Abstract
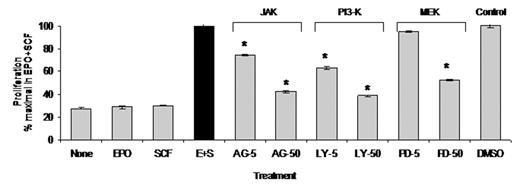
The adult bone marrow produces 2x1011 red blood cells daily to maintain the oxygen carrying capacity of peripheral blood. Certain pathologic conditions such as blood loss or hemolysis that result in increased oxygen demands lead to a physiologic bone marrow response characterized by increased rate of erythropoiesis and expansion of bone marrow proerythroblasts, the precursor cells that differentiate into mature red blood cells. We studied the mechanisms by which erythropoietin (EPO) and stem cell factor (SCF) regulate the expansion of primary human proerythroblasts. Using liquid cultures of peripheral blood CD34+ cells isolated from healthy volunteers, we generated uniform populations of transferrin receptor (CD71)+ human proerythroblasts. We established a serum-free culture model to study the effect of EPO and SCF on the survival and proliferative capacity of the cells. Primary proerythroblasts failed to survive in the presence of EPO or SCF alone, but exhibited marked synergistic proliferation in response to EPO plus SCF, exhibiting one log expansion in 5 days under serum-free conditions. Characterization of EPO receptor (EPOR) and SCF receptor (KIT)-mediated signal transduction in proerythroblasts revealed a requirement for EPOR, but not KIT, signaling for tyrosine phosphorylation of STAT5 (Tyr694), a downstream target for the cytoplasmic tyrosine kinase JAK2. MAP kinases ERK 1/2 were phosphorylated (Thr202/Tyr204) in response to either EPO or SCF alone, with phosphorylation of ERK1/2 induced predominantly by SCF. We found increased phosphorylation of ERK 1/2 when proerythroblasts were treated with both EPO and SCF. Phosphorylation of protein kinase B/Akt (Ser473), a signaling molecule downstream of phosphatidylinositol 3-kinase (PI3K), was observed following SCF treatment. Treatment with kinase inhibitors targeting JAK, PI3K and MAP kinase kinase (MEK1) during EPO and SCF stimulation revealed that JAK inhibitor AG490 attenuated STAT5 tyrosine phosphorylation, MEK inhibitor PD98059 abolished ERK 1/2 phosphorylation and the PI3K inhibitor LY294002 inhibited Akt phosphorylation. To determine the contribution of specific signaling pathways to synergistic proliferation of proerythroblasts in response to cooperative effects of EPO and SCF, we performed proliferation assays with increasing concentrations of each inhibitor (5 and 50 μM) or DMSO vehicle. We found significant, dose-dependent inhibition of proerythroblast proliferation in response to all three JAK, PI3K or MEK inhibitors (figure 1, *P<0.001 by ANOVA, n=6). We conclude that 1)- EPO or SCF alone fail to support proerythroblast survival, 2)- The cooperative and synergistic effects of EPO and SCF are required for the expansion of primary erythroid precursors, 3)- EPOR but not KIT signaling mediates tyrosine phosphorylation of STAT5 in proerythroblasts, 4)- Phosphorylation of Akt and ERK1/2 in proerythroblasts is mediated primarily by SCF, 5)- EPO and SCF together lead to increased phosphorylation of ERK 1/2, and 6)- The cooperative effect of EPO and SCF that mediates synergistic erythroid precursor expansion requires activation of multiple signaling pathways, including the JAK-STAT, PI3K and MAP kinase pathways.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal