Abstract
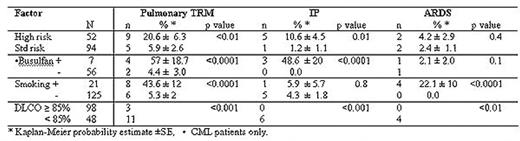
Between 7/1997 and 8/2004, 146 consecutive patients with hematological malignancies received a T cell depleted peripheral blood stem cell transplant (PBSCT) from an HLA identical sibling using three successive conditioning regimens: (A): 13.6Gy total body irradiation (TBI) + cyclophosphamide 120mg/kg (Cy) (n=85) , (B) 12.0Gy TBI with lung shielding to 9.0 Gy + Cy + fludarabine 125mg/m2 (Flu) , n= 35, (C) 12.0Gy TBI with lung shielding to 6.0 Gy + Cy + Flu, n= 26. Ninety-four (65.4%) had standard risk disease (transplant in first complete remission of acute leukemia, CML in chronic phase, and MDS-RA); the remainder had more advanced disease or unfavorable diagnoses. Actuarial transplant related mortality (TRM) was 16.4 ± 3%, at a median time of 103 days (range 23–238). Of the 21 transplant related deaths 14 (67%) were from pulmonary causes (6, idiopathic interstitial pneumonia (IP), 4 acute respiratory distress syndrome (ARDS), and pneumonia from CMV (2), RSV (1) and bacterial origin (1). Median time to death from IP and ARDS was 71 and 66 days post-transplant respectively. Kaplan-Meier analysis was used to study factors affecting pulmonary TRM. Pre-transplant characteristics predictive for pulmonary-related TRM are shown in the table. Patients with high risk disease and CML patients who had received busulfan for more than one month were at significantly greater risk of developing both pulmonary TRM and IP. Patients who smoked were significantly more at risk to develop ARDS and fatal pulmonary infection. Pre-transplant pulmonary function tests were highly predictive for pulmonary TRM. Diffusion capacity of the lung for carbon monoxide (DLCO), vital capacity and FEV-1 were highly correlated, but the most predictive parameter was DLCO: Of 48 patients with <85% normal vs. 98 with >85% of normal diffusion capacity, 6 vs 0 had IP, p<0.001, 4 vs 0 had ARDS, p=0.01 and 11 vs 3 had pulmonary TRM, p=0.0003. The death from all pulmonary causes was highest in protocol A (no lung shielding) and significantly less in conditioning regimens B+C (lung shielding) (12/85 vs 2/61 deaths, p = 0.05). These results indicate that after TBI and PBSCT, pulmonary causes contribute significantly to TRM, but can be predicted by patient characteristics and pulmonary function tests and may be reduced by lung shielding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal