Abstract
Retinoic acid (RA) overcomes the maturation block in t(15:17) acute promyelocytic leukemia (APL), leading to granulocytic differentiation. Patients receiving RA alone invariably develop RA resistance. RA-resistant cells can serve as useful models for the development of treatments for both APL and other leukemias. Previously, we showed that RA and tumor necrosis factor (TNF) promote monocytic differentiation of the APL cell line NB4 and U937 monoblastic cells. Here, we report that combining TNF with RA leads to maturation of several RA-resistant APL cells along a monocytic pathway, whereas UF-1, a patient-derived RA-resistant cell line, showed characteristics of granulocytic differentiation. We found distinct differences in gene regulation between UF-1 cells and cells showing monocytic differentiation. Although IRF-7 was up-regulated by TNF and RA in all cells tested, expression of c-jun and PU.1 correlated with monocytic differentiation. Furthermore, synergistic induction of PU.1 DNA binding and macrophage colony-stimulating factor receptor (m-CSF-1R) mRNA was observed only in cells differentiating into monocytes. Using neutralizing antibodies against m-CSF-1R or its ligand, we found that inhibiting this pathway strongly reduced CD14 expression in response to RA and TNF, suggesting that this pathway is essential for their synergy in RA-resistant leukemia cells. (Blood. 2004;104:3335-3342)
Introduction
Acute promyelocytic leukemia (APL) arises from a block in the normal maturation process of myeloid progenitors at the promyelocyte stage. This leukemia is unique in its ability to respond to retinoic acid (RA), which overcomes the block in differentiation and restores normal granulopoiesis. Treatment with RA alone results in resistance,1 but combination treatments with RA have proven very successful.2 However, resistance to conventional therapies can still occur, and finding novel means to overcome this resistance remains an important goal in the treatment of APL. RA-sensitive and -resistant APL cells have served as a useful model for studying retinoid receptor function and for developing novel differentiation therapies, such as cyclic adenosine monophosphate (cAMP) analogs3,4 and histone deacetylase inhibitors,5 that may have relevance to other leukemias.6,7 However, the clinical applicability of these therapies remains to be determined.
Although pharmacologic mechanisms may play a role in vivo,8 acquired mutations in the PML/retinoic acid receptor α (RARα) oncoprotein are a common means by which RA resistance arises both in vivo and from in vitro-derived cell models of RA-resistant APL. Resistance mutations often result in a dysfunctional PML/RARα that is unable to efficiently promote transcription of RA target genes.9,10 The mutations can work through several mechanisms, including altering the affinity of PML/RARα for its ligand and reducing its ability to recruit coactivators. There are also several models of APL resistance that currently have undefined mechanisms of resistance.11,12
Combination therapies that can overcome this resistance may act either by reactivating a blocked RA pathway or, alternatively, by way of stimulation of novel pathways that can mediate a differentiation response. Major challenges hamper the rational development of combination therapies with RA against APL or other malignancies. These include understanding the downstream effectors of both RA and different pathways that may be activated in response to the combination of RA and other agents. Finding these effectors could provide new specific therapeutic targets to release the differentiation block. A few targets of RA that appear to play an important role in granulopoiesis include the CCAAT enhancer-binding protein (C/EBP) family of transcription factors,13,14 the cell cycle inhibitor p21,15 and possibly interferon family members.16 However, there are almost certainly other targets involved.
To date, most agents that act in synergy with RA to overcome RA resistance, such as trichostatin A (TSA) and cAMP, appear to restore normal PML/RARα transcriptional activity,10,17 resulting in granulocytic differentiation. In contrast, tumor necrosis factor (TNF) synergizes with RA to activate an alternate pathway, resulting in monocytic differentiation.18 Here, we report that costimulation with RA and TNF can overcome resistance to RA in cells having mutant PML/RARα proteins and also in resistant cells where the mechanism of resistance is ill defined. TNF is a cytokine involved in numerous cellular processes, including apoptosis, inflammation, and differentiation.19-23 The strong inflammatory response to TNF in vivo has limited its use clinically. However, recent articles suggest that inflammatory shock can be overcome and that TNF can sensitize tumors to other antitumor drugs in vivo.24,25 TNF acts through its cognate receptors (TNFR1 and 2) that relay downstream signals through at least 2 distinct pathways. The first signal activates apoptosis through a cascade, beginning with caspase 8 and leading to DNA cleavage and cell death.26 The second pathway stimulates translocation of the nuclear factor-κB (NF-κB) family of transcription factors into the nucleus, where they activate numerous target genes. Activation of NF-κB inhibits TNF-induced apoptosis and mediates many of the nonapoptotic functions of the TNF pathway. The importance and versatility of the NF-κB pathway are underscored by the ability of numerous extracellular stimuli,27 including RA,18 to activate its transcription. It remains to be determined how each stimulus activates a distinct subset of NF-κB targets.
Previously, we described cross talk between RA and TNF pathways that led to monocytic differentiation of NB4 and U937 cells.18 Coincident with their effects on differentiation was an increase in NF-κB transcriptional activation and a synergistic induction of several NF-κB target genes, including TNFR2 and BCL-3. Here, we show that TNF and RA promote monocytic differentiation of several in vitro-derived RA-resistant APL cell lines, as well as granulocytic differentiation of a patient-derived RA-resistant APL cell line, UF-1. We found distinct differences between cells maturing along a monocytic lineage and UF-1. Expression of the transcription factors c-jun and PU.1 correlated with monocytic differentiation. Furthermore, synergistic induction of the macrophage colony-stimulating factor receptor (m-CSF-1R) mRNA and DNA binding of PU.1 were all observed in monocytic-differentiating cells but not in UF-1 cells.
The m-CSF-1R pathway has been shown to regulate and maintain a balance between the differentiation, survival, and proliferation of monocytes and macrophages.28,29 Using neutralizing antibodies, we investigated whether this pathway is necessary in carrying out the promonocytic differentiation response of TNF and RA.
Materials and methods
Materials
RPMI-1640 and fetal bovine serum (FBS) were purchased from Invitrogen (Burlington, Canada). RA was obtained from Sigma (Oakville, ON, Canada). Recombinant human TNF was obtained from PeproTech (Princeton, NJ). Nitroblue tetrazolium dye was supplied by Sigma (Oakville, Canada). α32P adenosine triphosphate (ATP) was from PerkinElmer (Wellesley, MA). For Western blotting, anti-RAR, c-jun, and PU.1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Neutralization studies used anti-m-CSF-1R, (Upstate Biotechnology, Lake Placid, NY), anti-m-CSF (Research Diagnostics, Flanders, NJ), and rabbit IgG (PeproTech). Both neutralization antibodies inhibit binding of the ligand to its receptor as determined by the manufacturer. Antibodies were replenished at day 3 of the 5-day treatments for MR2, MR6, and MRA1 cell lines.
Cell culture
Derivation of the RA-resistant cell lines MR2, MR4, MR6, and MRA1 from the parental APL cell line NB4 was previously described.5,11 These cells were maintained in RPMI-1640 supplemented with 10% FBS (Invitrogen, Burlington, ON, Canada). UF-1 cells, obtained from M. Kizaki (Keio University, Tokyo, Japan),30 were grown in RPMI-1640 supplemented with 10% FBS (HyClone, Logan, UT). Cell growth was quantified by using standard hemocytometer technique with trypan blue exclusion assay. Cells were treated with 10-6 M RA and 10 ng/mL TNF unless otherwise specified.
Differentiation assays
Cells to be used in both nitroblue tetrazolium (NBT) reduction assays and for fluorescence-activated cell sorting (FACS) analysis of differentiation markers were seeded at 3 × 105 cells/well in 6-well plates. NBT assays were performed as previously described.31 Briefly, approximately 2 × 105 cells were placed in RPMI-1640 medium and mixed with an equal volume of NBT (1.0 μg/mL). The mixture was then rotated at 37°C for 45 minutes. Fraction of NBT-positive cells was determined by using a hemocytometer. Cell surface expression of CD14 and CD11b was determined by flow-assisted cell sorting performed according to antibody manufacturer's specifications (Pharmingen, San Diego, CA) using FACSCAN cytometer (Becton Dickinson Labware, Bedford, MA). Note, cell surface levels of m-CSF-1R were determined in similar fashion by using the aforementioned m-CSF-1R antibody. For m-CSF and m-CSF-1R neutralization experiments, 5 μL antibody was added per 5 mL media and subsequently replenished 3 days after treatment. Terminal differentiation was determined by the inability of cells to repopulate after an initial period of treatment with RA and TNF. Briefly, treated cells were washed in phosphate-buffered saline (PBS), reseeded in RPMI containing full serum, and counted. Cultures that did not increase in cell number after 5 days were said to be terminally differentiated.
mRNA analysis
Total RNA was isolated by using guanidinium thiocyanate extraction as previously described.32 For Northern blotting, 20 μg RNA was electrophoresed on a 1% formaldehyde agarose gel and blotted onto Zeta probe (BioRad, Mississauga, ON, Canada) transfer membranes. cDNA probes were labeled by random priming (Amersham-Pharmacia, Piscataway, NJ). Hybridization and autoradiography were performed as previously described.33 Full-length cDNAs encoding interferon regulatory factor 7 (IRF-7) and m-CSF-1R were generous gifts of Dr John Hiscott (Jewish General Hospital and McGill University, Montreal, QC, Canada) and Dr Martine Roussel (St Jude Children's Research Hospital, Memphis, TN), respectively.
Western analysis
Cell lysate enriched for nuclear proteins was used for Western analyses of PML/RARα, c-jun, and PU.1. Cells were washed in PBS after which they were resuspended in 400 μL buffer A (10 mM Tris (tris(hydroxymethyl)aminomethane)-Cl pH 7.8, 10 mM KCl, 5 mM MgCl2, 0.1 mM EDTA (ethylenediaminetetraacetic acid), 300 mM sucrose, 0.5 mM dithiothreitol (DTT) plus protease inhibitors) and chilled on ice for 10 minutes. Five microliters 10% NP-40 was added to each sample and mixed by vortex to lyse cells. Nuclei were isolated through the sucrose gradient by centrifugation for 1 minute at approximately 50g. Supernatant was removed, and the nuclei were resuspended in 50 μL buffer B (20 mM Tris-Cl pH 7.8, 5 mM MgCl2, 320 mM KCl, 0.2 mM EDTA, 25% glycerol, 0.5 mM DTT plus protease inhibitors). Tubes were chilled on ice for 10 minutes with occasional tapping followed by centrifugation for 15 minutes at top speed to remove debris. Equal amounts of protein were separated on 10% polyacrylamide gels containing 0.1% sodium dodecyl sulfate (SDS) and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Membranes were probed with antibodies at a dilution of 1:500. Antibody binding was detected with the enhanced chemiluminescence (ECL) system (Amersham-Pharmacia).
Electrophoretic gel mobility shift analyses
Gel shift analyses for PU.1 were performed as described elsewhere.34 Reactions were carried out for 30 minutes by using the consensus PU.1 oligonucleotide 5′-GGGCTGCTTGAGGAAGTATAAGAAT-3′ with 12 μg nuclear extracts (isolated as previously described). Supershift reactions were done using anti-PU.1 antibodies from Santa Cruz. Activator protein-1 (AP-1) gel shift analysis was performed by using a probe corresponding to an AP-1 consensus element 5′-CGCTTGATGACTCAGCCGGAA-3′. Mutant AP-1 oligonucleotides had the CA underlined in the aforementioned oligo substituted for TG.
Results
RA and TNF can combine to overcome RA-resistant APL
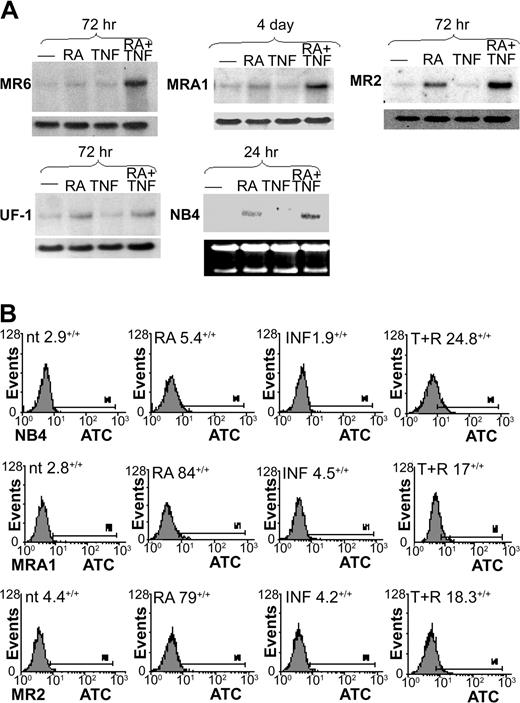
Previously, we described cross talk between TNF and RA pathways that synergistically induced maturation of NB4 APL cells, as well as U937 monoblastic cells. Here, we examined whether this effect could be extended to RA-resistant cells by using several RA-resistant APL cell models. We have previously isolated RA-resistant sublines from parental NB4 cells designated MR2, MR4, MR6, and MRA1,5,11 and the patient-derived RA-resistant cell line UF-1 has been described elsewhere.30 All these lines express full-length PML/RARα oncoprotein, but it is found in a mutated form in MR4, MRA1, and UF-1 (Table 1). These mutations are thought to play a central role in the ability of these 3 cell lines to resist RA-induced maturation. The cell lines MR2 and MR6 have acquired resistance through an undetermined mechanism, but both display altered protein binding profiles from high-performance liquid chromatography (HPLC) pulldowns with labeled RA.11 This suggests that a different complex of proteins associates with the PML/RARα in R2 and R6 cells than in the NB4 parental line. NBT analyses indicated a prodifferentiation response to TNF and RA in all cell lines tested regardless of PML/RARα status (Figure 1). Increased maturation in response to RA and TNF could be observed within 4 to 5 days after treatment, except for UF-1, which was the most sensitive to RA and TNF, showing almost complete differentiation after only 3 days of treatment. Even MR4, the most resistant line to TNF and RA, showed a weak but synergistic response to the combination. Interestingly, of the cell lines tested, this cell line also shows the weakest transcriptional response to RA alone5,10 (and M.W., W.H.M., unpublished data, December 2002). Terminal differentiation of these cells lines was also supported by the inability of cells treated with RA and TNF to repopulate in control media after treatment for 3 (UF-1 cells) or 5 (MRA1, MR2, MR4, MR6) days (data not shown).
Overview of response of RA-resistant cells to RA and TNF or HDAC inhibitor
Cell line . | Mutation position/observed change . | Result of mutation . | ATRA activation of RARB . | PML/RAR degraded in response to 72 h of RA? . | Differentiate in response to RA and TNF? . | Differentiate in response to inhibitors of HDAC and RA? . |
|---|---|---|---|---|---|---|
| UF-1 | 276 LBD | Arg-Trp | +/− | — | + | — |
| MRA1 | 410 LBD | Ile-Thr | +/− | +/− | + | + |
| MR4 | 398 LBD | Leu-Pro | — | — | +/− | +/− |
| MR2 | Altered PML/RAR binding profile | — | + | + | + | +/− |
| MR6 | Altered PML/RAR binding profile | — | + | + | + | +/− |
Cell line . | Mutation position/observed change . | Result of mutation . | ATRA activation of RARB . | PML/RAR degraded in response to 72 h of RA? . | Differentiate in response to RA and TNF? . | Differentiate in response to inhibitors of HDAC and RA? . |
|---|---|---|---|---|---|---|
| UF-1 | 276 LBD | Arg-Trp | +/− | — | + | — |
| MRA1 | 410 LBD | Ile-Thr | +/− | +/− | + | + |
| MR4 | 398 LBD | Leu-Pro | — | — | +/− | +/− |
| MR2 | Altered PML/RAR binding profile | — | + | + | + | +/− |
| MR6 | Altered PML/RAR binding profile | — | + | + | + | +/− |
Compilation of data showing genetic lesions in RA-resistant APL cells and their sensitivity to RA and TNF or histone deacetylase (HDAC) inhibitor treatment. Results show that several cell lines resistant to RA and HDAC inhibitors were found to respond well to RA and TNF treatment. Table is based on both new and previously published data.5,35 ATRA indicates all-trans retinoic acid; RARB, retinoic acid receptor β; LBD, ligand-binding domain; +/−, weak response; and—, no response.
RA and TNF induced differentiation of RA-resistant APL cells. Differentiation of cells in response to RA and TNF for varying times. Results are representative of 3 experiments performed in triplicate. (A) Results of NBT reduction assay performed on RA-resistant APL cells treated with RA and TNF for the indicated time periods. Synergy in the number of NBT-positive cells could be observed at concentrations of 10-6 M RA and 10 ng\mL TNF. (B) Cytofluorimetric analysis of surface marker expression. Percentage of cells expressing the monocyte-specific CD14 and myeloid-specific CD11b cell surface markers of differentiation was determined by using monoclonal fluorescein isothiocyanate (FITC; CD14) and phycoerythrin (PE; CD11b) labeled antibodies. Results are representative of 1 experiment of 3 performed in triplicate.
RA and TNF induced differentiation of RA-resistant APL cells. Differentiation of cells in response to RA and TNF for varying times. Results are representative of 3 experiments performed in triplicate. (A) Results of NBT reduction assay performed on RA-resistant APL cells treated with RA and TNF for the indicated time periods. Synergy in the number of NBT-positive cells could be observed at concentrations of 10-6 M RA and 10 ng\mL TNF. (B) Cytofluorimetric analysis of surface marker expression. Percentage of cells expressing the monocyte-specific CD14 and myeloid-specific CD11b cell surface markers of differentiation was determined by using monoclonal fluorescein isothiocyanate (FITC; CD14) and phycoerythrin (PE; CD11b) labeled antibodies. Results are representative of 1 experiment of 3 performed in triplicate.
Except for UF-1, all cells, including MR4, displayed a significant increase in CD14 when cotreated with RA and TNF (Figure 1B). This marker is strongly up-regulated during the differentiation of precursors into monocytes, indicating that the combination of TNF and RA specifically induced differentiation along a monocytic route, as was previously observed in NB4 and U937 cells.18 Although TNF and RA increased expression of CD11b and CD18 after only 3 days in UF-1 cells, there was no increase in CD14 expression. In fact, RA decreased the basal level of CD14 expression of these cells. Thus, unlike the NB4-derived subclones, these cells did not differentiate into monocytes. Morphologic analyses also demonstrated that UF-1 cells, unlike MRA1, MR2, and MR6 cells treated with TNF and RA, showed characteristics of granulocyte differentiation (data not shown). The ability of RA and TNF to overcome the differentiation block in these heterogeneous cell types suggests that this combination can more broadly activate differentiation than the combination of TSA with RA, which has not been effective in promoting differentiation in UF-1 cells (Table 1).
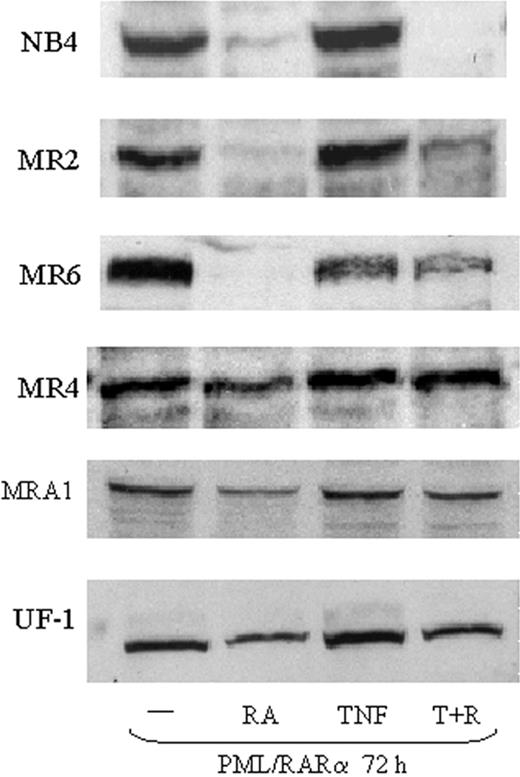
Effects of TNF and RA on PML/RARα expression
Several studies have suggested that targeted removal of PML/RARα is an essential step in overcoming RA-resistant APL.35 Therefore, we examined PML/RARα protein levels in resistant cells treated with TNF and RA. RA treatment of NB4 cells results in a full loss of PML/RARα within 12 to 24 hours. In MR2 and MR6 cells, PML/RAR was down-regulated by RA treatment alone, but only after 72 hours (Figure 2). RA had no significant effect on PML/RARα degradation in MR4 cells and only a slight reduction was seen in MRA1 and UF-1 cells. This is consistent with the MR4 cell line being the least sensitive to RA. We did not find that TNF enhanced RA-mediated PML/RARα degradation in any of the cell lines tested (Figure 2). In fact, TNF appeared to impair RA-induced proteolysis of PML/RARα after 72 hours of treatment in all the resistant cells tested (Figure 2). A similar decrease in PML/RARα degradation was seen when the tyrosine kinase inhibitor STI571 was used to promote APL differentiation with RA.36
Expression of PML/RARα in RA-resistant APL cells. Western blotting showing expression of PML/RAR in RA-resistant APL cells. Nuclear extracts (50 μg) were used for each analysis. Results show that TNF did not augment the degradation of PML/RARα in response to RA.
Expression of PML/RARα in RA-resistant APL cells. Western blotting showing expression of PML/RAR in RA-resistant APL cells. Nuclear extracts (50 μg) were used for each analysis. Results show that TNF did not augment the degradation of PML/RARα in response to RA.
These data indicate that TNF does not promote differentiation through effects on PML/RAR degradation and that cell sensitivity to the cooperative effects of TNF and RA does not depend on regulation of PML/RARα. Consistent with these data, and unlike other agents such as histone deacetylase (HDAC) inhibitors, TNF did not promote transcription through a retinoic acid response element (RARE)-linked chloramphenicol acetyltransferase (CAT) reporter construct (data not shown) in any of the resistant cells. This suggests the hypothesis that RA may be priming the cells for TNF-mediated differentiation rather than an effect of TNF on the RA pathway.
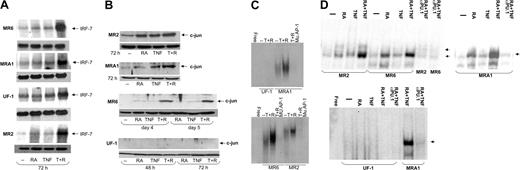
TNF and RA regulation of transcription factors involved in monocytic differentiation
Granulocytes and monocytes develop from common progenitors along closely linked pathways. There is much evidence that hematopoiesis is regulated through expression and controlled activation of lineage-specific transcription factors.37,38 Because most RA-resistant cells developed into a monocytic phenotype when treated with RA and TNF, we examined the expression of 3 transcription factors previously found to be involved in monocyte/macrophage differentiation.
IRF-7 has been reported to play a role in differentiation,39,40 but its target genes remain to be identified. Increased transcription of cytokines belonging to the interferon family may mediate the effects of IRF-7, but there are likely other targets, because it is not clear that interferon family members alone can initiate differentiation of leukemia cells.39,41 In RA-resistant APL cells, we found a large increase of IRF-7 mRNA levels in all cell lines tested within 3 days of treatment with TNF and RA (Figure 3A). Neither agent alone had a significant effect on IRF-7 levels. Thus, IRF-7 is synergistically induced in cells responding to TNF and RA.
Regulation of transcription factors involved in monocytic differentiation in RA-resistant APL cells. (A) Northern blotting of IRF-7 mRNA levels in RA-resistant APL cells. IRF-7 levels were seen to increase dramatically in cells treated with RA and TNF but not in response to either agent alone. The top panel for each cell line shows IRF-7 levels, and the bottom panel represents actin mRNA levels. (B) Western blotting showing c-jun expression in RA-resistant APL cells. Results show a positive correlation between c-jun expression and cells undergoing monocytic maturation. (C) Electrophoretic mobility shift assay (EMSA) analyses of AP-1 binding in RA-resistant APL cells in response to TNF and RA. Binding to an AP-1 site was seen to increase in response to these 2 agents in all cells tested except for UF-1. (D) EMSA analysis of PU.1 DNA binding in RA-resistant APL cells in response to RA and TNF at 72 hours after treatment. Binding data demonstrate an increased binding affinity of PU.1 in response to TNF and RA in cells undergoing monocytic differentiation. The presence of PU.1 in binding complexes was confirmed in reactions using anti-PU.1 antibodies. Complexes specifically inhibited by the PU.1 antibody are denoted with an arrow. Binding reactions were performed with a consensus PU.1 oligonucleotide and 12 μg nuclear extracts.
Regulation of transcription factors involved in monocytic differentiation in RA-resistant APL cells. (A) Northern blotting of IRF-7 mRNA levels in RA-resistant APL cells. IRF-7 levels were seen to increase dramatically in cells treated with RA and TNF but not in response to either agent alone. The top panel for each cell line shows IRF-7 levels, and the bottom panel represents actin mRNA levels. (B) Western blotting showing c-jun expression in RA-resistant APL cells. Results show a positive correlation between c-jun expression and cells undergoing monocytic maturation. (C) Electrophoretic mobility shift assay (EMSA) analyses of AP-1 binding in RA-resistant APL cells in response to TNF and RA. Binding to an AP-1 site was seen to increase in response to these 2 agents in all cells tested except for UF-1. (D) EMSA analysis of PU.1 DNA binding in RA-resistant APL cells in response to RA and TNF at 72 hours after treatment. Binding data demonstrate an increased binding affinity of PU.1 in response to TNF and RA in cells undergoing monocytic differentiation. The presence of PU.1 in binding complexes was confirmed in reactions using anti-PU.1 antibodies. Complexes specifically inhibited by the PU.1 antibody are denoted with an arrow. Binding reactions were performed with a consensus PU.1 oligonucleotide and 12 μg nuclear extracts.
C-jun has long been known to play a role in monocytic differentiation of leukemia cells.42,43 Therefore, we examined its expression in RA-resistant APL cells treated with RA and TNF. Western blot analyses showed moderate c-jun expression in MR2 cells, less in MRA1 and MR6 cells, and none in UF-1 cells. C-jun up-regulation was seen in all cells that developed monocytic characteristics. Both MRA1 and MR2 cells had increased c-jun levels within 72 hours after treatment. R6 showed very little c-jun until 4 days after treatment, at which time a strong synergistic response to TNF and RA was observed. No c-jun expression was observed in the granulocytic-differentiating UF-1 cell line (Figure 3B). Levels of c-jun determined using Western blotting also correlated with gel shift analyses of protein binding to an AP-1 site, with c-jun binding in all cells undergoing monocytic differentiation, but not in UF-1 cells (Figure 3C). The basal level of c-jun binding in MR2 and MR6 cells did not correlate perfectly with levels observed from Western blotting. This may represent a difference in phosphorylation levels of c-jun in the 2 cell lines under resting conditions. Overall, these data clearly show a correlation between c-jun induction and monocytic differentiation. Consistent with these results, there was also no increase in c-jun expression during granulocytic differentiation of NB4 cells induced by RA (data not shown). However, TNF alone could induce c-jun levels by 2 hours after treatment (data not shown). This induction could be observed until 2 days after treatment. These data are again consistent with our conclusion that c-jun expression correlates with monocytic differentiation induced by RA and TNF.
The actions of c-jun on differentiation may be in part through its ability to act as a coactivator of PU.1-dependent transcription.44 PU.1 is a master regulator of myeloid differentiation, and its expression and regulation by interacting partners are thought to be important in initiating differentiation.45 To assess the activity of PU.1 in the presence of TNF and RA, we performed gel mobility shift assays using a PU.1 consensus oligonucleotide (Figure 3D). The gel shift analyses showed a small increase in PU.1 binding in response to RA alone, similar as to what is seen in NB4 cells (data not shown). Interestingly, a similar, small increase of PU.1 protein was seen on Western blots when these cells were treated with RA (data not shown). The combination of TNF and RA lead to a large increase in the DNA binding capacity of PU.1, demonstrating that the agents cooperate to activate this transcription factor. This synergy was not seen in NB4 cells (data not shown). However, Western blotting showed that TNF had no effect on PU.1 protein levels (data not shown). The presence of PU.1 in the binding complex was confirmed with supershift experiments using anti-PU.1 antibodies. In UF-1 cells, which did not display monocytic characteristics, this PU.1 complex could not be detected. These data were also supported by Western blotting that showed low to nondetectable levels of PU.1 in UF-1 cells (data not shown). Thus, increased PU.1 binding and c-jun expression are linked to monocytic differentiation driven by RA and TNF.
TNF and RA stimulation of the m-CSF pathway
Knock-out mouse and other studies have clearly defined a pivotal role for the m-CSF-1R in monocyte differentiation.46 Because the RA-resistant APL cells showed primarily a monocytic response to TNF and RA, we studied the regulation of this receptor in RA-resistant APL cells.
Northern blotting showed very low levels of m-CSF-1R mRNA in all cell lines tested (Figure 4A). Previous reports have shown m-CSF-1R levels to be increased in response to RA through an unidentified mechanism.47,48 We found a moderate increase in m-CSF-1R mRNA levels in MR2 cells and a slight increase in UF-1 cells in response to RA but not in MR6 or MRA1 cells. Previous reports described the RA induction of m-CSF-1R as a more rapid event than we observed in these RA-resistant cell lines. Consistent with these results, we found m-CSF-1R induction in the RA-sensitive cell line NB4 to be apparent within 24 hours of RA treatment.
m-CSF-1R expression in RA-resistant APL cells. (A) Northern blotting was used to examine m-CSF-1R levels in response to RA and TNF. m-CSF-1R levels were observed to increase in response to RA in only UF-1 and MR2 cells within 72 hours of treatment. The combination of TNF and RA induced a large increase in m-CSF-1R levels in all RA-resistant cells undergoing monocytic differentiation but not in UF-1 cells. (B) FACS analysis showing heightened cell surface expression of m-CSF-1R in response to 3-day treatments with TNF and RA. Percentages indicate positive staining.
m-CSF-1R expression in RA-resistant APL cells. (A) Northern blotting was used to examine m-CSF-1R levels in response to RA and TNF. m-CSF-1R levels were observed to increase in response to RA in only UF-1 and MR2 cells within 72 hours of treatment. The combination of TNF and RA induced a large increase in m-CSF-1R levels in all RA-resistant cells undergoing monocytic differentiation but not in UF-1 cells. (B) FACS analysis showing heightened cell surface expression of m-CSF-1R in response to 3-day treatments with TNF and RA. Percentages indicate positive staining.
In all cells showing monocytic maturation, RA and TNF induced m-CSF-1R mRNA synergistically within 3 to 4 days. FACS analyses demonstrated that cell surface expression of the receptor correlated well with mRNA levels (Figure 4B), with receptor levels being enhanced in all cells tested on RA and TNF treatment for 72 hours. In contrast, m-CSF-1R was slightly induced in UF-1 cells by RA alone, but no further response could be observed when TNF was added. These data indicate a correlation between increased expression levels of the m-CSF receptor and monocytic differentiation of APL cells. Therefore, we next sought to determine the mechanistic significance of this correlation.
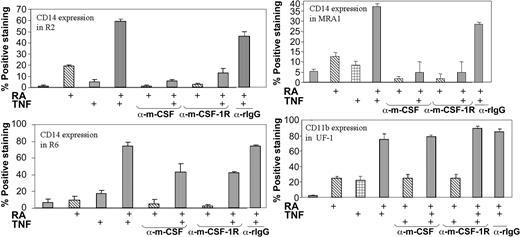
Neutralization of m-CSF-1 and m-CSF-1R ablates TNF and RA induced monocytic differentiation
Previous studies have shown the effectiveness of neutralizing antibodies directed against m-CSF-1R and its ligand in blocking signaling through this pathway.49-51 We also used this approach to determine whether the m-CSF-1R pathway is an essential mediator of the promonocytic effects of TNF and RA. As shown in Figure 1, the monocyte-specific marker CD14 was increased in a synergistic fashion on treatment with TNF and RA. In MRA1, MR2, and MR6, antibodies blocking either the m-CSF-1R or its ligand effectively blocked CD14 induction (Figure 5). Although no CD14 induction was observed in UF-1 cells, we also added neutralization antibodies to UF-1 to assess its effects on CD11b expression. Neither inhibition of the receptor nor its ligand blocked the induction of CD11b expression by RA and TNF. Thus, inhibition of the CD14 monocytic marker by these antibodies was specific, implicating the m-CSF pathway as being essential for specifying differentiation along a monocytic lineage in response to TNF and RA.
Neutralization of either the m-CSF-1R or its ligand negates CD14 induction in response to TNF and RA. Cytofluorimetric analysis of CD14 levels on RA-resistant APL cells in response to treatment with RA and TNF. UF-1 cells were treated for 3 days, while others were treated for 5. Antibodies and reagents were replenished at day 3 of the 5-day treatments. The synergy observed between ATRA and TNF observed 5 days after treatment was ablated on addition of neutralizing antibodies to m-CSF-1R or m-CSF. The antibodies did not decrease CD11b levels in UF-1. Control experiments using rabbit immunoglobulin G (rIgG) antibodies showed very little effect on CD14 expression.
Neutralization of either the m-CSF-1R or its ligand negates CD14 induction in response to TNF and RA. Cytofluorimetric analysis of CD14 levels on RA-resistant APL cells in response to treatment with RA and TNF. UF-1 cells were treated for 3 days, while others were treated for 5. Antibodies and reagents were replenished at day 3 of the 5-day treatments. The synergy observed between ATRA and TNF observed 5 days after treatment was ablated on addition of neutralizing antibodies to m-CSF-1R or m-CSF. The antibodies did not decrease CD11b levels in UF-1. Control experiments using rabbit immunoglobulin G (rIgG) antibodies showed very little effect on CD14 expression.
Discussion
RA-resistant cells have served as useful models to evaluate combination therapies for RA-resistant APL. The combination of RA and inhibitors of HDAC activity is one such example. This approach to overcoming RA resistance has been widely studied in vitro, as well as in clinical trials.52 However, the combination of RA and HDAC inhibitors has had limited clinical success,52,53 and, certainly, novel approaches are required. Our data demonstrate that TNF and RA can more effectively force differentiation of a variety of RA-resistant APL cells than, in our experience, the combination of HDAC and RA (Table 1). Unlike the differentiation resulting from RA and HDAC inhibitors, the maturation process described here was monocytic in nature in all but UF-1 cells, as evidenced by CD14 expression (Figure 1B) and morphology (data not shown).
It is well known that the PML/RARα oncoprotein plays a major role in the maturation block found in APL. It is, therefore, interesting that TNF and RA could bypass this block without enhancing PML/RARα degradation (Figure 2). This insensitivity to PML/RARα expression represents a difference between the monocytic pathway initiated by TNF and RA and the granulocytic pathway induced by other means. Previous studies using retinoic acid response elements linked to CAT reporter constructs have shown that of the cell lines tested MR4 cells have the lowest level of RA-induced transcription11 (M.W., W.H.M., unpublished data, November 2001). These cells are also most resistant to TNF and RA mediated differentiation, as measured by NBT-positive staining (Figure 1A). This suggests that a minimum level of RA-induced transcription is necessary for the resistant cells to achieve full maturation. However, the addition of TNF did not augment RA-mediated transcription of CAT constructs containing various RA response elements. Thus, activation of the RA transcriptional pathway is necessary but not the primary mediator of the monocytic response to RA and TNF.
It is widely accepted that myelomonocytic differentiation is dependent on the activation of specific transcription factors. One of the classic transcriptional activators involved in monocyte maturation is c-jun. Using Western blotting and electrophoretic mobility shift assay (EMSA), we found that c-jun was induced in all cell lines undergoing monocytic differentiation but not in UF-1 cells (Figure 3B-C). This suggests that c-jun may be necessary for TNF and RA to promote monocyte-specific maturation, a hypothesis also supported by a recent report showing that down-regulation of c-jun is critical for granulocyte lineage commitment.54 PU.1 is another transcription factor whose expression strongly correlates that of the monocyte-specific marker CD14 (Figure 3D). PU.1 is involved in myeloid differentiation along both monocytic and granulocytic routes. Knock-out mouse studies have demonstrated that PU.1 plays mainly a regulatory role in neutrophil development but is essential for the appearance of monocytes.55,56 PU.1 expression has also been correlated with monocytic differentiation of APL cells.48 We found that TNF and RA treatment resulted in a large increase in PU.1 DNA binding in all cells that showed monocytic specificity but not in UF-1 cells (Figure 3D). These data indicate that both c-jun and PU.1 are specifically regulated in cells undergoing monocytic differentiation. Previous studies have shown that c-jun is a transcriptional partner of PU.144 and could play a role in determining the target gene specificity of PU.1. Thus, coordinated up-regulation of these 2 transcription factors could be important in specifying a monocytic cell fate.
IRF-7 is a transcription factor that is involved in viral response57 and has also been shown to be directly involved in monocytic maturation.39 IRF-7 mRNA was synergistically increased by TNF and RA in all cell lines tested (Figure 3A). This suggests that IRF-7 is involved in the differentiation process of granulocytic as well as monocytic differentiation. There is evidence that IRF-7 can increase transcription synergistically when combined with c-jun.58 One mode through which RA and TNF could act synergistically to induce differentiation is to activate 2 separate pathways, such as those induced by c-jun and IRF-7, that in turn interact. These targets could then play a role in committing cells to a monocytic or granulocytic fate.
The role of the m-CSF-1 pathway in monocyte differentiation is well established. m-CSF-1R has been shown to be regulated during monocytic differentiation of leukemia59 cells, and its transcription has been shown to be increased by RA in breast cancer cells.47 Because of its pivotal role in differentiation, we investigated its role in differentiation induced by TNF and RA. Through the use of neutralizing antibodies, we found that activation of the m-CSF-1 pathway is necessary for TNF and RA to induce CD14 expression (Figure 5). These antibodies effectively shut down TNF and RA mediated induction of CD14 levels, but had no effect on CD11b expression. Thus, blocking this pathway inhibited an increase in a monocyte-specific cell surface marker but had no effect on the CD11b marker that is common to both monocyte and granulocyte lineages.
Previous work has demonstrated that the m-CSF-1 pathway cannot dictate monocytic differentiation in PU.1-negative cells.60 This may be important in understanding the different response of UF-1 cells to RA and TNF. Even though m-CSF-1R is expressed in UF-1 cells, our data indicate that PU.1 may also be required for TNF and RA to carry out promonocytic differentiation. The observation that PU.1 is expressed in NB4 (M.W., W.H.M., unpublished data, July 2002) and U93761 cells, where a strong promonocytic response is also observed in response to RA and TNF,18 supports this conclusion. However, knockdown studies are needed to confirm this hypothesis.
How TNF and RA interact to increase transcription remains to be answered. Our previous work showed that RA could activate NF-κB mediated transcription at late time periods and could combine with TNF at early time points to increase NF-κB DNA binding. Because both m-CSF-1R and IRF-7 can be regulated by NF-κB, it is possible that RA and TNF may regulate transcription of these genes through activation of NF-κB. To test this hypothesis we attempted to block NF-κB activity with specific inhibitors. However, at concentrations that proved to effectively inhibit NF-κB, these inhibitors were extremely toxic and very few of the cells survived for longer than 24 hours after treatment (data not shown). Therefore, it was difficult to determine the effect of NF-κB inhibition on RA and TNF mediated differentiation. Previously, we found that RA and TNF could activate NF-κB in NB4 cells.18 We found that combining RA with TNF could synergistically activate NF-κB DNA binding in RA-resistant APL cells including UF-1 (data not shown). This suggests that NF-κB may be involved in initiation of differentiation in response to RA and TNF but may not play a role in specificity. This is consistent with our previous data showing RA alone could activate NF-κB binding during granulocytic differentiation.18
To date, there have been several reports of agents that combine with RA to overcome RA-resistant APL. However, mechanistic data on how the resistance has been overcome have been lacking. Our work indicates that targeting both the TNF and RA pathway represents a novel method to overcome RA-resistant APL and that the PU.1 and m-CSF pathways are important in mediating the synergy of these 2 pathways. Although TNF has been found to be toxic in vivo, recent research has been focusing on specifically targeting TNF to the tumor area or tumor cells,25 thereby reducing its toxicity. Our finding that RA-resistant APL cells respond well to the combination of TNF and RA may provide a basis for the rational design of novel therapies for not only RA-resistant APL but other more common leukemias that are resistant to RA as well.
Prepublished online as Blood First Edition Paper, July 15, 2004; DOI 10.1182/blood-2004-01-0023.
Supported by grants from the Leukemia Research fund of Canada and the Canadian Institutes of Health Research (CIHR). M.W. is supported by a National Cancer Institute of Canada (NCIC) studentship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal