Abstract
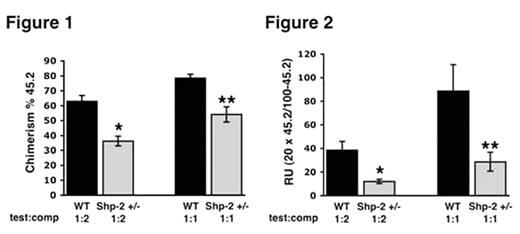
The protein tyrosine phosphatase, Shp-2, has been shown to be necessary for normal hematopoiesis based on embryonic stem (ES) cell-based assays; however, due to the early lethality of the homozygous Shp-2 mutant mice (Shp-2−/−) the role of Shp-2 in adult hematopoietic stem cell (HSC) function has never been examined. The Shp-2 heterozygous mice (Shp-2+/−) bear a mutant allele of the Shp-2 gene resulting in the production of a mutant protein lacking amino acids 46–110, which confers a loss of function. To test the hypothesis that Shp-2 is required for normal HSC activity, we compared the competitive repopulating ability of Shp-2+/− bone marrow-derived cells with WT cells. Total adult bone marrow low density mononuclear cells were isolated from Shp-2+/− and WT littermate controls (test cells, C57Bl/6 background, CD45.2+), mixed with a common pool of competitor (comp) cells (BoyJ background, CD45.1+), and administered to lethally irradiated (1100 cGy split dose) Gpi/BoyJ recipients. Based on peripheral blood chimerism, the repopulating ability of the Shp-2+/− cells was significantly lower than that of the WT cells (Figure 1, *p<0.0001 Shp-2+/− v. WT at ratio 1:2; **p=0.001 Shp-2+/− v. WT at ratio 1:1). We next converted the chimerism to repopulating units using the formula [competitor number x 105] X [% 45.2]/100 − [% 45.2] to quantitatively asses the repopulating defect in Shp-2+/− HSCs. We observed that the repopulating units of the Shp-2+/− cells was approximately 3-fold lower than that of the WT cells at both cell doses administered (Figure 2, *p=0.003 Shp-2+/− v. WT at ratio 1:2; **p=0.03 comparing Shp-2+/− v. WT at ratio 1:1). Multi-lineage analysis using two color fluorescence cytometry revealed a significantly lower contribution of Shp-2+/− cells to all lineages tested (B220, GR1, Mac, and CD4/8) compared to WT cells.
As Shp-2 has been shown to participate in cell migration, we sought to rule out a homing deficiency of the Shp-2+/− HSCs. We performed short term homing assays and observed no difference in spleen-homed or bone marrow-homed Shp-2+/− and WT lin- cells twenty hours following transplantation. To evaluate self-renewal potential, we conducted serial transplantation experiments. Total bone marrow low density mononuclear cells were isolated from primary or seconary recipient mice with equal chimerism and transplanted into lethally irradiated (1100 cGy split dose) Gpi/BoyJ recipients. While no significant difference was observed between Shp-2+/− and WT engraftement in secondary transplants, eight weeks following tertiary transplantation, engraftment of the Shp-2+/− cells is significantly lower than that of the WT cells (WT 68.9% +/− 9.5 v. Shp-2+/− 26.1% +/− 11.7, n=6, p<0.0001) suggesting that a self-renewal defect contributes to the decreased HSC activity of the Shp-2+/− cells. These data demonstrate that Shp-2 function is not only necessary within the progenitor compartment to support proficient hematopoiesis, but is also needed within the HSC compartment to support normal HSC self-renewal. These findings provide insight into how oncogenic Shp-2 potentially may contribute to the dysregulation of hematopoiesis and the pathogenesis of childhood leukemias.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal