Abstract
Outcomes of patients with active AML/MDS remain poor after HSCT. Treatment failure is in part a result of poor disease control with the preparative regimen. Here we report toxicity and efficacy of the immunoconjugate GO incorporated to a reduced intensity regimen.
Methods: Objective: determine which dose of GO between doses 2 and 4mg/m2 has the lowest toxicity(tox) probability with the highest response probability when added to FM (statistical method of Thall, Estey, Sung (1999)). Tox was defined as grade 3–4 organ tox, engraftment failure or early death (first 30 days post HSCT). Response was defined as engraftment, no tox and remission (CR) on day +30. Trial was planned with dose levels 4, 6 and 9 mg/m2 but given tox observed at 4mg/m2, the other dose levels were not explored and dose 2mg/m2 was added. Eligibility: patients (pts) age 12–75 years, ineligible for high-dose regimens or with high-risk CD33+ AML/MDS. Donors: related or unrelated (MUD). Treatment plan: GO 2 or 4mg/m2 day -12, fludarabine 30mg/m2 (days -5 to -2), melphalan 140mg/m2 (day -2); HSCT day zero. ATG was added in MUD HSCT. Graft-versus-host disease (GVHD) prophylaxis: tacrolimus and mini-methotrexate (5mg/m2 day+1,+3,+6,+11). GO was given on day -12 to avoid delay in engraftment and to decrease the number of committed myeloid progenitors prior to FM. Here we also included pts. treated under a compassionate IND and performed a non-randomized comparison with historic pts. treated with FM140 mg/m2 without GO.
Results: 29 pts. have been treated. Table shows baseline characteristics.
| GO dose . | 0 (n=49) . | 2 mg/m2 (n=21) . | 4 mg/m2 (n=8) . | P value . |
|---|---|---|---|---|
| Remission at BMT | 26% | 9% | 12% | 0.25 |
| MUD | 41% | 28% | 25% | 0.52 |
| Median age (years-range) | 54 (21–75) | 53 (13–72) | 30 (23–67) | 0.08 |
| Peripheral blood stem cell transplant | 47% | 86% | 100% | 0.0005 |
| High-risk cytogenetics | 43% | 33% | 37% | 0.83 |
| GO dose . | 0 (n=49) . | 2 mg/m2 (n=21) . | 4 mg/m2 (n=8) . | P value . |
|---|---|---|---|---|
| Remission at BMT | 26% | 9% | 12% | 0.25 |
| MUD | 41% | 28% | 25% | 0.52 |
| Median age (years-range) | 54 (21–75) | 53 (13–72) | 30 (23–67) | 0.08 |
| Peripheral blood stem cell transplant | 47% | 86% | 100% | 0.0005 |
| High-risk cytogenetics | 43% | 33% | 37% | 0.83 |
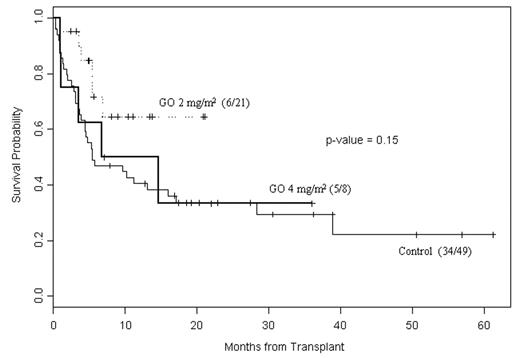
Median follow-up is 10 months (range 2.5–36) for GOFM alive patients (n=18). CR rate: GO 2mg/m2 =90%; GO 4 mg/m2= 71%; control=71%. There was only 1 case of moderate, reversible hepatic VOD (GO 2mg/m2). Grade III-IV tox and early death rates were: GO 2 mg/m2, 19% and 5% (n=1 due to pulmonary hemorrhage); GO 4mg/m2, 25% and 25% (n=2, due to pneumonia and multi-organ failure); control, 21% and 12% (n=6). Acute gd. II-IV GVHD rate was 44% for GO treated patients. All evaluable patients had engrafted by day +30; 8 pts have relapsed.
Comparison with historic controls: active disease and high-risk cytogenetics were significantly associated with worse event-free (EFS) and overall survival (unadjusted log-rank test). Cox proportional hazards model: risk of death or relapse was increased with older age (RR 1.02; P=0.06), MUD vs related donor (RR 1.91; P=0.03), active disease vs CR (RR 0.23; P=0.001), and having GO 4 mg/m2 or zero vs 2 mg/m2 (RR 0.46, P=0.04).Figure shows survival.
Conclusions: The addition of GO at the 2 mg/m2 dose level is promising and merits evaluation in a larger, controlled trial.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal