Abstract
The outcome of 63 consecutive submyeloablative allografts (27–66 y, median 52) performed for hematologic malignancies after 100 mg/m2 melphalan without (n=21; prior autograft) or with (n=42; no prior autograft) 50 mg/kg cyclophosphamide was analyzed to study the effect of pre-transplant characteristics. GVHD prophylaxis comprised cyclosporine-mycophenolate (n=37; HLA-identical sibling donors) or tacrolimus-mycophenolate (n=26, 1-locus mismatched sibling or 9–10/10 allele-matched unrelated). No growth factors were administered routinely post-transplant and supportive care was uniform. 14 patients experienced transplant-related mortality (TRM), and 32 relapsed. 24 relapsing patients died, and 7 of the other 8 are alive in CR or with declining disease. The following factors were analyzed in a Cox model for their effect on TRM and relapse: chemosensitive (n=25) vs refractory disease (n=38), age ≤55 (n=44) vs >55 (n=19), normal (n=32) vs abnormal (n=31) LDH, HLA match (n=56) vs mismatch (n=7), prior autograft or not, performance status 0–1 (n=47) vs 2–3 (n=16).
| Outcome . | Favorable factor . | RR (95% CI) . | P . |
|---|---|---|---|
| TRM | Age ≤55 | 0.20 (0.04–0.86) | 0.03 |
| HLA matched | 0.21 (0.05–0.89) | 0.04 | |
| Performance status 0-1 | 0.25 (0.06–0.99) | 0.05 | |
| Relape | Chemosensitive disease | 0.28 (0.11–0.73) | 0.01 |
| Outcome . | Favorable factor . | RR (95% CI) . | P . |
|---|---|---|---|
| TRM | Age ≤55 | 0.20 (0.04–0.86) | 0.03 |
| HLA matched | 0.21 (0.05–0.89) | 0.04 | |
| Performance status 0-1 | 0.25 (0.06–0.99) | 0.05 | |
| Relape | Chemosensitive disease | 0.28 (0.11–0.73) | 0.01 |
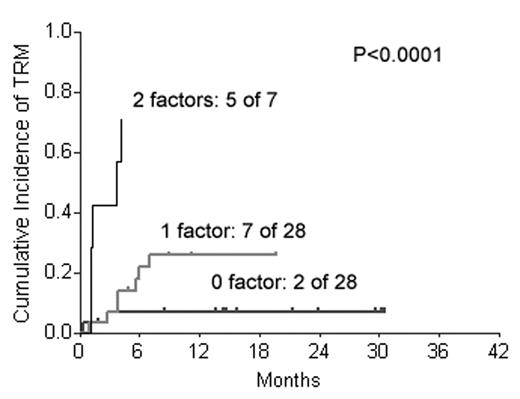
Fig 1 shows TRM for patients with 0, 1 or 2 high-risk factors for TRM.
Fig 1 shows TRM for patients with 0, 1 or 2 high-risk factors for TRM.
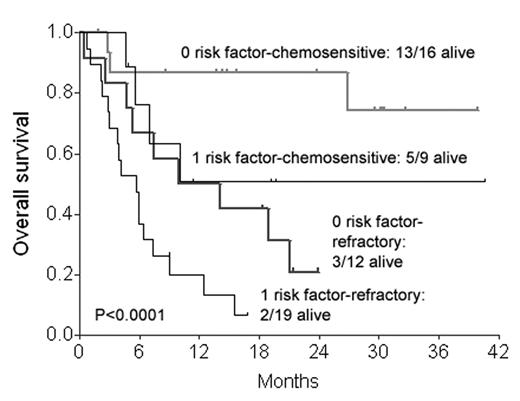
Fig 2 shows overall survival (OS) for patients with 0 or 1 high-risk factors for TRM by disease chemosensitivity.
Fig 2 shows overall survival (OS) for patients with 0 or 1 high-risk factors for TRM by disease chemosensitivity.
Table 2 shows the causes of death by risk factors for TRM and disease chemosensitivity.
| Group (n) . | Alive . | TRM . | Death from disease . |
|---|---|---|---|
| A: 2 risk factors for TRM (7) | 1 (14%) | 5 (71%) | 1 (14%) |
| B: 1 risk factor for TRM + Refractory (19) | 2 (11%) | 6 (32%) | 11 (58%) |
| C: 1 risk factor for TRM + Sensitive (9) | 5 (56%) | 1 (11%) | 3 (33%) |
| D: 0 risk factor for TRM + Refractory (12) | 3 (25%) | 1 (8%) | 8 (67%) |
| E: 0 risk factor for TRM + Sensitive (16) | 13 (81%) | 1 (6%) | 2 (13%) |
| Group (n) . | Alive . | TRM . | Death from disease . |
|---|---|---|---|
| A: 2 risk factors for TRM (7) | 1 (14%) | 5 (71%) | 1 (14%) |
| B: 1 risk factor for TRM + Refractory (19) | 2 (11%) | 6 (32%) | 11 (58%) |
| C: 1 risk factor for TRM + Sensitive (9) | 5 (56%) | 1 (11%) | 3 (33%) |
| D: 0 risk factor for TRM + Refractory (12) | 3 (25%) | 1 (8%) | 8 (67%) |
| E: 0 risk factor for TRM + Sensitive (16) | 13 (81%) | 1 (6%) | 2 (13%) |
These data suggest that while the current treatment approach is optimal for patients falling in Group E, modified approaches are needed for other patients. Based on the causes of failure, the following modifications appear to be warranted. Group A: A completely non-ablative regimen to reduce toxicity. Group B: A completely non-ablative regimen to reduce toxicity with augmentation of graft-vs-tumor effects by elective donor leukocyte infusions and/or abbreviated immunosuppression. Group C: Augmentation of graft-vs-tumor effects by elective donor leukocyte infusions and/or abbreviated immunosuppression. Group D: Conventional-intensity rather than reduced-intensity allogeneic HSCT.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal