Abstract
Background and Methods: Donor-derived CTL that respond to tumor antigens emerge after SCT, particularly in association with the status of immune recovery and disease remission. To analyze the frequency of CTL against WT1, PR1 and PRAME after SCT, tetramer-based analysis was performed in 69 samples taken from 34 patients with the HLA-A02 phenotype, including 2 with RCC (10 MDS, 8 AML, 6 NHL, 4 ALL, 2 CML, 2 RCC, 1 ATL, 1 PCL). Peripheral blood samples were stained with WT1 126, PR1 169, and PRAME 300 tetramers, and CMV pp65 tetramer was used as a positive control. Antigen -specific CTL was defined as CD3+, CD13−, CD19−, CD4−, CD8+, and tetramer + cells present in the lymphocyte gate, and the cut-off level was defined as 0.02% per CD8+ lymphocytes.
Results: All samples from CMV-seropositive patients were positive for CMV-CTL with high titers (average 2.3%). Regarding PR1, only 1 sample showed the presence of tetramer-positive cells (0.04%). Similarly in PRAME, only 3 samples from 3 different patients were sporadically positive with low titers (<0.05%), but positive results were not found at different occasions. Regarding WT1-CTL, positive results were detected in 23 samples taken from 2 RCC, 2 NHL, 1 AML, 1 ALL, 1 ATL, and 1 PCL patient. A higher positive rate for WT1-CTL assay suggests that WT1 may be a potential candidate antigen for wider application compared to the other two antigens.
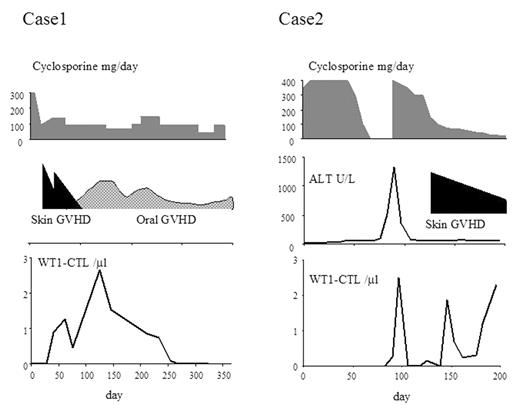
Case monitoring: Based on these results, we performed serial analyses of WT1-CTL during the clinical course in 2 patients with RCC who underwent reduced-intensity stem cell transplantation (RIST). Before RIST, both RCC patients had undergone resection of the primary disease, but had developed multiple lung metastases, which were progressive despite interferon therapy. WT1-CTL in one of the RCC patients was detected at day 40 post-SCT after the development of skin GVHD, and the peak formation of WT1-CTL occurred on day 128 when oral chronic GVHD was noted. WT1-CTL became undetectable after day 268, but the disease has not progressed up to day 359. The other patient became positive for WT1-CTL on day 90, one week after the onset of liver acute GVHD. Cyclosporine was started at a dose of 400 mg/body and WT1-CTL disappeared. However, when cyclosporine was tapered to 100 mg/body, WT1-CTL became detectable again on day 146 following the development of skin GVHD. While the number of WT1-CTL was correlated with the presence of allo-reaction and cyclosporine treatment, the total lymphocyte count and CMV-CTL counts did not change significantly. This patient is currently showing stable disease beyond day 200. This is the first report of the precise monitoring of WT1-CTL after SCT in patients with RCC, and of the correlation of WT1-CTL with the clinical response. Further studies will be needed to evaluate the correlation between WT1-CTL and disease control.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal