Abstract
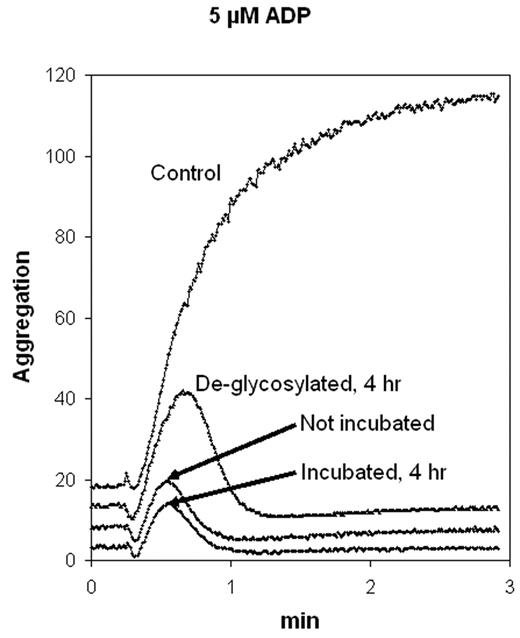
To study thrombogenesis in wild-type and transgenic mice we engineered a recombinant, soluble form of murine ENTPDase1/CD39 for use as a therapeutic agent. ENTPDase1 contains N- and C-terminal intracellular and transmembrane regions flanking an extracellular domain containing the 5 enzymatically active apyrase-conserved regions. The soluble portion of murine CD39 was amplified using PCR with 5′-XhoI and 3′-XbaI linked primers and cloned into the XhoI/XbaI-digested yeast expression vector pGAPZα. The vector, which features the constitutive GAP promoter and α factor secretion signal, was modified by elimination of the Ste13 cleavage sites and by introduction of a 5′-6His tag separated from the murine solCD39 coding sequence by an enterokinase cleavage site. Stably expressing clones were selected. Aliquots of 30-fold concentrated conditioned medium, obtained following 72 h culture of a yeast clone, were assayed for ADPase and ATPase activity and for the ability to inhibit platelet reactivity. Nucleotidase activity was monitored by native gel electrophoresis and staining using ATP metabolism to phosphate and conversion of Ca-phosphate into PbS, as well as by radio-TLC analyses. Platelet aggregation was quantitated using ADP and collagen as stimuli in heparinized and citrated human platelet-rich plasma (PRP). SolCD39 produced in mammalian cells (CHO, or COS-1) yields an active enzyme that is heavily N-glycosylated. Similarly, Pichia pastoris-produced (murine) solCD39 yields a comparably active protein that is also heavily glycosylated, albeit with the yeast-specific high mannose-type N-glycans. Its ADPase to ATPase activity ratio was ~1.2. Yeast-derived murine solCD39 was treated with PNGase F, an amidase that cleaves between the innermost N-acetyl glucosamine of complex oligosaccharides and asparagine residues of glycoproteins. This removed all saccharides from solCD39, as shown by PAS staining, and by mobility shifts by Western blot analysis and Pb staining in native gels. Interestingly, the deglycosylated protein retained ~60% of both enzymatic and biological activities as compared to the parent molecule. The figures shows platelet aggregation to 5 μM ADP in citrated PRP for control (no murine solCD39), isolated solCD39, and equal quantites of solCD39 that was deglycosylated, as well as subjected to the deglycosylation reaction in the absence of PNGase F. These data are similar for the present murine yeast-produced molecule and COS-produced human solCD39. Our data indicate the possibility of high scale production of solCD39 in yeast, with excellent yield, stability and antithrombotic activity.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal