Abstract
Lymphocytes are targeted to inflamed sites by specific “homing” and chemokine receptors. Most of them, including ligands for P- and E-selectin, are absent from naive CD4+ T cells and become induced after activation and differentiation in effector/memory cells. Polarized effector cells are characterized by the rapid production of distinct cytokines upon restimulation. Their cytokine memory is in part controlled by epigenetic imprinting during differentiation. Here we ask whether a similar mechanism could regulate selectin ligand expression, mediating entry into inflamed sites, notably within the skin. We report that acquisition of selectin ligands by naive but not memory CD4+ cells depends on progression through the G1/S phase of the cell cycle—a phase susceptible to modification of the chromatin structure. Cell-cycle arrest prevented transcriptional activation of glycosyltransferases involved in the generation of selectin ligands, suggesting that progression through the cell cycle is required to unlock their genes. Artificial DNA demethylation strongly increased the frequency of selectin ligand-expressing cells, suggesting that DNA methylation keeps transferase genes inaccessible in naive T cells. Due to these findings we propose that selectin-dependent inflammation-seeking properties are imprinted by epigenetic modifications upon T-cell differentiation into effector cells.
Introduction
Activation of naive T lymphocytes by specific antigen under appropriate conditions leads to functional differentiation into subsets of effector cells.1 This differentiation is accompanied by changes in homing receptor expression resulting in memory subsets displaying distinct homing properties. While naive T cells recirculate between lymphoid organs, fractions of effector/memory T cells gain access to peripheral inflamed sites.2,3 In contrast to naive T cells, these effector cells express receptors for inflammatory chemokines and ligands for E- and P-selectin but lack L-selectin expression.4-7
E- and P-selectin ligands are fucosylated oligosaccharides decorating distinct carrier proteins such as P-selectin glycoprotein binding protein-1 (PSGL-1) and enable trafficking of leukocytes and T cells into inflamed areas, particularly within the skin.8-10 The oligosaccharide epitopes become induced upon differentiation into effector/memory cells on a fraction of cells.6,11 A related E-selectin binding epitope involved in skin-specific homing of T cells has been termed “cutaneous lymphocyte antigen” (CLA).12
The generation of selectin ligands depends on the coordinated action of a set of glycosyltransferases, which determines both tissue-specific and differentiation-dependent generation of selectin-binding epitopes.8 Particularly in T lymphocytes, expression of fucosyltransferase (FucT) VII and core-2 glycosaminyltransferase (C2 GlcNAcT) appears to correlate with the expression of selectin ligands, and deletion of these enzymes abrogates the inducible expression on T cells.13,14
Secondary immune responses are more efficient than primary responses due to the presence of effector cells, which can rapidly recall their effector functions. Those cells evolved from naive T cells, which after activation by an appropriate peptide–major histocompatibility complex (peptide-MHC) underwent clonal expansion and programmed differentiation. This differentiation is associated with the commitment to produce particular effector cytokines such as interferon-γ (IFN-γ) (“Th1” cells) or interleukin-4 (IL-4) (“Th2” cells), which are recalled upon subsequent stimulation.15,16
Permanent differentiation of cell lineages is often associated with chromatin modification controlling gene expression in a heritable, clonal manner. Key mediators of epigenetic changes are postsynthetic modifications of DNA itself or of proteins, which are closely attached to the DNA.17,18
Changes in the pattern of DNA methylation and histone acetylation of regulatory regions of the IL-4 and IFN-γ gene suggest that similar mechanisms are involved in the stabilization of the functional polarization of effector lymphocytes.19-21 The cell cycle, providing a window for chromatin remodeling, is a major checkpoint for the acquisition of effector functions. Thus, arrest of naive T cells after T-cell receptor (TCR) engagement prevented functional differentiation without affecting the levels of lineage-determining transcription factors.22-24 As a result, the model emerged that master transcription factors interfere with the action of specific DNA methyltransferases like DNA methyltransferase 1, which are recruited to replication forks at S phase where they establish symmetric CpG methylation of hemimethylated substrates, effectively duplicating the DNA methylation pattern on newly synthesized DNA strands.25
The rapid recruitment of effector/memory cells to sites of inflammation is a prerequisite for an effective immune response. It implies that subsets of effector/memory cells retain inflammation-seeking homing properties for longer times or rapidly recall this property. We hypothesized that epigenetic mechanisms regulating gene accessibility could be involved in the fixation of such an inflammation-seeking homing pattern. Therefore, we determined whether induction of selectin ligands after activation of naive T cells is controlled by the cell cycle, and show that their upregulation and induction of the involved glycosyltransferases are indeed strictly dependent on entry into the cell cycle in naive but not memory T cells. This indicates that the respective gene loci are closed in naive T cells and become permanently accessible after priming in a part of T cells. DNA methylation appears to be involved in the control of accessibility of the genes, because expression of the selectin ligands is strongly enhanced by treatment with methylation inhibitors. These findings provide first evidence for the involvement of epigenetic mechanisms resulting in a stable imprinting of homing properties on effector T cells.
Materials and methods
Mice
DO11.10 mice (a kind gift from D.Y. Loh, Washington University School of Medicine, St Louis, MO) carrying a transgenic TCR specific for the ovalbumine (OVA)–peptide 323-339 and BALB/c mice were bred under specific pathogen-free conditions in the Bundesinstitut für Risikobewertung, Berlin, Germany. All animal experiments were performed in accordance with institutional, state, and federal guidelines.
Isolation and cell purification
CD4+ T cells were purified from pooled peripheral and mesenteric lymph nodes of DO11.10 mice either by panning using anti-CD8 (53-672), anti-CD25 (PC/6), anti–Mac-1 (M1/70), and anti-FcR II/III (2.4G2) antibodies or by direct isolation of CD4+ cells by anti-CD4–fluorescein isothiocyanate (anti-CD4-FITC) (GK1.5) and anti-FITC multisort–magnetic cell separation (MACS) beads (Miltenyi Biotec, Bergisch Gladbach, Germany) to a purity of at least 98%. Naive CD4+ CD62L+ cells were positively selected with anti-CD62L microbeads (Miltenyi Biotec) to a purity of at least 98%. Antigen-presenting cells (APCs) were prepared by depletion of CD90+ cells from spleen cells or purified CD4 negative fraction using anti-CD90 MACS microbeads (Miltenyi Biotec). APCs were irradiated (30 Gy) before culture. For cultures containing naive and memory cells, CD4+ cells were isolated from peripheral lymph nodes with anti-CD4 (L3T4) microbeads (Miltenyi Biotec).
CFSE labeling
Labeling of naive CD4+ CD62L+ cells with 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was performed as described.22 In brief, washed cells were resuspended at 1 × 107/mL in phosphate-buffered saline (PBS), CFSE (5 μM final concentration) was added, and cells were incubated for 3 minutes at room temperature. The reaction was stopped by washing with RPMI 1640 containing 10% fetal calf serum (FCS).
Cell culture
Cell culture was set up with 2 × 106 cells per milliliter in complete RPMI 1640 containing 10% FCS and 10 μM 2-mercaptoethanol (2-ME) (Life Technologies, Bethesda, MD). CD4+ CD62L+ T cells from OVA TCR transgenic mice and APCs were cultured at a ratio of 1:4 in the presence of OVA323-339 peptide (Biochemistry Department, Charité Universitätsmedizin Berlin, Germany) at 0.5 μM. Cell cultures containing total CD4+ cells were stimulated with 0.1 μM OVA323-339 peptide, supporting better survival of memory cells. Cell cultures were supplemented with recombinant murine IL-12 (R&D Systems, Minneapolis, MN) at 5 ng/mL, IFN-γ (R&D Systems) at 20 ng/mL, and neutralizing anti–IL-4 antibodies at 5.5 μg/mL (11B11) as indicated. For mRNA detection, 1 × 106/mL naive T cells were activated with plate-bound anti-CD3 (1 μg/mL) and soluble anti-CD28 (1 μg/mL).
Inhibitors
Progression of cells through the cell cycle was inhibited by addition of either 300 μM l-mimosine (ICN, Santa Ana, CA), 2 μg/mL aphidicolin (Sigma Chemical, St Louis, MO), 200 nM paclitaxel (ICN), or mycophenolic acid at 1 μM (Sigma Chemical) to the cell culture.
To study the possible role of DNA methylation, 5-aza-2-deoxycytidine (Sigma Chemical), as an inhibitor of cytosine methylation, was added to the cell culture at concentrations of 5 μM for 48 hours.
Cytometric analysis and cell sorting
For cytometric analysis dead cells were excluded by staining with 4,6 diamidino-2-phenylindole (Sigma Chemical). OVA TCR transgenic T cells were identified by the clonotype-specific monoclonal antibody KJ1-26.1. P-selectin binding ligands were detected by P-selectin–human immunoglobulin G (IgG) chimeric protein (provided by Dr D. Vestweber, Max-Planck-Institut für Vaskuläre Biologie, Münster, Germany) and phycoerythrin (PE)–conjugated anti–human IgG antibody F(ab′)2 (Dianova, Hamburg, Germany) as secondary reagent, as previously described.11 Naive and effector/memory cells were identified by the expression of CD45RB (clone 16A, Pharmingen, San Diego, CA). The state of activation was determined by fluorochrome-conjugated monoclonal antibody to CD25 (Pharmingen). Cytometric analysis was performed using an LSR or FACSCalibur and CellQuest research software (BD Biosciences). Cell sorting was done using a MoFlo fluorescence-activated cell sorter (FACS; Cytomation, Fort Collins, CO).
RNA isolation and real-time PCR analysis
For determination of mRNA expression, about 1 × 106 cells were lysed and RNA was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany). After DNase treatment RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (M-MLV-RT) (Gibco, Carlsbad, CA) using oligo(dT) primer (Amersham Pharmacia Biotech, Piscataway, NJ). For quantitative PCR the following primer (TIB-Molbiol, Berlin, Germany) and probes (Metabion, Planegg-Martinsried, Germany) were used. Primer: hypoxanthine phosphoribosyl transferase (HPRT) reverse: 5′-TTG AGC ACA CAG AGG GCC A; HPRT forward: 5′-ATC ATT ATG CCG AGG ATT TGG AA; FucT VII reverse: 5′-CAG ATG CAC CCT CTA GTA CTC TGG; FucT VII forward: 5′-TGC ACT GTC CTT CCA CAA CC; C2 GlcNAcT reverse: 5′-TGC CAG TTT ATC AGC GGG AC; C2 GlcNAcT forward: 5′-CAG GAG TCA GAG CCT CAA CAG A. Probes: HPRT: 5′-FAM-TGG ACA GGA CTG AAA GAC TTG CTC GAG ATG-TAMRA; FucT VII: 5′-FAM-CCT GCG CCC AGT GTA CAG TCT GCA-TAMRA; C2 GlcNAcT: 5′-FAM-TCA GGC TGC CTG TGA TTC TAA ACG TGA TAT C-TAMRA.
Quantitative PCR was done in a GeneAmp 5700 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany) using 10 to 100 ng cDNA and qPCR mastermix (Eurogentec, Seraing, Belgium) with the respective primers (900 nM) and probes (250 nM).
Results
Proliferation enhances P-lig expression on CD4+ lymphocytes
In vivo studies have shown that after activation of naive T cells the frequency of cells expressing P-selectin ligands (P-lig) increases with proliferation.26 We found similar results after activation of naive CD4+ cells in vitro under appropriate conditions11,27 —that is, in the presence of IL-12, IFN-γ, and anti-IL-4: among T cells that had not divided, only 5% to 15% expressed P-lig while completion of the first mitosis and further divisions significantly increased the likelihood of P-lig expression on day 3 after activation (Figure 1).
Proliferation enhances P-lig expression on activated CD4+ lymphocytes. Naive CD4+ T cells from DO11.10 mice were CFSE labeled and stimulated with OVA323-339 peptide and APCs plus IL-12, IFN-γ, and αIL-4. On day 3, individual generations (arrows, nos.) of undivided and divided KJ1-26.1+ cells were identified by loss of CFSE staining. (A) An example of control and P-lig staining in combination with CFSE. (B) The frequency of P-lig–positive cells within indicated generations of KJ1-26.1+ cells is summarized from 7 independent experiments (*P < .05).
Proliferation enhances P-lig expression on activated CD4+ lymphocytes. Naive CD4+ T cells from DO11.10 mice were CFSE labeled and stimulated with OVA323-339 peptide and APCs plus IL-12, IFN-γ, and αIL-4. On day 3, individual generations (arrows, nos.) of undivided and divided KJ1-26.1+ cells were identified by loss of CFSE staining. (A) An example of control and P-lig staining in combination with CFSE. (B) The frequency of P-lig–positive cells within indicated generations of KJ1-26.1+ cells is summarized from 7 independent experiments (*P < .05).
Proliferation enhances mRNA expression of FucT VII and C2 GlcNAct
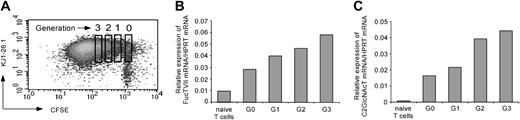
To verify if glycosyltransferases involved in the generation of selectin ligands are the target of cell-cycle–dependent regulation, we analyzed mRNA expression of FucT VII and C2 GlcNAcT. Therefore, we activated CFSE-labeled naive T cells from OVA TCR transgenic D011.10 mice for 3 days by OVA323-339 peptide and APCs in the presence of IL-12, IFN-γ, and αIL-4. Then, transgenic T cells were stained by the clonotype-specific antibody KJ1-26.1 and sorted into different generations according to their CFSE content (Figure 2A). Expression of FucT VII and C2 GlcNAcT mRNA was determined by quantitative PCR. Cells that had undergone one or more divisions showed a dramatically increased FucT VII mRNA and C2 GlcNAcT mRNA expression compared with naive T cells and, to a lesser degree, with cells from the same culture that had not divided yet (Figure 2B-C).
Proliferation enhances FucT VII and C2 GlcNAcT mRNA expression in activated CD4+ lymphocytes. On day 3 after activation as in Figure 1, transgenic KJ1-26.1+ T cells were sorted according to the CFSE stain into individual generations (G0 to G3) (A). FucT VII mRNA and C2 GlcNAcT mRNA expression in relation to HPRT as a housekeeping gene was determined by quantitative PCR within these sorted fractions and in naive T cells (B-C). One representative of 2 experiments is shown.
Proliferation enhances FucT VII and C2 GlcNAcT mRNA expression in activated CD4+ lymphocytes. On day 3 after activation as in Figure 1, transgenic KJ1-26.1+ T cells were sorted according to the CFSE stain into individual generations (G0 to G3) (A). FucT VII mRNA and C2 GlcNAcT mRNA expression in relation to HPRT as a housekeeping gene was determined by quantitative PCR within these sorted fractions and in naive T cells (B-C). One representative of 2 experiments is shown.
Arresting the cells in G1/S phase of the cell cycle abrogates P-lig induction, which is accompanied by inhibition of FucT VII and C2 GlcNAcT mRNA induction
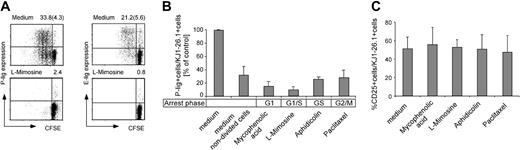
A small but significant fraction of nondivided cells expressed P-lig; these cells might have entered but not yet completed the cell cycle. To clarify this, cell-cycle inhibitors were used to stop mitosis at different phases of the cell cycle. The presence of inhibitors during activation strongly suppressed the induction of P-lig as shown in Figure 3A-B. Arrest in an early phase of the cell cycle (G1 phase by mycophenolic acid and G1/S phase by l-mimosine) almost completely abrogated the induction of P-lig in naive cells (2% to 4% P-lig–positive cells, Figure 3A), and arrest in later phases (S or G2/M phase) by aphidicolin or paclitaxel allowed P-lig expression at levels comparable to untreated cells that had not completed cell division (Figure 3B). Furthermore, we confirmed the absence of functional P-selectin ligands on l-mimosine–treated cells in a nonstatic adhesion assay and found that adhesion of G1/S-arrested T cells to P-selectin was reduced to only 5% (± 0.75%) of that of untreated Th1 cells (data not shown).
Inhibitors of the cell cycle suppress expression of P-lig but not other activation-induced molecules. Naive CD4+ T cells were stimulated as in Figure 1 in the absence or presence of the indicated drugs arresting the T cells in different phases of the cell cycle. The percentage of selectin lig-positive cells within CD4+ KJ1-26.1+ cells was determined on day 3. (A) A representative dot plot of P-lig and E-lig expression among total KJ1-26.1+. The percentages of selectin lig-positive cells among all and among undivided (in parentheses) cells is given. (B) Four separate experiments are summarized, and the mean + SD is given. To compare these experiments, the frequency of P-lig–positive cells induced in the absence of cell-cycle inhibitors is set to 100% for each experiment, and the relative frequency within undivided and inhibitor-treated cultures was determined. (C) CD25 expression on KJ1-26.1+ cells at day 1 after activation. The mean + SD from 3 separate experiments is given.
Inhibitors of the cell cycle suppress expression of P-lig but not other activation-induced molecules. Naive CD4+ T cells were stimulated as in Figure 1 in the absence or presence of the indicated drugs arresting the T cells in different phases of the cell cycle. The percentage of selectin lig-positive cells within CD4+ KJ1-26.1+ cells was determined on day 3. (A) A representative dot plot of P-lig and E-lig expression among total KJ1-26.1+. The percentages of selectin lig-positive cells among all and among undivided (in parentheses) cells is given. (B) Four separate experiments are summarized, and the mean + SD is given. To compare these experiments, the frequency of P-lig–positive cells induced in the absence of cell-cycle inhibitors is set to 100% for each experiment, and the relative frequency within undivided and inhibitor-treated cultures was determined. (C) CD25 expression on KJ1-26.1+ cells at day 1 after activation. The mean + SD from 3 separate experiments is given.
Induction of E-selectin ligands appears to be regulated in parallel as shown in Figure 3A: undivided T cells showed a low frequency of E-lig–positive cells, and l-mimosine treatment abrogated induction of E-lig.
Therefore, entry into the S phase seems to be indeed a critical determinant in the regulation of the relevant genes. In contrast, expression of activation markers such as CD25 is observed rapidly after activation and is not blocked by inhibition of mitosis (Figure 3C). The same applies to CD69, L-selectin down-regulation, and IL-2 secretion 1 or 2 days after stimulation.22 This confirms unimpaired activation in the inhibitor-treated cultures and the peculiar type of gene regulation involved in P-lig induction.
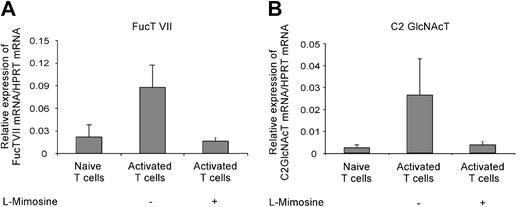
The impact of the cell cycle on the mRNA expression of fucosyltransferase VII (FucT VII) and core-2 glycosaminyltransferase (C2 GlcNAct) was determined 3 days after activation of naive T cells in the presence or absence of l-mimosine. Naive T cells were activated by plate-bound αCD3 and soluble αCD28 to avoid contamination by mRNA from APCs. In line with its effects on P-lig expression, l-mimosine treatment prevented the upregulation of mRNA expression for FucT VII and C2 GlcNAct seen in untreated cultures (Figure 4). This indicated that, in contrast to most molecules induced after activation, transcription of both FucT VII and C2 GlcNAcT genes not only requires activating signals but also entry into the cell cycle. The strict link between expression and mitosis points to the involvement of chromatin remodeling.
Cell-cycle arrest prevents up-regulation of FucT VII and C2 GlcNAcT mRNA expression. A total of 1 × 106 naive T cells were activated by plate-bound anti-CD3 and soluble anti-CD28 plus IL-12, IFN-γ, and anti-IL-4 for 3 days. The relative expression of FucT VII (A) and C2 GlcNAcT (B) mRNA to housekeeping gene HPRT was determined for naive T cells and cells activated in the presence or absence of l-mimosine. The mean (+ SD) from 4 individual experiments is shown.
Cell-cycle arrest prevents up-regulation of FucT VII and C2 GlcNAcT mRNA expression. A total of 1 × 106 naive T cells were activated by plate-bound anti-CD3 and soluble anti-CD28 plus IL-12, IFN-γ, and anti-IL-4 for 3 days. The relative expression of FucT VII (A) and C2 GlcNAcT (B) mRNA to housekeeping gene HPRT was determined for naive T cells and cells activated in the presence or absence of l-mimosine. The mean (+ SD) from 4 individual experiments is shown.
Most memory/effector cells can express P-selectin ligands independent of cell cycling
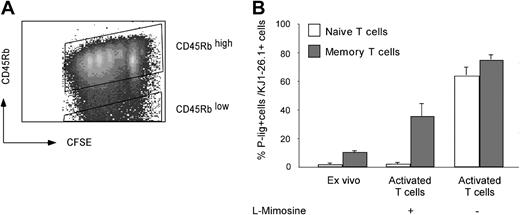
If imprinting by chromatin remodeling is involved in glycosyltransferase regulation, at least subfractions of memory cells primed under P-lig–permissive conditions should carry the ligand-generating genes either permanently active or in an accessible, cell cycle–independent state. To investigate this prediction, CFSE-labeled CD4+ T cells isolated from peripheral lymph nodes of DO11.10 mice were activated in the presence or absence of l-mimosine, arresting the cells in G1/S phase of the cell cycle. For identification of naive and effector/memory cells, we used a combination of CFSE labeling and CD45RB staining, which traces the proliferation-associated down-regulation of CD45RB and allows discrimination of naive and effector/memory cells for up to 3 days after activation (Figure 5A). In line with previous observations, only negligible numbers of naive, CD45RBhigh cells expressed P-lig when arrested in G1/S phase on day 3 after activation (Figure 5B). In contrast, memory cells contained about 10% P-lig–expressing cells after isolation ex vivo, and an additional fraction of 20% to 35% up-regulated the ligands independent of cell cycling. To exclude the possibility that selective survival of P-lig–positive memory/effector cells results in enhanced frequencies of P-lig–positive cells under these conditions, we sorted P-lig–positive and P-lig–negative CD45RBlow CD4+ cells and activated the cells as described in “Materials and methods.” Comparable survival rates of 20% and 25% at day 3, respectively, excluded a preferential enrichment of P-lig–positive compared with P-lig–negative memory T cells (data not shown).
Cell-cycle arrest prevents induction of P-lig in naive but not in memory cells. Expression of P-lig was measured on naive and memory/effector cells before and 3 days after activation by 0.1 μM OVA323-339 peptide and cytokines (IL-12, IFN-γ, and anti-IL-4) in the presence or absence of l-mimosine. Naive (CD45RBhigh) and memory T cells (CD45RBlow) were identified on day 3 after activation by combining CFSE staining and CD45RB expression (A). (B) The frequency of P-lig–positive cells within KJ1.26-1+ naive and memory/effector cells (mean + SD from 3 separate experiments).
Cell-cycle arrest prevents induction of P-lig in naive but not in memory cells. Expression of P-lig was measured on naive and memory/effector cells before and 3 days after activation by 0.1 μM OVA323-339 peptide and cytokines (IL-12, IFN-γ, and anti-IL-4) in the presence or absence of l-mimosine. Naive (CD45RBhigh) and memory T cells (CD45RBlow) were identified on day 3 after activation by combining CFSE staining and CD45RB expression (A). (B) The frequency of P-lig–positive cells within KJ1.26-1+ naive and memory/effector cells (mean + SD from 3 separate experiments).
Thus, more than 50% of memory cells able to express P-lig, according to nontreated culture, did so independent of cell cycling, indicating that they carry the P-lig–generating loci in an open conformation.
Artificial demethylation enhances induction of selectin ligands
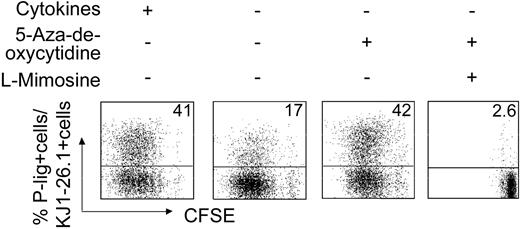
The above data do not provide clues as to the mechanisms that stabilize accessibility of the transferase genes. Demethylation of DNA is a key mechanism in epigenetic regulation of gene expression. To elucidate a possible role in the regulation of P-selectin ligand expression, naive T cells were exposed to 5-aza-2-deoxycytidine, a cytosine methylation inhibitor, during activation under the least permissive conditions for P-lig induction—that is, in the absence of IL-12 (Figure 6). Indeed, artificial DNA demethylation was accompanied by induction of a high frequency of P-lig–expressing cells similar to what is observed under optimal in vitro conditions containing IL-12. If cell cycling was simultaneously blocked by l-mimosine treatment, no significant induction of P-lig was seen, excluding unspecific effects. This indicates that methylation keeps P-lig–generating genes inaccessible in naive cells and that chromatin remodeling has to occur with primary induction of selectin ligands under permissive conditions.
Artificial DNA demethylation up-regulates P-lig expression on effector T cells. Naive transgenic CD4+ cells were activated with OVA323-339 peptide and APCs either with or without cytokine supplement (IL-12, IFN-γ, and αIL-4) as indicated and in the presence or absence of the DNA methylation inhibitor 5-aza-2-deoxycytidine (5 μM) and cell-cycle inhibitor l-mimosine. Data from 1 of 4 similar experiments are shown. Numbers in top right corners indicate the percentage of P-lig–positive cells in culture.
Artificial DNA demethylation up-regulates P-lig expression on effector T cells. Naive transgenic CD4+ cells were activated with OVA323-339 peptide and APCs either with or without cytokine supplement (IL-12, IFN-γ, and αIL-4) as indicated and in the presence or absence of the DNA methylation inhibitor 5-aza-2-deoxycytidine (5 μM) and cell-cycle inhibitor l-mimosine. Data from 1 of 4 similar experiments are shown. Numbers in top right corners indicate the percentage of P-lig–positive cells in culture.
Discussion
Expression of appropriate homing receptors on effector cells allows their accumulation at the site of infection, which, in concert with the polarized effector functions, ensures a rapid and efficient immune response during secondary infection. Here, we provide first evidence that an inflammation-seeking homing potential might be imprinted by epigenetic gene modification, a mechanism shown to be involved in the fixation of functional differentiation of effector cells.
First, we demonstrate that the cell cycle is a major checkpoint for the regulation of P-lig expression in lymphocytes. In general, proliferation enhances the frequency of cells expressing P-selectin ligands as shown here and upon in vivo activation.26 This also applies to mRNA expression of FucT VII and C2 GlcNAct, which were induced only in activated T cells progressing through the cell cycle. Arrest of the cells in early phases of the cell cycle completely prevented acquisition of P-selectin ligands as well as E-selectin ligands and up-regulation of fucosyltransferase VII and core-2 glycosaminyltransferase, showing that mRNA expression of both enzymes is sensitive to cell cycling.
Secondly, artificial demethylation by 5-aza-2-deoxycytidine, a cytosine methylation inhibitor, enhances the induction of selectin ligands under the least permissive in vitro conditions to levels observed under optimal inductive conditions. This effect of DNA demethylation and the requirement to complete early phases of the cell cycle that are vulnerable to chromatin remodeling strongly suggest that induction of selectin ligands is epigenetically modulated in lymphocytes during differentiation. Therefore, our data indicate that methylation keeps P-lig–generating genes inaccessible in naive cells and that chromatin remodeling has to occur with primary induction of selectin ligands under permissive conditions and might be involved in keeping the genes permanently accessible. The cell-cycle–linked expression of FucT VII and C2 GlcNAct mRNA suggests that epigenetic regulation occurs in the regulatory regions of these genes; however, not entirely excluded at present is an indirect mechanism where imprinting resides on the level of a proximal regulatory element such as an undetermined transcription factor.
The above model let us predict that effector/memory cells primed in vivo under P-lig–permissive conditions carry the ligand-generating genes permanently accessible, resulting either in constitutive expression or cell cycle–independent re-expression. Indeed, whereas the frequency of cells constitutively expressing selectin ligands is low among resting memory cells, more than 40% expressed the ligands independently from the cell cycle. This suggests that the transferase genes are not switched on permanently but remain accessible. Whether this state results in distinct, possibly less stringent requirements for transcriptional activation is presently under investigation.
The observed frequency of memory cells expressing P-lig without the need for mitosis resembles that among CD4+ effector cells found in vivo during acute inflammation28 and most likely represents the fraction of memory cells initially primed to express P-lig.
In conclusion, the findings of this study reveal a striking similarity between induction of the cytokine memory and generation of effector cell subsets displaying selective, inflammation-seeking trafficking properties. Dependency on cell cycling, a role of DNA methylation, and the capacity to recall a functionally polarized state are hallmarks of epigenetic imprinting. Thus, epigenetic modifications might provide the basis for a long-term inflammation-seeking homing pattern of effector/memory cells, ensuring a rapid secondary immune response within peripheral extralymphoid sites.
Prepublished online as Blood First Edition Paper, August 5, 2004; DOI 10.1182/blood-2003-09-3047.
Supported by the Deutsche Forschungsgemeinschaft (SFB 366).
U.S. and S.J. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank D. Vestweber for providing the P-selectin–IgG chimera, M. Loehning for helpful discussions and comments on the manuscript, and P. Bertram for excellent performance of quantitative PCR analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal