Abstract
Interleukin 2 (IL-2) rescued human natural killer (NK) KHYG-1 cells from apoptosis along with a reduction of ceramide. Conversely, an increase of ceramide inhibited IL-2-rescued survival. IL-2 deprivation-induced activation of acid sphingomyelinase (SMase) and inhibition of glucosylceramide synthase (GCS) and sphingomyelin synthase (SMS) were normalized by IL-2 supplementation. A phosphatidyl inositol-3 (PI-3) kinase inhibitor, LY294002, inhibited IL-2-rescued survival, but a mitogen-activated protein kinase inhibitor, PD98059, and an inhibitor of Janus tyrosine kinase/signal transducer and activator of transcription pathway, AG490, did not. LY294002 inhibited IL-2-induced reduction of ceramide through activation of acid SMase and inhibition of GCS and SMS, suggesting the positive involvement of PI-3 kinase in ceramide reduction through enzymatic regulation. Indeed, a constitutively active PI-3 kinase enhanced growth rate and ceramide reduction through inhibition of acid SMase and activation of GCS and SMS. Further, LY294002 inhibited IL-2-induced changes of transcriptional level as well as mRNA and protein levels in acid SMase and GCS but did not affect the stability of the mRNAs. These results suggest that PI-3 kinase-dependent reduction of ceramide through regulation of acid SMase, GCS, and SMS plays a role in IL-2-rescued survival of NK cells. (Blood. 2004;104:3285-3293)
Introduction
It is well known that natural killer (NK) cells play a critical role in elimination of infected and transformed cells. However, little is known about the mechanism to regulate growth and survival of NK cells. Interleukin 2 (IL-2) has been reported to be involved in clonal expansion and functional differentiation of NK cells.1-5 Among downstream signals of IL-2, the implication of antiapoptotic protein kinases such as Src-kinase, Janus tyrosine kinase/signal transducer and activator of transcription (Jak/stat) system, spleen tyrosine kinase (Syk) kinase, mitogen-activated protein (MAP) kinase, and phosphatidylinositol 3 (PI-3) kinase has been well described.6-12 In contrast, involvement of proapoptotic molecules such as Bcl-2-associated X protein (BAX) family, caspases, DNA fragmentation factor 40 (DFF40)/caspase-activated DNase (CAD), and ceramide in IL-2-induced cell survival were rarely known.
Ceramide, a key molecule in both synthesis and degradation of complex sphingolipids, has been reported to be involved in the signal transduction of extracellular stimuli leading to apoptotic cell death, differentiation, and growth arrest.13-16 It was reported that IL-1β mediated prostaglandin E2 production through ceramide generation by sphingomyelin (SM) hydrolysis17-19 and that increase of ceramide content was correlated with IL-6 and IL-8 production in fibroblasts and gastric cancer cells, respectively.20,21 These results suggest the potential involvement of ceramide in cytokine-induced cell growth. In fact, IL-2 was previously shown to induce a reduction of ceramide content and an increase of DNA synthesis in Jurkat T cells,22 but it remains to be clarified the molecular mechanism by which ceramide content is regulated in IL-2-induced NK cell growth and survival.
Intracellular ceramide is known to be generated by SM hydrolysis through activation of acid or neutral sphingomyelinase (SMase) in response to various kinds of proapoptotic stress such as Fas antibody, irradiation, heat shock, and anticancer reagents.13,16,23 In addition to SMase(s), glucosylceramide synthase (GCS), generating glucosylceramide by transferring a uridine diphosphate (UDP)-glucose to ceramide,24 and sphingomyelin synthase (SMS), generating SM by transferring phosphocholine from phosphatidylcholine to ceramide, are suggested to play a role in ceramide regulation. For example, overexpression of GCS developed the resistance against adriamycin-induced apoptosis in MCF-7 breast cancer cells25 and HL-60 leukemia cells,26 and the transfection of anti-sense oligonucleotides for GCS restored drug-sensitivity in adriamycin-resistant MCF-7 cells.27 SMS was reported to be activated in simian virus 40 (SV-40)-transformed human lung fibroblasts28,29 and drug-resistant HL-60/ADR cells.26 Reduction of ceramide through activation of SMS was suggested in basic fibroblast growth factor (bFGF)-induced increase of DNA synthesis in primary astrocytes.30 These results suggest that regulation of ceramide content through ceramide-related enzymes is closely involved in apoptosis and cell growth.
PI-3 kinase has been reported to mediate IL-2-induced cell growth and survival,4,31-33 and recently cannabinoids were shown to inhibit ceramide-induced apoptosis in astrocytes through activation of PI-3 kinase.34 As a mechanism by which PI-3 kinase induces inhibition of tumor necrosis factor-α-induced apoptosis in primary astrocytes, PI-3 kinase-dependent inhibition of ceramide generation through SM hydrolysis was shown.35 In addition, ceramide generation by neutral SMase was inhibited by a constitutively active PI-3 kinase.36
Thus, the involvement of PI-3 kinase in ceramide regulation through inhibition of neutral SMase is suggested, but it remains to be clarified whether and how ceramide-related enzymes such as acid SMase, GCS, and SMS are regulated through IL-2-mediated PI-3 kinase. We here show that IL-2 restores NK cell survival with a reduction of ceramide, but this rescue is hindered by an increase of intracellular ceramide. A PI-3 kinase inhibitor, LY294002, blocks IL-2-induced reduction of ceramide through activation of acid SMase and inhibition of GCS and SMS. In addition, a constitutively active PI-3 kinase enhances growth rate and reduction of ceramide through regulation of acid SMase, GCS, and SMS. We further show that PI-3 kinase regulates IL-2-induced changes of protein, mRNA, and transcriptional levels in acid SMase and GCS but does not affect mRNA degradation level. These results suggest the role of PI-3 kinase-dependent reduction of ceramide through acid SMase, GCS, and SMS in IL-2-rescued NK cell survival.
Materials and methods
Materials
Human KHYG-1 cells37 were a kind gift from Dr H. Umehara (Kyoto University, Kyoto, Japan). [γ-32P]-ATP (adenosine triphosphate; 6000 Ci/mmol [22200 × 1010 Bq/mmol]) was purchased from Amersham Biosciences (Piscataway, NJ). Human recombinant IL-2 (Imunase 35) was obtained from Shionogi Pharmaceutical (Osaka, Japan). D-erythro-C6-NBD-ceramide and D-erythro-C6-NBD-sphingomyelin were obtained from Matreya (Pleasant Gap, PA). Antiacid SMase and anti-β-actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Akt and antiphospho-Akt (Ser 473) antibodies were purchased from New England Biolabs (Beverly, MA). Anti-GCS antibody was a kind gift from Dr Y. Hirabayashi (RIKEN, Wako, Japan). Horseradish peroxidase-conjugated anti-rabbit goat immunoglobulin was purchased from TAGO Immunologicals (Burlingame, CA). LY294002, AG490, and PD98059 were purchased from CALBIOCHEM (San Diego, CA). Other chemicals, unless mentioned otherwise, were purchased from Sigma Chemical (St Louis, MO). Thinlayer chromatography (TLC) plates were purchased from Whatman (Clifton, NJ). Human cDNAs of GCS and β-actin were obtained from RIKEN DNA bank. A human cDNA of acid SMase was a kind gift from Dr K. Sandhoff (Universitat Bonn, Germany).
Cell culture
Human KHYG-1 cells were grown in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (FBS; Sigma), kanamycin sulfate (80 ng/mL), and IL-2 (100 U/mL) (IL-2-containing medium) at 37°C in a humidified atmosphere containing 5% CO2. The viable cell numbers were counted by a trypan blue dye exclusion method at the indicated period. Monkey COS-7 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, kanamycin sulfate (80 ng/mL) at 37°C in a humidified atmosphere containing 5% CO2.
Measurement of apoptotic cells
The cells were fixed in 1% glutaraldehyde for 30 minutes at room temperature and stained with DAPI (4′, 6-diamino-2-phenylindole) as described before.38 At least 200 cells were counted under a fluorescent microscope in each determination, and the cells with nuclear condensation and fragmentation were judged as apoptotic cells.
IL-2 rescue experiment
The KHYG-1 cells cultured in IL-2-containing medium were washed twice with IL-2-deprived medium (RPMI-1640 supplemented with 10% FBS [Sigma] and 80 ng/mL kanamycin sulfate) and cultured without IL-2 at a concentration of 3 × 105 cells/mL. IL-2 (100 U/mL) was supplied to the medium after 12 hours of deprivation, and the cells were cultured thereafter (IL-2- → +). In contrast to IL-2-rescue experiment, some cells were cultured in the absence (IL-2-) or presence (IL-2+) of IL-2 for 36 hours.
Ceramide quantification
After extracting the lipids according to the Bligh and Dyer method as described before,39 ceramide levels in the cells were measured enzymatically by using Escherichia coli diacylglycerol kinase (DGK), which converts ceramide and diacylglycerol to ceramide 1-phosphate and phosphatidic acid, respectively, and the amounts of ceramide were corrected by phospholipid phosphate. The solvent system used to separate ceramide phosphate was chloroform/acetone/methanol/acetic acid/distilled water (DW) = 10:4:3:2:1.40
RNA preparation and Northern blot analysis
Total RNA was prepared by using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Total RNA (7 μg) was denatured at 65°C in 50% formamide and separated by 1% agarose-formamide gel electrophoresis. RNA was transferred onto a Hybond-N+ (Amersham Biosciences, Piscataway, NJ) nylon membrane and fixed with UV cross-linker. Human GCS, acid SMase, and β-actin cDNA probes were labeled with [γ-32P] ATP by random primer methods (Bca BEST Labeling Kit; Takara, Osaka, Japan) according to the manufacturer's protocol. RNA-fixed membrane was hybridized to the [γ-32P]-labeled cDNA probe at 65°C in a hybridization buffer (Rapid-Hyb Buffer; Amersham Biosciences) for 12 hours. Then the membrane was washed 3 times in 2 × SSC (standard saline citrate; 1 × SSC containing 0.15 M NaCl and 15 mM sodium citrate) and 0.1% SDS (sodium dodecyl sulfate) at 60°C for 15 minutes. The membrane was exposed to x-ray film (Fuji Photo Film, Tokyo, Japan) with the intensifying screens at -80°C for 1 or 2 days. Equal loading of RNA was confirmed by expression of β-actin.
Assay procedure for acid and neutral SMase activities
KHYG-1 cells were harvested; washed with phosphate-buffered saline (PBS); sonicated in 150 μL lysis buffer containing 10 mM Tris (tris (hydroxymethyl)aminomethane)-HCl (pH 7.5), 1 mM EDTA (ethylenediaminetetraacetic acid), 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL pepstatin A, and 100 μg/mL aprotinin; and left on ice for 30 minutes. The homogenate was centrifuged at 10 000g for 10 minutes at 4°C, and the supernatant was used as an enzyme source. The assay mixture for the measurement of magnesium-dependent neutral SMase contained 0.1 M Tris-HCl (pH 7.5), 10 μM C6-NBD SM, 10 mM MgCl2, 0.1% Triton X-100, 5 mM dithiothreitol, and 50 μg enzyme source in a final volume of 0.1 mL. The assay mixture for acid SMase contained 0.1 M sodium acetate (pH 5), 10 μM C6-NBD SM, 0.1% Triton X-100, and 50 μg enzyme source in a final volume of 0.1 mL. In this assay, SMase catalyzes hydrolysis of C6-NBD-SM to produce C6-NBD-ceramide. Incubation was carried out at 37°C for 2 hours. The reaction was stopped by addition of 2 mL chloroform/methanol (2:1, vol/vol). Then, 0.9 mL DW was added to the tubes and mixed by vortex. The tubes were centrifuged at 1000g for 5 minutes to separate the 2 phases. After the upper phase was removed, the organic phase was dried down under N2 gas, and reaction products were applied to the TLC plate. The solvent system to separate reaction products was chloroform/methanol/12 mM MgCl2 in DW (65:25:4, vol/vol) and analyzed by FluorImager (excitation = 475 nm, emission = 525 nm; Molecular Dynamics, Sunnyvale, CA). Protein concentration was determined by using a protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Assay for GCS and SMS activities
KHYG-1 cells were harvested, washed with phosphate-buffered saline, and dispersed in 150 μL hypotonic buffer containing 20 mM Tris-HCl (pH 7.5), 2 mM EDTA, 10 mM EGTA (ethylene glycol tetraacetic acid), 1 mM PMSF, and 2.5 μg/mL leupeptin. After left on ice for 10 minutes, the cells were lysed by passing through a 27-gauge needle at least 10 times. The lysate was centrifuged at 800g for 5 minutes at 4°C. The supernatant was used as the whole-cell extract. In this assay GCS catalyzes the transfer of glucose residue from UDP-glucose to C6-NBD-ceramide. SMS catalyzes the addition of phosphocholine to ceramide to produce C6-NBD-sphingomyelin. Protein (50 μg) was added to 100 μL reaction buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10 μg/mL C6-NBD ceramide, 100 μg/mL phosphatidylcholine, and 500 μM UDP-glucose) and incubated at 37°C for 2 hours.
Western blot analysis
KHYG-1 cells were harvested, washed with phosphate-buffered saline, and mixed by vortex in 100 μL lysis buffer containing 10 mM Tris-HCl (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 1% Triton X-100, 1 mM PMSF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. After being left on ice for 30 minutes, the lysate was centrifuged at 10 000g for 15 minutes at 4°C. The supernatant was used as loading sample. The samples (50 μg) were denatured by boiling in Laemmli sample buffer for 5 minutes, subjected to SDS-polyacrylamide gel electrophoresis using 10% running gel, and electroblotted to Immobilon Transfer Membrane (Millipore, Bedford, MA) as described before.41 Nonspecific binding was blocked by incubation of the membrane with PBS containing 5% skim milk and 0.1% Tween 20 for more than 12 hours. The membrane was incubated with a 1:1000 dilution of anti-GCS, acid SMase, Akt, phospho-Akt (Ser 473), and β-actin antibodies in PBS-T (PBS and 0.1% Tween 20) containing 5% skim milk for 2.5 hours. The membrane was washed 3 times in PBS-T for 10 minutes each and incubated with 1:4000 dilution of anti-goat or rabbit immunoglobulin conjugated with horseradish peroxidase in PBS-T containing 5% skim milk for 1 hour. After washing the membrane 3 times for 10 minutes each in PBS-T, detection was performed by using enhanced chemiluminescence (ECL) Western blotting detection reagent (Amersham Biosciences) according to the manufacturer's protocol.
Expression constructs and transfection
The expression construct, pEF-Bos vector for PI-3 kinase, contained the catalytic p110 subunit of PI-3 kinase, Myc-Tagged at C-terminus, which has been previously described.42 COS-7 cells were transiently transfected by electroporation method. Expression plasmid cDNA (15 μg) and 100 μL Nucleofector Solution V (Amaxa Biosystems, Gaithersburg, MD) were mixed with 1 × 106 cells and exposed to a pulse (program A-24) by using Nucleofector (Amaxa Biosystems). The transfected cells were cultured in RPMI 1640 media supplemented with 10% fetal bovine serum and kanamycin sulfate (80 ng/mL) and incubated at 37°C in a humidified atmosphere containing 5% CO2. To determine transfection efficiency, pSV-β-Galactosidase Control Vector (Promega, Madison, WI) was transfected to COS-7 cells as described earlier. Twenty-four hours after transfection, these cells were fixed with 0.25% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactoside.43
Nuclear run-on assay
KHYG-1 cells (5 × 107) were harvested, resuspended in 4 mL ice-cold lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.5% Nonidet P-40), and left on ice for 10 minutes. Nuclei were pelleted by centrifugation at 500g for 5 minutes and resuspended in 100 μL glycerol buffer (50 mM Tris-HCl, pH 8.3, 40% glycerol, 5 mM MgCl2, 0.1 mM EDTA). An equal volume of reaction buffer (100 mM KCl, 0.5 mM ATP, CTP [cytidine triphosphate], and GTP [guanosine triphosphate]) was added to the nuclear suspension, and reaction mixture was incubated with 100 μCi [3.7 MBq] [α-32P] UTP (3000 Ci/mmol [11100 × 1010 Bq]) at 30°C for 20 minutes. The reaction was then terminated by the addition of 100 μL stop buffer (10 mM Tris-HCl, pH 8, 1 mM EDTA, 100 mM NaCl, 20 mM MgCl2, 150 U/mL RNase inhibitor, 40 U/mL DNase) and incubated at 30°C for 15 minutes. Then proteinase K (750 μg/mL) and 1% SDS were added and incubated at 37°C for 30 minutes. RNA was isolated by TRIzol reagent according to the manufacturer's protocol. Human acid SMase, GCS, and β-actin cDNAs (10 μg each) were denatured by heating in a boiling water bath for 5 minutes and chilled rapidly on ice. These denatured DNAs were applied to the Hybond-N+ nylon membrane with the pipette tip. These membranes were fixed by UV cross-linking. These membranes were prehybridized in hybridization buffer (5 × Denhardt solution, 40% formamide, 4 × SSC, 5 mM EDTA, 0.4% SDS, and 100 μg/mL yeast tRNA) at 42°C for 12 hours. Hybridization was performed with 107 cpm 32P-labeled RNA/mL hybridization buffer at 42°C for 72 hours. Then the membrane was washed 3 times in 2 × SSC with 0.1% SDS at 37°C for 10 minutes and 0.1 × SSC with 0.1% SDS at 50°C for 10 minutes. Signals were detected by Fuji Imaging Analyzer (BAS2000; Fuji Photo Film) after 12 hours of exposure.
Results
IL-2-deprivation-induced apoptosis with an increase of ceramide and its restoration by IL-2 supplementation in KHYG-1 cells
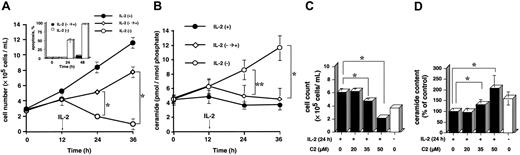
When KHYG-1 cells were cultured in the medium containing 100 U/mL IL-2 and 10% FCS, cell growth was exponentially increased, and its doubling time was approximately 16 to 18 hours (Figure 1A). In IL-2-deprived condition, the cells slightly decreased growth rate in the first 12 hours and thereafter underwent apoptotic cell death (Figure 1A and inset). IL-2 deprivation for 48 hours induced apoptosis up to 90%, but IL-2 supplementation after 12 hours of deprivation rescued the cells from apoptosis (Figure 1A and inset). Intracellular ceramide levels in IL-2-deprived condition were significantly higher than those in IL-2-supplied condition (Figure 1B; P < .01 each). Ceramide contents increased from 4.5 ± 0.5 pmol/nmol phosphate to 6.2 ± 1.0, 8.5 ± 1.3, and 11.6 ± 1.7 pmol/nmol phosphate after 12, 24, and 36 hours, respectively, of IL-2 deprivation (Figure 1B). However, increase of ceramide content (6.2 ± 1.0 pmol/nmol phosphate) returned close to the control level (4.9 ± 1.5 pmol/nmol phosphate) after 24 hours of IL-2 supplementation (Figure 1B).
Effects of IL-2 supplementation on cell growth, apoptosis, and ceramide content and inhibition of IL-2-induced cell growth by exogenous C2-ceramide in KHYG-1 cells. (A) KHYG-1 cells were cultured in the presence (•, IL-2+) or absence (○, IL-2-) of IL-2 (100 U/mL) for 36 hours. After IL-2 deprivation for 12 hours, cells were rescued with IL-2 (100 U/mL) supplementation and further cultured for 24 hours (⋄, IL-2-→+). Cells were harvested at the indicated times (0, 12, 24, and 36 hours). Viable cell numbers and the percentage of apoptotic cells (inset) were counted by a trypan blue dye exclusion method and DAPI method under fluorescent microscope, respectively. (B) Intracellular ceramide was measured by DGK assay after lipid extraction by Bligh and Dyer method as described in “Materials and methods.” (C-D) KHYG-1 cells at an initial concentration of 3 × 105 cells/mL were treated with various concentrations of C2-ceramide (0, 20, 35, and 50 μM) in the presence or absence of IL-2 for 24 hours. Viable cell number and ceramide content were assessed by trypan blue dye exclusion method and DGK assay, respectively. The results were obtained from 3 different experiments. The bars indicate 1 SD. The significance of difference of cell count was determined by analysis of variance (ANOVA) test. *P < .01.
Effects of IL-2 supplementation on cell growth, apoptosis, and ceramide content and inhibition of IL-2-induced cell growth by exogenous C2-ceramide in KHYG-1 cells. (A) KHYG-1 cells were cultured in the presence (•, IL-2+) or absence (○, IL-2-) of IL-2 (100 U/mL) for 36 hours. After IL-2 deprivation for 12 hours, cells were rescued with IL-2 (100 U/mL) supplementation and further cultured for 24 hours (⋄, IL-2-→+). Cells were harvested at the indicated times (0, 12, 24, and 36 hours). Viable cell numbers and the percentage of apoptotic cells (inset) were counted by a trypan blue dye exclusion method and DAPI method under fluorescent microscope, respectively. (B) Intracellular ceramide was measured by DGK assay after lipid extraction by Bligh and Dyer method as described in “Materials and methods.” (C-D) KHYG-1 cells at an initial concentration of 3 × 105 cells/mL were treated with various concentrations of C2-ceramide (0, 20, 35, and 50 μM) in the presence or absence of IL-2 for 24 hours. Viable cell number and ceramide content were assessed by trypan blue dye exclusion method and DGK assay, respectively. The results were obtained from 3 different experiments. The bars indicate 1 SD. The significance of difference of cell count was determined by analysis of variance (ANOVA) test. *P < .01.
Increase of endogenous ceramide by C2-ceramide inhibited IL-2-rescued cell growth
Next, we examined whether cell-permeable, synthetic ceramide, N-acetylsphingosine (C2-ceramide), affects IL-2-induced cell growth by increasing endogenous ceramide. Cell growth as judged by viable cell numbers was inhibited from 6 × 105 cells/mL to 6.2 × 105, 4.8 × 105, and 2.2 × 105 cells/mL after 24 hours of treatment with 20, 35, and 50 μM C2-ceramide, respectively, in the presence of IL-2 (Figure 1C). As shown in Figure 1D, treatment with 20, 35, and 50 μM C2-ceramide for 24 hours increased intracellular ceramide contents to 99%, 132%, and 208% of the control level, respectively. The results suggest that endogenous ceramide levels were positively correlated with the extent of cell growth inhibition.
Changes of enzymatic activities in GCS, SMS, and SMases in IL-2-induced survival
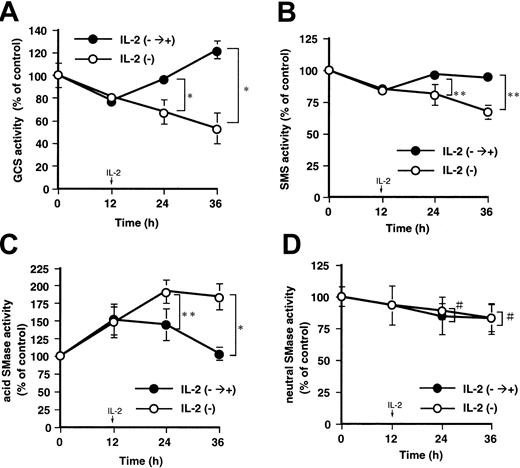
We compared the activities in ceramide-related enzymes, such as GCS and SMS, and acid and neutral SMases between IL-2-deprived and -supplied conditions. The activities of GCS and SMS decreased to 77% ± 4% and 83% ± 3% of the control, respectively, 12 hours after deprivation, and subsequent IL-2 supplementation for 24 hours restored the activities to 123% ± 7% and 94% ± 3% of the control, respectively (Figure 2A-B). After 36 hours of deprivation, GCS and SMS activities decreased to 53% ± 13% and 67% ± 5% of the control, respectively. However, acid SMase was activated to 150% ± 22% of the control after 12 hours of deprivation. Subsequent supplementation of IL-2 for 24 hours returned increase of acid SMase activity close to the control level, but consecutive deprivation for 36 hours further increased acid SMase to 175% ± 18% of the control (Figure 2C). Neutral SMase activity was not affected in the presence or absence of IL-2 (Figure 2D). These results suggest that IL-2 supplementation induces a reduction of ceramide content through inhibition of acid SMase and activation of GCS and SMS.
Effects of IL-2 supplementation on the activities in GCS, SMS, and acid and neutral SMases. KHYG-1 cells were cultured in the absence (○, IL-2-) or presence of IL-2 supplementation (100 U/mL) after 12 hours of deprivation (•, IL-2-→+). The cells were harvested at indicated times (0, 12, 24, and 36 hours), and after extraction of the proteins the activity of each enzyme was assessed. The activities of GCS (A), SMS (B), acid (C), and neutral (D) SMases were measured by using C6-NBD-ceramide and C6-NBD-SM as the substrates as described in “Materials and methods.” The activities of each enzyme detected in IL-2-supplied control cells were 789 ± 66, 41 ± 1.6, 579 ± 25, and 917 ± 91 pmol/mg protein/hour for GCS, SMS, acid, and neutral SMases, respectively. The bars indicate 1 SD. The significance of differences of enzyme activities was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
Effects of IL-2 supplementation on the activities in GCS, SMS, and acid and neutral SMases. KHYG-1 cells were cultured in the absence (○, IL-2-) or presence of IL-2 supplementation (100 U/mL) after 12 hours of deprivation (•, IL-2-→+). The cells were harvested at indicated times (0, 12, 24, and 36 hours), and after extraction of the proteins the activity of each enzyme was assessed. The activities of GCS (A), SMS (B), acid (C), and neutral (D) SMases were measured by using C6-NBD-ceramide and C6-NBD-SM as the substrates as described in “Materials and methods.” The activities of each enzyme detected in IL-2-supplied control cells were 789 ± 66, 41 ± 1.6, 579 ± 25, and 917 ± 91 pmol/mg protein/hour for GCS, SMS, acid, and neutral SMases, respectively. The bars indicate 1 SD. The significance of differences of enzyme activities was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
Inhibition of PI-3 kinase by LY294002 blocked IL-2-induced survival, but AG490 and PD98059 did not
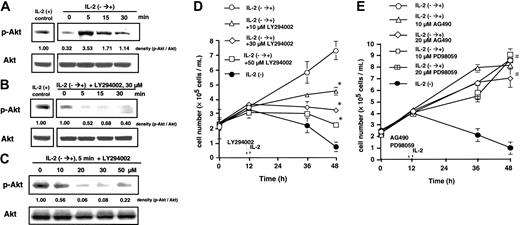
Western blot analysis using antiphospho-Akt antibody showed that the amount of phosphorylated Akt on Serine 473 was decreased to 29% of the IL-2-supplied control after 12 hours of IL-2 deprivation, but IL-2 supplementation immediately returned the decreased phosphorylation of Akt to the control level (Figure 3A). When the cells were pretreated with various concentrations of LY294002 (10, 20, 30, and 50 μM) 1 hour before supplementation, IL-2-induced Akt phosphorylation was effectively inhibited in a dose- and time-dependent manner (Figure 3B-C). Treatment with various concentrations of LY294002 (10, 30, and 50 μM) dose dependently decreased IL-2-restored cell growth from 7.4 × 105 cells/mL to 4.6 × 105, 3.2 × 105, and 2.0 × 105 cells/mL, respectively (Figure 3D). Thus, because phosphorylation of Serine 473 of Akt was a direct evidence of PI-3 kinase activation,44,45 these results suggest that the activation of PI-3 kinase is required for IL-2-restored NK cell growth. In addition to PI-3 kinase, the possible involvement of Jak/stat or MAP kinase pathways in IL-2-mediated growth was examined. Treatment with AG490 or PD98059 did not affect IL-2-restored increase of cell growth (Figure 3E), suggesting little involvement of Jak/stat and MAP kinase pathways in IL-2-restored cell growth.
Inhibition of IL-2-induced Akt phosphorylation and cell growth by LY294002 but not by AG490 and PD98059. KHYG-1 cells at an initial concentration of 2.5 × 105/mL were cultured in the absence of IL-2 for 12 hours, rescued with IL-2 (100 U/mL) supplementation (IL-2-→+), or treated with various concentrations of LY294002, AG490, or PD98059 for 1 hour in the absence of IL-2 before IL-2 supplementation. (A) At the indicated times after IL-2 supplementation (0, 5, 10, and 30 minutes), the protein levels of phosphorylated Akt kinase (Serine 473) and Akt kinase were determined by Western blot analysis. Equal loading of the samples was confirmed by protein levels of Akt. (B-C) Cells were treated with the indicated concentrations of LY294002 (B: 30 μM; C: 0, 10, 20, 30, and 50 μM) for 1 hour in the absence of IL-2 and harvested at the indicated times (B: 0, 5, 10, and 30 minutes; C: 5 minutes) after IL-2 supplementation. Western blot analysis was performed for Akt and phosphorylated-Akt. The ratio of phosphorylated-Akt to Akt was calculated by National Institutes of Health (NIH) image software and shown at the bottom of each panel. The results were representative of 3 independent experiments. (D-E) Cells were treated with the indicated concentrations of LY294002, AG490, and PD98059 for 1 hour in the absence of IL-2 and harvested 0, 24, and 36 hours after IL-2 supplementation. Viable cell numbers were assessed by a trypan blue dye exclusion method. The bars indicate 1 SD. The significance of cell number was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
Inhibition of IL-2-induced Akt phosphorylation and cell growth by LY294002 but not by AG490 and PD98059. KHYG-1 cells at an initial concentration of 2.5 × 105/mL were cultured in the absence of IL-2 for 12 hours, rescued with IL-2 (100 U/mL) supplementation (IL-2-→+), or treated with various concentrations of LY294002, AG490, or PD98059 for 1 hour in the absence of IL-2 before IL-2 supplementation. (A) At the indicated times after IL-2 supplementation (0, 5, 10, and 30 minutes), the protein levels of phosphorylated Akt kinase (Serine 473) and Akt kinase were determined by Western blot analysis. Equal loading of the samples was confirmed by protein levels of Akt. (B-C) Cells were treated with the indicated concentrations of LY294002 (B: 30 μM; C: 0, 10, 20, 30, and 50 μM) for 1 hour in the absence of IL-2 and harvested at the indicated times (B: 0, 5, 10, and 30 minutes; C: 5 minutes) after IL-2 supplementation. Western blot analysis was performed for Akt and phosphorylated-Akt. The ratio of phosphorylated-Akt to Akt was calculated by National Institutes of Health (NIH) image software and shown at the bottom of each panel. The results were representative of 3 independent experiments. (D-E) Cells were treated with the indicated concentrations of LY294002, AG490, and PD98059 for 1 hour in the absence of IL-2 and harvested 0, 24, and 36 hours after IL-2 supplementation. Viable cell numbers were assessed by a trypan blue dye exclusion method. The bars indicate 1 SD. The significance of cell number was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
Inhibition of PI-3 kinase by LY294002 blocked IL-2-induced reduction of ceramide and changes of activities in acid SMase, GCS, and SMS
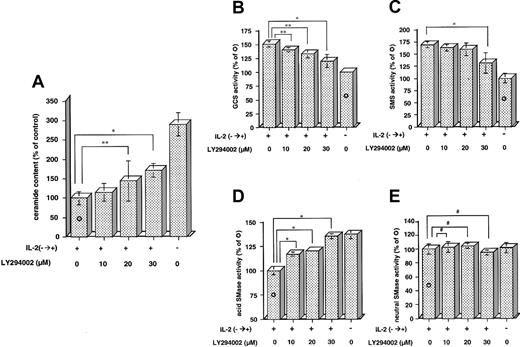
Treatment with various concentrations of LY294002 (10, 20, and 30 μM) inhibited IL-2-induced reduction of ceramide (Figure 4A). Ceramide content was increased to 170% ± 14% of the control by treatment with 30 μM LY294002 even after 24 hours of IL-2 supplementation. At the same condition, changes in GCS, SMS, and acid and neutral SMase activities were examined (Figure 4B-E). IL-2-induced activation of GCS and SMS was dose dependently inhibited by LY294002. For example, 30 μM LY294002 decreased IL-2-induced activation of GCS and SMS from 151% ± 6% and 169% ± 7% to 120% ± 11% and 131% ± 20% of the control level, respectively (Figure 4B-C). In contrast, 30 μM LY294002 increased IL-2-inhibited activity of acid SMase to the control level (Figure 4D). Neutral SMase was not affected by treatment with LY294002 (Figure 4E). These results suggest that inhibition of PI-3 kinase by LY294002 is involved in IL-2-induced ceramide reduction through the regulation of ceramide-related enzymes, GCS, SMS, and acid SMase in KHYG-1 NK cells.
Inhibition by LY294002 of IL-2-induced ceramide reduction and enzymatic changes in GCS, SMS, and acid and neutral SMases. KHYG-1 cells were cultured in the absence of IL-2 for 36 hours or rescued with IL-2 supplementation (100 U/mL, IL-2-→+) and treated with various concentrations of LY294002 (0, 10, 20, and 30 μM) for 24 hours after 12 hours of deprivation. Ceramide content (A) was assessed by DGK assay, and the activities of GCS (B), SMS (C), acid (D), and neutral (E) SMases were measured by using C6-NBD-ceramide and C6-NBD-SM as the substrates as described in “Materials and methods.” Ceramide content after 24 hours of IL-2 supplementation was 4.6 ± 0.8 pmol/nmol phosphate. The activity of each enzyme detected after 24 hours of IL-2 supplementation was 789 ± 66, 41 ± 1.6, 579 ± 25, and 917 ± 91 pmol/mg protein/hour for GCS, SMS, acid, and neutral SMases, respectively. The bars indicate 1 SD. The significance of differences between the control ( ) and LY294002-treated samples was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
) and LY294002-treated samples was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
Inhibition by LY294002 of IL-2-induced ceramide reduction and enzymatic changes in GCS, SMS, and acid and neutral SMases. KHYG-1 cells were cultured in the absence of IL-2 for 36 hours or rescued with IL-2 supplementation (100 U/mL, IL-2-→+) and treated with various concentrations of LY294002 (0, 10, 20, and 30 μM) for 24 hours after 12 hours of deprivation. Ceramide content (A) was assessed by DGK assay, and the activities of GCS (B), SMS (C), acid (D), and neutral (E) SMases were measured by using C6-NBD-ceramide and C6-NBD-SM as the substrates as described in “Materials and methods.” Ceramide content after 24 hours of IL-2 supplementation was 4.6 ± 0.8 pmol/nmol phosphate. The activity of each enzyme detected after 24 hours of IL-2 supplementation was 789 ± 66, 41 ± 1.6, 579 ± 25, and 917 ± 91 pmol/mg protein/hour for GCS, SMS, acid, and neutral SMases, respectively. The bars indicate 1 SD. The significance of differences between the control ( ) and LY294002-treated samples was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
) and LY294002-treated samples was determined by ANOVA test. *P < .01; **P < .05; #P > .05.
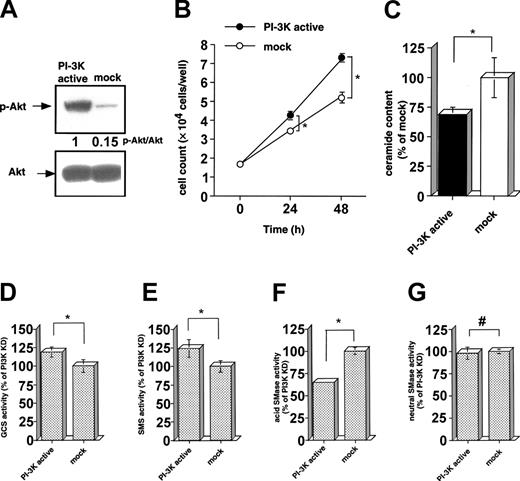
Increase of cell growth along with a reduction of ceramide through activation of GCS and SMS, and inhibition of acid SMase by a constitutively active PI-3 kinase
To confirm the involvement of PI-3 kinase on ceramide reduction through enzymatic regulation in KHYG-1 cells, the effects of constitutively active PI-3 kinase on cell growth and ceramide regulation were examined in PI-3 kinase-overexpressing COS-7 (PI-3K active) cells. The extent of Akt phosphorylation in PI-3K active cells was 6 times higher as compared with the mock cells (Figure 5A). As shown in Figure 5B, the PI-3K active cells showed higher growth rate and lower ceramide content than the mock cells. The viable cell number in PI-3K active cells was increased to 4.2 ± 2 × 104 cells/well as compared with that in the mock cells (3.1 ± 1 × 104 cells/well, P < .01) after 24 hours of seeding at 2.4 × 104 cells/well. The baseline of ceramide content in PI-3K active cells was 27% lower than that in the mock cells (Figure 5C). GCS and SMS activities (119% ± 7% and 124% ± 12%, respectively) in PI-3K active cells were significantly higher than those in the mock cells (Figure 5D-E, P < .01). However, acid SMase activity in PI-3K active cells was 64% ± 2% of the mock control (Figure 5F). There was no significant difference in neutral SMase activity between PI-3K active and mock cells (Figure 5G). These results show that activation of PI-3 kinase is closely involved in a reduction of ceramide through modulation of SMS, GCS, and acid SMase.
Effects of a constitutively active PI-3 kinase on cell growth, ceramide content, and activities in GCS, SMS, and acid and neutral SMases in COS-7 cells. The expression vector for constitutively activated PI-3 kinase (PI-3K active) was transiently transfected to the COS-7 cells by electroporation method as described in “Materials and methods.” (A) PI-3 kinase-dependent protein expression of phosphorylated Akt (Serine 473) was determined by Western blot analysis, using antibody for Akt and phospho-Akt (Serine 473) antibodies. (B) Transfected COS-7 cells were seeded in the 24-well culture dish at an initial concentration of 2 × 104 cells/wells and cultured for 48 hours. Viable cell numbers were counted by a trypan blue dye exclusion method at the indicated times (0, 24, and 48 hours). (C) Ceramide content in COS-7 cells 24 hours after transfection was measured by DGK assay as described in “Materials and methods.” Ceramide content in the mock cells was shown as the control (5.8 ± 1.2 pmol/nmol phosphate). The activities of GCS (D), SMS (E), and acid (F) and neutral (G) SMases in COS-7 cells after 24 hours of transfection were measured by using C6-NBD-ceramide and C6-NBD-SM as described in “Materials and methods.” The activities of each enzyme in the mock cells were shown to be 1564 ± 133, 359 ± 28, 664 ± 28, and 30 ± 0.84 pmol/mg protein/hour for GCS, SMS, and acid and neutral SMases, respectively. In these experiments 75% ± 9% was a transfection efficiency as judged by expression of transfected β-galactosidase. The results were obtained from 3 different experiments. The bars indicate 1 SD. The significance of difference was determined by ANOVA test. *P < .01.
Effects of a constitutively active PI-3 kinase on cell growth, ceramide content, and activities in GCS, SMS, and acid and neutral SMases in COS-7 cells. The expression vector for constitutively activated PI-3 kinase (PI-3K active) was transiently transfected to the COS-7 cells by electroporation method as described in “Materials and methods.” (A) PI-3 kinase-dependent protein expression of phosphorylated Akt (Serine 473) was determined by Western blot analysis, using antibody for Akt and phospho-Akt (Serine 473) antibodies. (B) Transfected COS-7 cells were seeded in the 24-well culture dish at an initial concentration of 2 × 104 cells/wells and cultured for 48 hours. Viable cell numbers were counted by a trypan blue dye exclusion method at the indicated times (0, 24, and 48 hours). (C) Ceramide content in COS-7 cells 24 hours after transfection was measured by DGK assay as described in “Materials and methods.” Ceramide content in the mock cells was shown as the control (5.8 ± 1.2 pmol/nmol phosphate). The activities of GCS (D), SMS (E), and acid (F) and neutral (G) SMases in COS-7 cells after 24 hours of transfection were measured by using C6-NBD-ceramide and C6-NBD-SM as described in “Materials and methods.” The activities of each enzyme in the mock cells were shown to be 1564 ± 133, 359 ± 28, 664 ± 28, and 30 ± 0.84 pmol/mg protein/hour for GCS, SMS, and acid and neutral SMases, respectively. In these experiments 75% ± 9% was a transfection efficiency as judged by expression of transfected β-galactosidase. The results were obtained from 3 different experiments. The bars indicate 1 SD. The significance of difference was determined by ANOVA test. *P < .01.
Inhibition of PI-3 kinase by LY294002 blocked IL-2-induced changes of mRNA and protein levels in GCS and acid SMase
As shown in Figure 6A, IL-2 deprivation induced a decrease of GCS and an increase of acid SMase at mRNA level as compared with β-actin (Figure 6A-B). The levels of GCS and acid SMase mRNAs were changed from 100% each to 49% and 122% of the control unit, respectively, after 12 hours of IL-2 deprivation. Subsequent supplementation with IL-2 for 12 hours attenuated the levels of GCS and acid SMase mRNAs close to the control level, eg, from 49% and 122% to 145% and 73% of the control level, respectively (Figure 6A). Treatment with 30 μM LY294002 blocked IL-2-induced changes of GCS and acid SMase mRNA levels from 145% and 73% to 118% and 103% of the control level, respectively (Figure 6A). In addition, IL-2 supplementation induced similar changes of GCS and acid SMase at the protein level (Figure 6B). Treatment with 30 μM LY294002 also blocked IL-2-induced increase of GCS protein and decrease of acid SMase protein (Figure 6B).
Effects of LY294002 on IL-2-induced changes of mRNA and protein levels in GCS and acid SMase. KHYG-1 cells were cultured in the absence (IL-2-) of IL-2 (100 U/mL) for 12 hour or 36 hours, or rescued with IL-2 supplementation for 24 hours after IL-2 deprivation for 12 hours (IL-2-→+). In the indicated cases, 30 μM LY294002 was added 1 hour before the rescue by IL-2 supplementation. (A) After extraction of total mRNA, Northern blot analysis was performed for GCS, acid SMase, and β-actin mRNAs as described in “Materials and methods.” Each lane contains 7 μg total mRNA. Equal loading was confirmed by mRNA level of β-actin in each lane. (B) The protein levels of GCS and acid SMase were detected by Western blot analysis as described in “Materials and methods.” Equal amounts of loading were confirmed by protein level of β-actin. The ratios to β-actin in GCS and acid SMase at mRNA and protein levels were calculated by NIH image software and shown at the bottom of each panel. The results were representative of 3 independent experiments.
Effects of LY294002 on IL-2-induced changes of mRNA and protein levels in GCS and acid SMase. KHYG-1 cells were cultured in the absence (IL-2-) of IL-2 (100 U/mL) for 12 hour or 36 hours, or rescued with IL-2 supplementation for 24 hours after IL-2 deprivation for 12 hours (IL-2-→+). In the indicated cases, 30 μM LY294002 was added 1 hour before the rescue by IL-2 supplementation. (A) After extraction of total mRNA, Northern blot analysis was performed for GCS, acid SMase, and β-actin mRNAs as described in “Materials and methods.” Each lane contains 7 μg total mRNA. Equal loading was confirmed by mRNA level of β-actin in each lane. (B) The protein levels of GCS and acid SMase were detected by Western blot analysis as described in “Materials and methods.” Equal amounts of loading were confirmed by protein level of β-actin. The ratios to β-actin in GCS and acid SMase at mRNA and protein levels were calculated by NIH image software and shown at the bottom of each panel. The results were representative of 3 independent experiments.
Effects of inhibition of PI-3 kinase by LY294002 on the stability and transcriptional rate of acid SMase and GCS mRNAs
To investigate further mechanisms by which PI-3 kinase modulates the levels of acid SMase and GCS mRNAs, we analyzed the effects of LY294002 on the stability and transcriptional rate of these mRNAs after IL-2 supplementation. For the stability, KHYG-1 cells cultured in IL-2-deprived condition for 12 hours were first treated with 10 μg/mL actinomycin D to inhibit de novo synthesis of mRNAs. Then, after IL-2 supplementation changes in acid SMase and GCS mRNA levels were examined by Northern blot analysis. As shown in Figure 7A-B, treatment with 30 μM LY294002 did not affect the degradation rate of acid SMase and GCS mRNAs, suggesting little involvement of IL-2-activated PI-3 kinase in the stability of these mRNAs. Next, we assessed the effects of LY294002 on the transcriptional rate in acid SMase and GCS mRNAs by nuclear run-on assay. IL-2 deprivation for 12 hours induced an increase of acid SMase and a decrease of GCS as compared with β-actin at mRNA transcription level (Figure 7D-E). By subsequent IL-2 supplementation, the transcriptional levels of acid SMase and GCS mRNAs were returned to those of consecutively IL-2-supplied control (Figure 7D,F). In contrast, treatment with 30 μM LY294002 blocked IL-2-induced changes of mRNA transcription levels in GCS and acids SMase (Figure 7G).
Effect of LY294002 on the stability of mRNA and transcriptional rate in acid SMase and GCS. KHYG-1 cells were treated with (LY294002 + IL-2+) or without (IL-2+) 30 μM LY294002 in the presence of IL-2 (100 U/mL) after IL-2 deprivation. A newly synthesized mRNA was blocked by addition of 10 μg/mL actinomycin D 10 minutes before IL-2 supplementation, and then the cells were harvested at the indicated time (0, 1, 2, 3, and 4 hours). Northern blot analysis was performed to detect the changes of acid SMase (A) and GCS mRNA levels (B) as described in “Materials and methods.” The amount of 18 S ribosomal RNA was visualized by bromide staining under UV illuminator to confirm the equal amounts of loading (C). KHYG-1 cells were also cultured in the presence (D) or absence (E) of IL-2 (100 U/mL) for 20 hours or rescued with IL-2 supplementation (100 U/mL) after 12 hours of deprivation and further cultured for 8 hours without (F) or with (G) 30 μM LY294002. LY294002 was added 1 hour before IL-2 supplementation. The nuclear run-on assay was performed to assess the transcriptional rate of acid SMase, GCS, and β-actin genes as described in “Materials and methods.” The results were representative of 2 independent experiments. Dot intensity was measured by NIH image, and the average of relative intensity of acid SMase and GCS to β-actin was plotted under each panels. The bars indicate 1 SD.
Effect of LY294002 on the stability of mRNA and transcriptional rate in acid SMase and GCS. KHYG-1 cells were treated with (LY294002 + IL-2+) or without (IL-2+) 30 μM LY294002 in the presence of IL-2 (100 U/mL) after IL-2 deprivation. A newly synthesized mRNA was blocked by addition of 10 μg/mL actinomycin D 10 minutes before IL-2 supplementation, and then the cells were harvested at the indicated time (0, 1, 2, 3, and 4 hours). Northern blot analysis was performed to detect the changes of acid SMase (A) and GCS mRNA levels (B) as described in “Materials and methods.” The amount of 18 S ribosomal RNA was visualized by bromide staining under UV illuminator to confirm the equal amounts of loading (C). KHYG-1 cells were also cultured in the presence (D) or absence (E) of IL-2 (100 U/mL) for 20 hours or rescued with IL-2 supplementation (100 U/mL) after 12 hours of deprivation and further cultured for 8 hours without (F) or with (G) 30 μM LY294002. LY294002 was added 1 hour before IL-2 supplementation. The nuclear run-on assay was performed to assess the transcriptional rate of acid SMase, GCS, and β-actin genes as described in “Materials and methods.” The results were representative of 2 independent experiments. Dot intensity was measured by NIH image, and the average of relative intensity of acid SMase and GCS to β-actin was plotted under each panels. The bars indicate 1 SD.
Discussion
It was previously reported that IL-2-induced DNA synthesis was accompanied by a reduction of ceramide,22 but the treatment with bacterial, neutral SMase did not affect DNA synthesis in lymphocytes.22 Therefore, it was suggested that ceramide regulation through acid SMase, GCS, and SMS is involved in cell growth and survival of lymphocytes. Here, we used IL-2-dependent KHYG-1 NK cells, which undergo apoptosis without IL-2 even in the medium containing 10% FCS (Figure 1). The survival of KHYG-1 cells was restored by IL-2 supplementation along with a reduction of ceramide content, whereas increase of endogenous ceramide by treatment with synthetic, cell permeable C2-ceramide inhibited IL-2-induced survival (Figure 1), suggesting that the reduction of ceramide is required for IL-2-induced rescue of NK cells from apoptosis.
Stimulation of neuronal growth by bFGF was reported to induce up-regulation of GCS activity,46 whereas inhibition of GCS by 1-phenyl-2-decanoylamino-3-morpholino-1-propanol (PDMP) resulted in cell cycle arrest through ceramide accumulation in NIH-3T3 cells.47 In addition, transformation of human lung fibroblasts by SV-40 was reported to induce an increase of SMS activity,28 resulting in activation of NF-κB by way of protein kinase C.29 An inhibitor of ceramidase, N-oleoylethanolamine inhibited platelet-derived growth factor (PDGF)-stimulated proliferation of mesangial cells, at least in part, through an increase of ceramide.48 Acid SMase-deficient lymphoblasts derived from a patient with Niemann-Pick disease (NPD) failed to undergo apoptosis with little generation of ceramide in response to Fas cross-linking.49 These results suggest that a diverse kind of ceramide-related enzyme play a role in apoptosis and cell survival through regulation of ceramide content. Riboni et al30,50 reported that bFGF increased a turnover of [3H]ceramide to [3H]SM, probably through activation of SMS in the rat primary astrocytes. We here showed that ceramide content was reduced through not only activation of SMS and GCS but also inhibition of acid SMase in IL-2-rescued NK cells (Figure 2A-C), although neutral SMase was not affected (Figure 2D). Ceramidase activity was too low to detect in KHYG-1 cells (data not shown). These results suggest the possible involvement of an efficient reduction of ceramide by the coordinated action of ceramide-related enzymes in IL-2-rescued NK cell survival.
We next investigated the involving signaling pathway in ceramide reduction in IL-2-rescued condition. IL-2 has been shown to activate the signaling molecules such as PI-3 kinase, Jak/stat system, and MAP kinase to induce cell growth.51 Because PI-3 kinase was known to phosphorylate Akt Serine 473, IL-2-induced increase of phosphorylation of Akt Serine 473 indicates the involvement of PI-3 kinase in IL-2-induced NK cell survival (Figure 3A), and the inhibition of phosphorylation of Akt Serine 473 showed a specific, inhibitory effect of LY294002 on PI-3 kinase at the concentration (10-30 μM) we used here (Figure 3B-C). As shown in Figure 3D, restoration of cell growth by IL-2 was significantly inhibited by LY294002 but not by AG490 and PD98059, which are the inhibitors for Jak/Stat kinase and MAP kinase, respectively (Figure 3E). Therefore, PI-3 kinase, as compared with Jak/stat and MAP kinase pathway, appeared to be more critical in IL-2-restored KHYG-1 cell survival.
Because inhibition of PI-3 kinase also blocked IL-2-induced reduction of ceramide and enzymatic changes in GCS, SMS, and acid SMase (Figure 4), ceramide reduction in IL-2-rescued NK cells seemed to be regulated by PI-3 kinase through inhibition of acid SMase and activation of GCS and SMS. This notion was further confirmed in PI-3 kinase-overexpressing COS-7 cells, where higher levels of growth increase and ceramide reduction were detected through activation of GCS and SMS, as well as inhibition of acid SMase as compared with the mock control cells (Figure 5). It was previously reported that exogenous SMase was partially suppressed by expression of a constitutively active PI-3 kinase and enhanced by inhibition of PI-3 kinase, and that the inhibition by IL-10 and IL-13 of tumor necrosis factor-α-mediated degradation of SM to ceramide is involved in the activation of PI-3 kinase. PI-3 kinase also negatively regulated p75NTR-dependent ceramide signaling through an inhibition of acid SMase activity in PC12 cells.52 Therefore, although these results are suggesting that cross-talk between PI-3 kinase and ceramide generation through SMase(s),52-54 this work is a first report, showing the coordinated involvement of acid SMase, GCS, and SMS through PI-3 kinase in a reduction of ceramide. In addition, we interestingly found that ceramide levels seem to be regulated through PI-3 kinase-dependent acid SMase, GCS, and SMS in IL-2-stimulated human primary NK cells (data not shown).
We next investigated the mechanism of PI-3 kinase to regulate acid SMase and GCS in IL-2-rescued NK cells. Northern and Western blot analyses showed that LY294002 clearly blocked IL-2-induced inhibition of acid SMase and activation of GCS at the mRNA and protein levels (Figure 6). As a molecular basis for the regulation of acid SMase and GCS mRNAs through PI-3 kinase has not been fully clarified, the stability and transcriptional rate of GCS and acid SMase mRNAs were further examined. The results showed that inhibition of PI-3 kinase by LY294002 did not affect the stability of GCS and acid SMase mRNAs (Figure 7) even though LY294002 was reported to increase lipopolysaccharide (LPS)-induced mRNA stability of cyclooxygenase 2 (COX-2) in human macrophages55 or to decrease the stability of the α1 collagen mRNA in lung fibroblasts.56 However, nuclear run-on assays showed that IL-2-induced transcriptional changes in acid SMase and GCS were blocked through inhibition of PI-3 kinase by LY294002. Thus, the transcriptional regulation of acid SMase and GCS through PI-3 kinase seems to be involved in a reduction of ceramide in IL-2-rescued cell survival.
In summary, these results suggest that IL-2 reduces ceramide content through PI-3 kinase-mediated regulation of GCS, SMS, and acid SMase at mRNA and protein levels. The transcriptional rate of GCS and acid SMase seems to be controlled through IL-2-activated PI-3 kinase, but the precise mechanism of regulation is at present unclear. PI-3 kinase has been known to mediate cell growth signals by a transcriptional regulation by ways of the transcription factors such as Forkhead family, FKHRL1 (Forkhead in rhabdomyosarcoma-like 1), CREB (cyclic AMP [adenosine monophosphate] response element binding protein) and Sp1 (specificity protein 1), and RNA polymerase I.57-60 Sp1 was reported to mediate induction of acid SMase during macrophage differentiation by phorbol 12-myristate-13-acetate (PMA) and 1,25-dihydroxy-vitamin D3.61 Sp1 is also suggested to be critical in a positive regulation of GCS activity.62 Therefore, in the future, the role should be investigated of transcription factor(s) regulated by PI-3 kinase in the regulation of ceramide-related enzymes such as GCS, SMS, and acid SMase.
Prepublished online as Blood First Edition Paper, July 22, 2004; DOI 10.1182/blood-2004-03-0900.
Supported by the 21st Century Center of Excellence Program of the Ministry of Education, Culture, Sports, Science and Technology to Graduate School of Biostudies and Institute for Virus Research, Kyoto University (Y.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr K. Sandhoff (Universitat Bonn, Germany) and Y. Hirabayashi (RIKEN, Wako, Japan) for their kind gifts of cDNA clone of human acid SMase and antiglucosylceramide synthase antibody, respectively. We also thank the members of the Leukemia Research Unit in the Department of Hematology and Oncology, Kyoto University.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal