various organs of DBA/2 mouse. (A) PB over the course of the disease progression. (B) Hematopoietic organs at death.
The infiltration of Kit-positive P815 cells into various organs of DBA/2 mouse. (A) PB over the course of the disease progression. (B) Hematopoietic organs at death.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal