Abstract
Heparin therapy decreases plasma factor VIIa (FVIIa) levels as measured by the soluble tissue factor (sTF)-based clotting assay, but it is unclear whether the measured decrease is a real in vivo effect, an in vitro artifact, or both. Because plasma samples are diluted tenfold and mixed 1:1 with FVII-deficient plasma, patient tissue factor pathway inhibitor (TFPI) usually contributes only a small portion of the TFPI in the assay. So, mild in vivo variation of TFPI has negligible effect on measuring FVIIa. However, marked in vivo elevations of TFPI during heparin therapy might interfere with the assay, thus artifactually decreasing measured FVIIa levels.
Methods: Plasma FVIIa was measured using the STAclot VIIa-rTF kit. In some tests, a blocking anti-TFPI monoclonal antibody (T4E2) was added to the sTF reagent (0 or 50 μg/ml IgG). Equal volumes of sample (test plasma or assay standard), FVII-deficient plasma, and sTF reagent were mixed and incubated at 37°C for 1 h, after which clotting was initiated by adding CaCl2. Free TFPI levels were measured with Asserachrom Free TFPI (Diagnostica Stago).
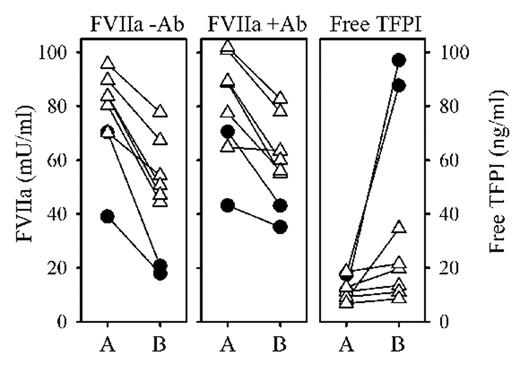
Results: Anti-TFPI IgG shortened the clotting times of the assay standards and normal pooled plasma (free TFPI concentration, 7.3 ng/ml), but resulted in no net change in measured FVIIa. Adding TFPI to pooled plasma interfered with FVIIa measurement only when free TFPI exceeded 70 ng/ml. FVIIa levels in plasma samples from ten normal individuals (28–141 mU/ml FVIIa) were measured with and without anti-TFPI IgG and again there was no significant change in measured FVIIa levels. Adding 100 ng/ml TFPI to these samples decreased their apparent FVIIa levels by 13–44% when measured in the absence of blocking anti-TFPI IgG, but this was completely abrogated by anti-TFPI IgG. Pre-heparin and post-heparin samples from normal individuals (see Figure) treated with either Dalteparin (D, n=6, triangles) or unfractionated heparin (U, n=2, circles) were then evaluated. [A= pre-heparin; B= post-heparin.] In each case, free-TFPI increased and FVIIa (measured -Ab) decreased after heparin administration. FVIIa levels measured without TFPI interference (+Ab) also decreased, although less profoundly.
Conclusions: Only very high levels (>70 ng/ml) of free TFPI interfere with the FVIIa assay. To properly assess TFPI interference, it is essential that blocking anti-TF IgG be added to both the samples and the assay calibrators from which the standard curve is prepared. TFPI levels > 70 ng/ml are generally not found in normal individuals or in the majority of disease conditions, but can exist in patients receiving heparin. Removing the influence of TFPI on the FVIIa assay (using a blocking antibody) only partially accounts for the decrease in measured FVIIa levels in response to heparin. A portion of this decrease in FVIIa levels may therefore be attributed to the ability of heparin to directly accelerate the inactivation of factor VIIa by plasma protease inhibitors, including antithrombin.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal