Abstract
In Ph+ leukemia, the breakpoints within BCR cluster in 3 distinct breakpoint cluster regions (bcr). Rearrangements in the minor (m−), major (M−) and micro (m−) breakpoint cluster region (bcr) give rise to e1a2, e13/14a2 and e19a2 BCR-ABL fusion proteins that are associated with Ph+ ALL, CML and chronic neutrophilic leukemia, respectively. Atypical BCR-ABL fusions have also been reported, mostly in isolated cases. Here, we describe eight patients with an unusual e8a2 BCR-ABL transcript and provide an overview of their common characteristics. The index case (#1) was a 56 year old man diagnosed with CML in 1993. Therapy with busulfan and hydrea induced a complete hematologic remission (CHR) that was maintained until March 2001, when accelerated disease developed. Treatment with imatinib and subsequently AML-type chemotherapy were only transiently effective and the patient died from myeloid blast crisis in March 2003. Cytogenetics demonstrated a standard t(9;22)(q34;q11). Unexpectedly, FISH was consistent with an m-bcr rearrangement and multiplex PCR showed an unusually large band. Sequencing identified a fusion between BCR exon e8 and ABL exon a2, with a 55 bp insert corresponding to an inverted segment of ABL intron Ib (corresponding to nt 29861–29915, Genbank U07561). Immunoblotting of bone marrow mononuclear cells with anti-ABL antibody identified a 200 kDa protein (p200BCR-ABL).
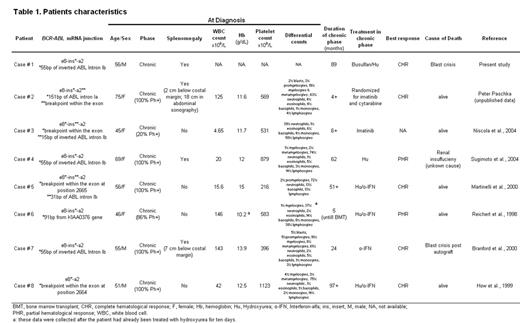
Seven additional patients with an e8a2 BCR-ABL fusion have been identified (table 1). These patients tend to have relatively high platelet counts (median 569 x 109/L, range 216 – 1123) and relatively low white counts (median 42 x 109/L, range 4.7 – 146) but no other distinguishing characteristics at diagnosis. With a median follow-up of 36 months (range, 4 –97), two patients had progressed to blast crisis and died, one died from an unrelated cause, and five were alive, one after allogeneic BMT. Remarkably, none of the patients treated with interferon-a achieved even a minor cytogenetic remission, similar to patients with p190-positive CML. Two patients besides case #1 have received imatinib, one has achieved a CHR at four months, and information on the other is not available. Compared to P190 and P210, p200 lacks the pleckstrin homology (PH) domain but retains the Dbl-like and CDC24 homology domains of BCR. Deletion of the PH domain may disrupt the function of the Dbl-like domain to serve as a GTP exchange factor for Rac, Cdc42 and Rho. This may lead to disruption of the actin cytoskeleton and would be expected to cause a disease that is biologically closer to p190 than to p210.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal