Abstract
Attenuated androgens like danazol are the current treatment of choice for long-term prophylaxis in patients with hereditary angioedema (HAE), who may experience life-threatening acute attacks. Unfortunately, this group of drugs bears the risk of severe side effects (e.g. depression, weight gain, hirsutism, transaminase elevations, liver adenoma and carcinoma, headaches, hypertension, menstrual abnormalities).
Objectives: We therefore explored the option to administer a pasteurized, plasma-derived C1-Inhibitor concentrate (C1-INH, Berinert®P) for individual replacement therapy (IRT) in patients with HAE in whom danazol induced severe side effects, was not effective or was contraindicated (e.g. children, pregnant women).
Methods: Prospective, observational, intra-individual cross over study, comparing efficacy, safety and quality of life of danazol (prophylaxis) vs. C1-INH (IRT) in 23 patients suffering from severe HAE.
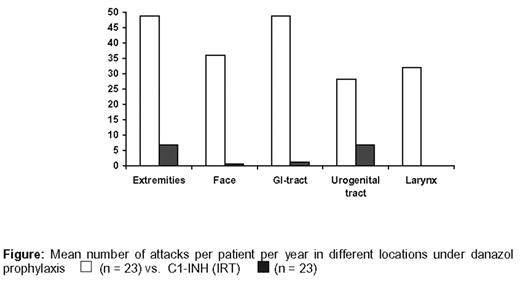
Results: Mean annual attack frequency decreased from 48.8 ± 37.1 (danazol) to 8.1 ± 22.1 (C1-INH); p<0.001. All patients who received pasteurized C1-INH were free of life threatening attacks (laryngeal edema) or adverse events, which did occur under long-term prophylaxis with danazol (Fig.). In addition, all quality of life parameters improved significantly; p<0.001. During the entire 20-year observation period with C1-INH in all patients there was no transmission of HIV type 1/2, hepatitis A-, B-, C-, G-viruses, or parvovirus B19.
Conclusions: Compared to danazol, IRT with pasteurized C1-INH in patients with severe HAE significantly reduces the frequency of HAE attacks, especially of life-threatening attacks, and significantly improves the quality of life.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal