Abstract
Hyperhomocysteinemia is associated with ischemic cardiovascular disease (ICD) and venous thromboembolism (VTE). We tested the hypothesis that methylenetetrahydrofolate reductase (MTHFR) C677T homozygosity with hyperhomocysteinemia is associated with ICD and VTE. First, 9238 randomly selected whites from the general population were followed for 23 years. Second, 2125 whites with ischemic heart disease and 836 whites with ischemic cerebrovascular disease were compared with 7568 controls from the general population. Plasma homocysteine was elevated 25% in homozygotes versus noncarriers (P < .001) and 19% in ICD/VTE cases versus controls (P < .001). In prospective studies adjusted hazard ratios for ICD and VTE for homozygotes versus noncarriers did not differ from 1.0. Furthermore, MTHFR C677T homozygosity was not associated with increased risk of ICD or VTE in subgroups after stratification for sex, age, cholesterol, high-density lipoprotein cholesterol, lipoprotein(a), fibrinogen, triglycerides, body mass index, smoking, diabetes mellitus, hypertension, and factor V Leiden genotype. Finally, in case-control studies odds ratios for ischemic heart disease and ischemic cerebrovascular disease in homozygotes versus noncarriers did not differ from 1.0. In conclusion, MTHFR C677T homozygosity with hyperhomocysteinemia is not associated with ICD or VTE; however, ICD/VTE is associated with hyperhomocysteinemia. Therefore, ICD and VTE may cause hyperhomocysteinemia, rather than vice versa.
Introduction
Recent meta-analyses suggest that hyperhomocysteinemia is a risk factor for ischemic cardiovascular disease (ICD) and venous thromboembolism (VTE)1-4 ; however, in prospective studies these associations are less consistent than in cross-sectional or case-control studies.3,5-7 It is therefore still difficult to conclude whether hyperhomocysteinemia causes ICD and VTE or vice versa. An alternative approach, therefore, is to study common genetic causes of hyperhomocysteinemia, like the C677T polymorphism in methylenetetrahydrofolate (MTHFR).2,8-11
MTHFR converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the primary circulatory form of folate.12 MTHFR thereby regulates the remethylation of homocysteine into methionine by 5-methyltetrahydrofolate. The C677T polymorphism renders the enzyme thermo-labile and less active, resulting in hyperhomocysteinemia in the homozygous state.13 Meta-analyses demonstrate overall that TT homozygosity is associated with ICD and VTE;2,4,5,11 however, this could not be documented in the subgroup of nested case-control studies from prospective studies.11
We tested the hypothesis that MTHFR C677T homozygosity is associated with ICD and VTE. To do so, we performed a prospective study in the adult Danish general population with 23 years of follow-up of 9238 individuals; 4035 of these individuals were re-examined for plasma homocysteine levels in 2001-2003. In addition, we also performed 2 case-control studies including 2125 ischemic heart disease (IHD) cases, 836 ischemic cerebrovascular disease (ICVD) cases, and 7568 controls. Our prospective studies constitute the only population-based prospective studies published on MTHFR C677T and risk of ICD and VTE. Our case-control studies constitute by far the largest of any such study published on MTHFR C677T and risk of IHD and ICVD.
Patients, materials, and methods
Study design
Prospective studies. Seven prospective studies were conducted within The Copenhagen City Heart Study. ICD was studied overall but also subclassified into IHD, ICVD, and intermittent claudication, while VTE was studied overall and subclassified into deep venous thrombosis (DVT) and pulmonary embolism (PE). The cohort was defined as participants in The Copenhagen City Heart Study who attended the 1991-1994 examination and gave blood for DNA analysis. All outcomes were recorded in the follow-up period from 1976 through 1999. Individuals diagnosed with ICD before entry into The Copenhagen City Heart Study were excluded from the relevant study, while participants with prior VTE were not excluded.
Case-control studies. Two case populations comprising 2125 IHD patients and 836 ICVD patients who attended the Copenhagen University Hospital were compared with 7568 control subjects from the Copenhagen City Heart Study; controls were free of IHD, ICVD, and intermittent claudication.
Subjects
The Copenhagen City Heart Study. This is a prospective cardiovascular study of individuals selected from the Central-Population-Register Code designed to reflect the adult Danish general population, including both men and women.14 Those invited were stratified into 5-year age groups from 20 to 95 years, with main emphasis placed on the 35- to 70-year-olds. In 1976-1978, a total of 19 329 individuals were invited, of whom 14 223 (74%) participated. In 1981-1983, the original cohort, supplemented with 500 in the 20- to 25-year-old group, was invited and 12 698 (70%) participated. In 1991-1994, the cohort, further supplemented with 3000 in the 20- to 49-year-old group, was invited and 10 135 (61%) participated. Finally, in 2001-2003 4035 individuals were re-examined for plasma homocysteine levels for the present study. More than 99% were whites of Danish descent. DNA was available on 9259 participants of whom 9238 were genotyped for MTHFR C677T.
Information on diagnoses of IHD (World Health Organization International Classification of Diseases, 8th ed. codes 410-414 and ICD-10 codes I21, I22), ICVD (ICD-8 codes 432-435) intermittent claudication (ICD-8 code 443.99 and ICD-10 code I73.9), DVT (ICD-8 codes 451.00, 451.08, 451.09, 451.90, 451.92, 671.01-671.09 and ICD-10 codes I80.1, I80.2, I80.3, O22.3, O87.1) and pulmonary embolism (ICD-8 codes 450.99, 673.99 and ICD-10 codes I26.0, I26.9, O88.2) were gathered until the end of 1999 (ICVD until the end of 1997) from the Danish National Hospital Discharge Register, the Danish National Register of Causes of Death, and medical records from general practitioners and hospitals.
The Danish ethics committees for the cities of Copenhagen, Frederiksberg, and Copenhagen County, as well as the institutional review board for Herlev University Hospital, approved the studies. Informed consent was obtained from participants.
Copenhagen University Hospital. Patients with IHD were identified among patients from the greater Copenhagen area referred for coronary angiography because of angina pectoris from 1991 through 2002. IHD was diagnosed by experienced cardiologists based on characteristic symptoms of stable angina pectoris,15 plus at least one of the following: stenosis on coronary angiography, previous myocardial infarction, or a positive exercise electrocardiography test. Two-thousand one-hundred and twenty-five IHD patients, aged 19-90, were genotyped for MTHFR C677T. More than 99% were whites of Danish descent.
Patients with ICVD were identified among patients from the greater Copenhagen area referred from 1994 to 2002 for outpatient ultrasonography of the carotid artery. Experienced neurologists and vascular surgeons diagnosed ICVD on the basis of sudden onset of focal neurologic symptoms, together with carotid artery stenosis of at least 50% on the symptomatic or most stenotic side. Patients had ischemic stroke (focal neurologic symptoms lasting more than 24 hours when hemorrhage was excluded on computed tomography), transient ischemic attack (focal neurologic symptoms lasting less than 24 hours), or amaurosis fugax (transient monocular blindness). Eight-hundred and thirty-six patients, aged 35-88, were genotyped for MTHFR C677T. More than 99% were whites of Danish descent.
Analysis
The C677T polymorphism is caused by the substitution of thymidine for cytosine in exon 4 of the MTHFR gene. The polymorphism was identified by polymerase chain reaction (PCR) followed by restriction enzyme digestion and separation on a 3% agarose gel. The following sense and antisense primers within exon 4 were used: 5′GCCCAGCCACTCACTGTTTTA3′ and 5′AGGACGGTGCGGTGAGAGTG3′. The PCR product of 418 bp was digested with HinFI, resulting in 77 and 341 bp bands for noncarriers, 77, 165, 176, and 341 bands for heterozygotes, and 77, 165, and 176 bands for homozygotes. To avoid misclassification of MTHFR genotype, all participants had their genotypes determined from the agarose gel by 2 independent investigators. Furthermore, data entry was performed in duplicate by 2 independent investigators. Genotyping for factor V Leiden was as previously described.16,17
Both measurement and detection of conventional cardiovascular risk factors was as described previously.18 Homocysteine was measured in EDTA (ethylenediaminetetraacetic acid)–plasma (separated from erythrocytes immediately after blood sampling) using the IMX system (Abbott Diagnostics, Abbott Park, IL).
Statistical analyses
We used SPSS software, version 10.0 (SPSS, Chicago, IL). Two-sided P values less than .05 were considered significant. Categorical variables were compared using Pearson chi-square test. Student t test, Mann-Whitney U test, and analysis of variance (ANOVA) compared continuous variables between groups.
Log-rank tests and Kaplan-Meier curves are given for prospective data. Cox regression analysis was used to estimate hazard ratios adjusted for sex and age or for sex, age, and medians of cholesterol, HDL-cholesterol, lipoprotein(a), fibrinogen, triglycerides, and body mass index, as well as smoking, diabetes mellitus, hypertension, and factor V Leiden genotype. AWald test was performed to test for trend in hazard ratios across MTHFR C677T genotypes. Unconditional logistic regression analyses assessed associations between MTHFR genotype and IHD and ICVD in case-control studies.
Results
In the sample from the adult Danish population used for prospective studies, MTHFR C677T genotype distribution did not differ from that predicted by the Hardy-Weinberg equilibrium (P = .38): there were 4429 noncarriers, 3961 heterozygotes, and 848 homozygotes (Table 1). None of the characteristics listed in Table 1 differed by MTHFR C677T genotype; however, risk factors differed between cases and controls in prospective as well as case-control studies (Table 2).
Characteristics of participants in prospective studies by MTHFR C677T genotype
. | Noncarriers . | Heterozygotes . | Homozygotes . |
|---|---|---|---|
| No. | 4429 | 3961 | 848 |
| Female, % | 56 | 55 | 55 |
| Age at entry, y | 45 ± 0.2 | 45 ± 0.2 | 45 ± 0.4 |
| Cholesterol level, mmol/L | 5.8 ± 0.02 | 5.8 ± 0.02 | 5.8 ± 0.04 |
| HDL-cholesterol level, mmol/L | 1.3 ± 0.01 | 1.3 ± 0.01 | 1.3 ± 0.01 |
| Lipoprotein(a) level, mg/dL | 309 ± 6 | 313 ± 6 | 296 ± 13 |
| Fibrinogen level, g/L | 3.1 ± 0.01 | 3.1 ± 0.01 | 3.1 ± 0.03 |
| Triglyceride level, mmol/L | 1.7 ± 0.02 | 1.7 ± 0.02 | 1.7 ± 0.04 |
| Body mass index, kg/m2 | 24.5 ± 0.1 | 24.7 ± 0.1 | 24.5 ± 0.2 |
| Smoking, % | 75 | 76 | 77 |
| Diabetes mellitus, % | 3 | 4 | 3 |
| Hypertension, % | 13 | 14 | 16 |
| Factor V Leiden | |||
| Noncarrier, % | 92 | 93 | 93 |
| Heterozygote, % | 8 | 7 | 7 |
| Homozygote, % | 0.2 | 0.3 | 0 |
. | Noncarriers . | Heterozygotes . | Homozygotes . |
|---|---|---|---|
| No. | 4429 | 3961 | 848 |
| Female, % | 56 | 55 | 55 |
| Age at entry, y | 45 ± 0.2 | 45 ± 0.2 | 45 ± 0.4 |
| Cholesterol level, mmol/L | 5.8 ± 0.02 | 5.8 ± 0.02 | 5.8 ± 0.04 |
| HDL-cholesterol level, mmol/L | 1.3 ± 0.01 | 1.3 ± 0.01 | 1.3 ± 0.01 |
| Lipoprotein(a) level, mg/dL | 309 ± 6 | 313 ± 6 | 296 ± 13 |
| Fibrinogen level, g/L | 3.1 ± 0.01 | 3.1 ± 0.01 | 3.1 ± 0.03 |
| Triglyceride level, mmol/L | 1.7 ± 0.02 | 1.7 ± 0.02 | 1.7 ± 0.04 |
| Body mass index, kg/m2 | 24.5 ± 0.1 | 24.7 ± 0.1 | 24.5 ± 0.2 |
| Smoking, % | 75 | 76 | 77 |
| Diabetes mellitus, % | 3 | 4 | 3 |
| Hypertension, % | 13 | 14 | 16 |
| Factor V Leiden | |||
| Noncarrier, % | 92 | 93 | 93 |
| Heterozygote, % | 8 | 7 | 7 |
| Homozygote, % | 0.2 | 0.3 | 0 |
Values are mean ± SE or percentage of individuals. HDL indicates high-density lipoprotein. Homozygotes and heterozygotes each were compared with noncarriers using Student t test, Mann-Whitney U-test, or χ2-test. No difference between genotypes was detected.
Characteristics of participants by disease status
. | Prospective studies . | . | . | Case-control studies . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Controls* . | ICD . | VTE . | Controls . | IHD . | ICVD . | ||||
| No. | 7656 | 1374 | 208 | 7568 | 2125 | 836 | ||||
| Female, % | 58 | 43† | 46‡ | 58 | 28† | 36† | ||||
| Age, y | 44 ± 0.1 | 53 ± 0.2† | 52 ± 0.6† | 56 ± 0.2 | 61 ± 0.2† | 64 ± 0.3† | ||||
| Cholesterol level, mmol/L | 5.7 ± 0.01 | 6.4 ± 0.03† | 6.2 ± 0.08† | 6.1 ± 0.01 | 5.6 ± 0.03† | 6.3 ± 0.04† | ||||
| HDL cholesterol level, mmol/L | 1.3 ± 0.01 | 1.1 ± 0.01† | 1.2 ± 0.03† | 1.6 ± 0.01 | 1.2 ± 0.01† | 1.4 ± 0.02† | ||||
| Lipoprotein(a) level, mg/dL | 300 ± 4 | 362 ± 12† | 352 ± 30§ | 300 ± 4 | 597 ± 21† | 499 ± 35† | ||||
| Fibrinogen level, g/L | 3.0 ± 0.01 | 3.5 ± 0.03† | 3.3 ± 0.06† | 3.0 ± 0.01 | ND | 3.8 ± 0.1† | ||||
| Triglyceride level, mmol/L | 1.6 ± 0.02 | 2.1 ± 0.04† | 1.7 ± 0.08 | 1.8 ± 0.02 | 1.7 ± 0.04 | 2.0 ± 0.05§ | ||||
| Body mass index, kg/m2 | 24.3 ± 0.04 | 25.8 ± 0.1† | 26.1 ± 0.03† | 25.4 ± 0.05 | ND | 25.2 ± 0.2 | ||||
| Smoking, % | 74 | 85† | 84‡ | 74 | 84† | 78§ | ||||
| Diabetes mellitus, % | 3 | 8† | 5§ | 3 | 10† | 14† | ||||
| Hypertension, % | 10 | 32† | 20† | 10 | 25† | 44† | ||||
| Factor V Leiden | ||||||||||
| Noncarrier, % | 92 | 93 | 80 | 92 | 91 | 93 | ||||
| Heterozygote, % | 8 | 7 | 18 | 8 | 9 | 7 | ||||
| Homozygote, % | 0.2 | 0.4 | 1.5 | 0.2 | 0.3 | 0.4 | ||||
. | Prospective studies . | . | . | Case-control studies . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | Controls* . | ICD . | VTE . | Controls . | IHD . | ICVD . | ||||
| No. | 7656 | 1374 | 208 | 7568 | 2125 | 836 | ||||
| Female, % | 58 | 43† | 46‡ | 58 | 28† | 36† | ||||
| Age, y | 44 ± 0.1 | 53 ± 0.2† | 52 ± 0.6† | 56 ± 0.2 | 61 ± 0.2† | 64 ± 0.3† | ||||
| Cholesterol level, mmol/L | 5.7 ± 0.01 | 6.4 ± 0.03† | 6.2 ± 0.08† | 6.1 ± 0.01 | 5.6 ± 0.03† | 6.3 ± 0.04† | ||||
| HDL cholesterol level, mmol/L | 1.3 ± 0.01 | 1.1 ± 0.01† | 1.2 ± 0.03† | 1.6 ± 0.01 | 1.2 ± 0.01† | 1.4 ± 0.02† | ||||
| Lipoprotein(a) level, mg/dL | 300 ± 4 | 362 ± 12† | 352 ± 30§ | 300 ± 4 | 597 ± 21† | 499 ± 35† | ||||
| Fibrinogen level, g/L | 3.0 ± 0.01 | 3.5 ± 0.03† | 3.3 ± 0.06† | 3.0 ± 0.01 | ND | 3.8 ± 0.1† | ||||
| Triglyceride level, mmol/L | 1.6 ± 0.02 | 2.1 ± 0.04† | 1.7 ± 0.08 | 1.8 ± 0.02 | 1.7 ± 0.04 | 2.0 ± 0.05§ | ||||
| Body mass index, kg/m2 | 24.3 ± 0.04 | 25.8 ± 0.1† | 26.1 ± 0.03† | 25.4 ± 0.05 | ND | 25.2 ± 0.2 | ||||
| Smoking, % | 74 | 85† | 84‡ | 74 | 84† | 78§ | ||||
| Diabetes mellitus, % | 3 | 8† | 5§ | 3 | 10† | 14† | ||||
| Hypertension, % | 10 | 32† | 20† | 10 | 25† | 44† | ||||
| Factor V Leiden | ||||||||||
| Noncarrier, % | 92 | 93 | 80 | 92 | 91 | 93 | ||||
| Heterozygote, % | 8 | 7 | 18 | 8 | 9 | 7 | ||||
| Homozygote, % | 0.2 | 0.4 | 1.5 | 0.2 | 0.3 | 0.4 | ||||
Values are mean ± SE or percentage of individuals. Values were calculated on a slightly varying number of individuals depending on availability of data.
HDL indicates high-density lipoprotein; ND, not determined.
Individuals who remained free from ICD and VTE throughout follow-up. Values in the prospective studies represent baseline values. In the prospective studies, individuals developing ICD or VTE were compared with individuals who remained free from both ICD and VTE using Student ttest, Mann-Whitney U-test, or χ2 test. In case-control studies, cases with IHD or ICVD were compared with controls examined in 1991 through 1994 who were free from both ICD and VTE.
P < .001.
P < .01.
P < .05.
Plasma homocysteine levels
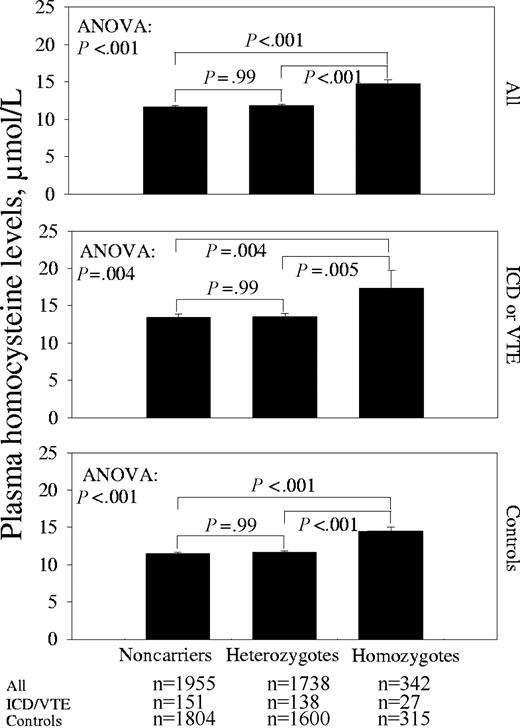
Among the 4035 persons re-examined in 2001-2003, homocysteine levels were 14.7 ± 0.5 (mean ± SE), 11.8 ± 0.1, and 11.7 ± 0.1 μmol/L in homozygotes, heterozygotes, and noncarriers, respectively (ANOVA: P < .001, Figure 1); on post hoc tests homocysteine levels were 25% higher in homozygotes compared with both heterozygotes and noncarriers (both P < .001). We observed similar differences in homocysteine levels by MTHFR C677T genotype in ICD/VTE cases and controls separately (Figure 1). Overall, ICD/VTE cases and controls had mean homocysteine levels of 14.0 ± 0.4 μmol/L and 11.8 ± 0.1 μmol/L, respectively (19% higher; t test: P < .001).
Plasma homocysteine levels by MTHFR C677T genotype. Bars represent mean ± SE.
Prospective studies
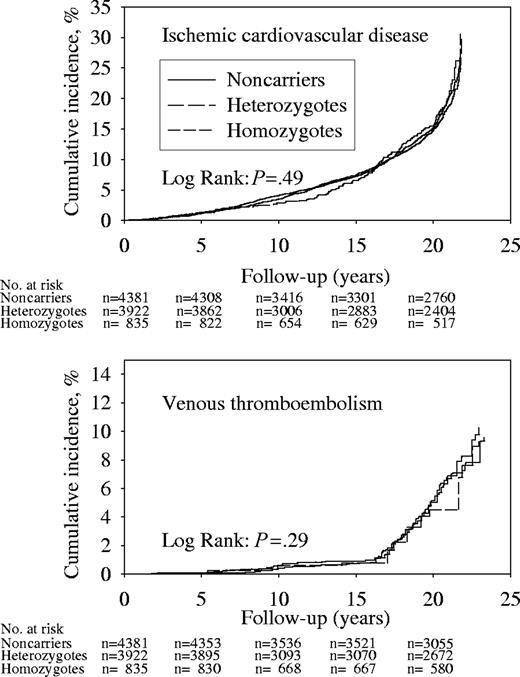
Cumulative incidences of ICD or VTE were similar for all genotypes (Figure 2); we observed 1374 and 208 ICD and VTE events during 165 913 and 173 125 person-years of follow-up. Hazard ratios for ICD and VTE in homozygotes or heterozygotes versus noncarriers did not differ from 1.0, when adjusted for sex and age or after multifactorial adjustment (Table 3). Test for trend in adjusted hazard ratios from noncarriers to heterozygotes to homozygotes were not significant for either end point.
Cumulative incidences during follow-up of ICD and VTE by MTHFR C677T genotype. Numbers at risk are at the beginning of each 5-year period.
Cumulative incidences during follow-up of ICD and VTE by MTHFR C677T genotype. Numbers at risk are at the beginning of each 5-year period.
Hazard ratios for ICD and VTE by MTHFR C677T genotype in prospective studies
. | Ischemic cardiovascular disease . | . | Venous thromboembolism . | . | ||
|---|---|---|---|---|---|---|
. | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | ||
| Sex and age adjustment | ||||||
| Noncarriers | 1.0 | .13 | 1.0 | .64 | ||
| Heterozygotes | 1.1 (1.0-1.2) | .13 | 1.1 (0.8-1.5) | .64 | ||
| Homozygotes | 1.1 (0.9-1.3) | .13 | 1.1 (0.6-1.7) | .64 | ||
| Multifactorial adjustment* | ||||||
| Noncarriers | 1.0 | .44 | 1.0 | .67 | ||
| Heterozygotes | 1.0 (0.9-1.2) | .44 | 1.1 (0.8-1.5) | .67 | ||
| Homozygotes | 1.1 (0.9-1.3) | .44 | 1.0 (0.6-1.7) | .67 | ||
. | Ischemic cardiovascular disease . | . | Venous thromboembolism . | . | ||
|---|---|---|---|---|---|---|
. | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | ||
| Sex and age adjustment | ||||||
| Noncarriers | 1.0 | .13 | 1.0 | .64 | ||
| Heterozygotes | 1.1 (1.0-1.2) | .13 | 1.1 (0.8-1.5) | .64 | ||
| Homozygotes | 1.1 (0.9-1.3) | .13 | 1.1 (0.6-1.7) | .64 | ||
| Multifactorial adjustment* | ||||||
| Noncarriers | 1.0 | .44 | 1.0 | .67 | ||
| Heterozygotes | 1.0 (0.9-1.2) | .44 | 1.1 (0.8-1.5) | .67 | ||
| Homozygotes | 1.1 (0.9-1.3) | .44 | 1.0 (0.6-1.7) | .67 | ||
Hazard ratio with 95% confidence interval (CI) was calculated with noncarriers versus heterozygotes or homozygotes using Cox regression. P values are for trend tests from noncarriers to heterozygotes to homozygotes.
Adjusted for sex, age, and medians of cholesterol, high-density lipoprotein cholesterol, lipoprotein(a), fibrinogen, triglycerides, and body mass index as well as smoking, diabetes mellitus, hypertension, and factor V Leiden genotype.
Subanalyses of end points showed no differences in cumulative incidences of IHD, ICVD, intermittent claudication, DVT, or pulmonary embolism between C677T genotypes (data not shown); we observed 1043, 454, 39, 137, and 98 events during 168 519, 155 997, 187 011, 173 487, and 174 002 person-years of follow-up, respectively. Hazard ratios for IHD, ICVD, intermittent claudication, DVT, or pulmonary embolism for homozygotes or heterozygotes versus noncarriers did not differ from 1.0, adjusted for sex and age or after multifactorial adjustment (Table 4). Tests for trend in adjusted hazard ratios across genotypes were not significant for any end point; although the P value was .04 for ischemic cerebrovascular disease after age and sex adjustment, this trend test became insignificant after multifactorial adjustment.
Hazard ratios for IHD, ICVD, intermittent claudication, DVT, and PE by MTHFR C677T genotype in prospective studies
. | Ischemic cardiovascular disease . | . | . | . | . | . | Venous thromboembolism . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IHD . | . | ICVD . | . | Intermittent claudication . | . | DVT . | . | PE . | . | ||||||||
. | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | ||||||||
| Sex and age adjustment | ||||||||||||||||||
| Noncarriers | 1.0 | .99 | 1.0 | .04 | 1.0 | .37 | 1.0 | .63 | 1.0 | .64 | ||||||||
| Heterozygotes | 1.0 (0.9-1.1) | .99 | 1.1 (0.9-1.4) | .04 | 1.2 (0.5-2.0) | .37 | 1.1 (0.8-1.6) | .63 | 0.9 (0.6-1.4) | .64 | ||||||||
| Homozygotes | 1.0 (0.8-1.2) | .99 | 1.4 (1.0-1.8) | .04 | 0.3 (0.03-2.0) | .37 | 0.9 (0.4-1.8) | .63 | 0.9 (0.4-1.8) | .64 | ||||||||
| Multifactorial adjustment* | ||||||||||||||||||
| Noncarriers | 1.0 | .51 | 1.0 | .12 | 1.0 | .39 | 1.0 | .78 | 1.0 | .61 | ||||||||
| Heterozygotes | 1.0 (0.9-1.1) | .51 | 1.1 (0.9-1.3) | .12 | 1.0 (0.5-2.0) | .39 | 1.2 (0.8-1.6) | .78 | 0.9 (0.6-1.4) | .61 | ||||||||
| Homozygotes | 0.9 (0.8-1.2) | .51 | 1.3 (0.9-1.8) | .12 | 0.3 (0.04-2.1) | .39 | 0.9 (0.5-1.8) | .78 | 0.8 (0.4-1.9) | .61 | ||||||||
. | Ischemic cardiovascular disease . | . | . | . | . | . | Venous thromboembolism . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | IHD . | . | ICVD . | . | Intermittent claudication . | . | DVT . | . | PE . | . | ||||||||
. | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | Hazard ratio (95% CI) . | Trend test, P . | ||||||||
| Sex and age adjustment | ||||||||||||||||||
| Noncarriers | 1.0 | .99 | 1.0 | .04 | 1.0 | .37 | 1.0 | .63 | 1.0 | .64 | ||||||||
| Heterozygotes | 1.0 (0.9-1.1) | .99 | 1.1 (0.9-1.4) | .04 | 1.2 (0.5-2.0) | .37 | 1.1 (0.8-1.6) | .63 | 0.9 (0.6-1.4) | .64 | ||||||||
| Homozygotes | 1.0 (0.8-1.2) | .99 | 1.4 (1.0-1.8) | .04 | 0.3 (0.03-2.0) | .37 | 0.9 (0.4-1.8) | .63 | 0.9 (0.4-1.8) | .64 | ||||||||
| Multifactorial adjustment* | ||||||||||||||||||
| Noncarriers | 1.0 | .51 | 1.0 | .12 | 1.0 | .39 | 1.0 | .78 | 1.0 | .61 | ||||||||
| Heterozygotes | 1.0 (0.9-1.1) | .51 | 1.1 (0.9-1.3) | .12 | 1.0 (0.5-2.0) | .39 | 1.2 (0.8-1.6) | .78 | 0.9 (0.6-1.4) | .61 | ||||||||
| Homozygotes | 0.9 (0.8-1.2) | .51 | 1.3 (0.9-1.8) | .12 | 0.3 (0.04-2.1) | .39 | 0.9 (0.5-1.8) | .78 | 0.8 (0.4-1.9) | .61 | ||||||||
Adjusted for sex, age, and medians of cholesterol, high-density lipoprotein cholesterol, lipoprotein(a), fibrinogen, triglycerides, body mass index as well as smoking, diabetes mellitus, hypertension and factor V Leiden genotype. Hazard ratios with 95% confidence intervals (CI) was calculated for heterozygotes or homozygotes versus noncarriers using Cox regression. P values are for trend tests, from noncarriers to heterozygotes to homozygotes.
Finally, MTHFR C677T genotype was not associated with increased risk of ICD or VTE in any subgroup after stratification for sex, age, cholesterol, HDL-cholesterol, lipoprotein(a), fibrinogen, triglycerides, body mass index, smoking, diabetes mellitus, hypertension, and factor V Leiden genotype (data not shown). Tests of multiplicative interaction showed no evidence for interaction between C677T genotype and the covariates listed above on ICD or VTE.
Case-control studies
Odds ratios for IHD and ICVD in heterozygotes or homozygotes relative to noncarriers did not differ from 1.0 (Table 5).
Odds ratios for IHD and ICVD by MTHFR C677T genotype in case-control studies
. | IHD . | . | ICVD . | . | ||
|---|---|---|---|---|---|---|
. | Odds ratio (95% CI) . | Trend test, P . | Odds ratio (95% CI) . | Trend test, P . | ||
| Unadjusted | ||||||
| Noncarriers | 1.0 | .84 | 1.0 | .72 | ||
| Heterozygotes | 0.9 (0.8-1.0) | .84 | 1.0 (0.8-1.1) | .72 | ||
| Homozygotes | 1.1 (0.9-1.3) | .84 | 1.0 (0.8-1.3) | .72 | ||
| Adjusted for age and sex | ||||||
| Noncarriers | 1.0 | .66 | 1.0 | .83 | ||
| Heterozygotes | 0.9 (0.8-1.0) | .66 | 1.0 (0.8-1.2) | .83 | ||
| Homozygotes | 1.0 (0.9-1.3) | .66 | 1.0 (0.7-1.3) | .83 | ||
. | IHD . | . | ICVD . | . | ||
|---|---|---|---|---|---|---|
. | Odds ratio (95% CI) . | Trend test, P . | Odds ratio (95% CI) . | Trend test, P . | ||
| Unadjusted | ||||||
| Noncarriers | 1.0 | .84 | 1.0 | .72 | ||
| Heterozygotes | 0.9 (0.8-1.0) | .84 | 1.0 (0.8-1.1) | .72 | ||
| Homozygotes | 1.1 (0.9-1.3) | .84 | 1.0 (0.8-1.3) | .72 | ||
| Adjusted for age and sex | ||||||
| Noncarriers | 1.0 | .66 | 1.0 | .83 | ||
| Heterozygotes | 0.9 (0.8-1.0) | .66 | 1.0 (0.8-1.2) | .83 | ||
| Homozygotes | 1.0 (0.9-1.3) | .66 | 1.0 (0.7-1.3) | .83 | ||
Odds ratios with 95% confidence intervals (CI) were calculated with noncarriers versus heterozygotes or homozygotes using unconditional logistic regression. P values are for trend tests, from noncarriers to heterozygotes to homozygotes. There were 2125 cases of IHD, 836 cases of ICVD, and 7568 controls.
Discussion
MTHFR C677T homozygosity was not associated with ICD or VTE in our prospective study of the adult Danish general population. In subanalyses on IHD, ICVD, intermittent claudication, DVT, and pulmonary embolism, homozygosity also was not associated with either end point. Stratification by 12 risk factors for ICD and/or VTE did not reveal any significant context dependent associations between MTHFR C677T homozygosity and ICD or VTE. Finally, MTHFR C677T homozygosity was not associated with IHD or ICVD in our case-control studies.
In accordance with previous studies,4,9,11,13 we found that MTHFR C677T homozygotes versus noncarriers had a 25% increase in plasma homocysteine levels and that ICD/VTE cases versus controls had a 19% increase in plasma homocysteine levels. It is puzzling, therefore, that while ICD and VTE cases had increased homocysteine levels, MTHFR C677T homozygotes with genetically elevated homocysteine levels did not have increased ICD or VTE risk. This data therefore suggest that hyperhomocysteinemia is not causally related to ICD or VTE, at least not in MTHFR C677T homozygotes. Our data, however, does not contradict the hypothesis that ICD and/or VTE cause hyperhomocysteinemia.
The 2 largest meta-analyses on MTHFR C677T homozygosity and IHD risk both reach the conclusion that MTHFR C677T homozygosity is associated with a common odds ratio for IHD of 1.2 relative to noncarriers (Table 6).2,11 The largest meta-analysis on MTHFR C677T homozygosity and ICVD risk by Kelly et al4 reached a similar conclusion with a common odds ratio of 1.2. Finally, Wald et al2 reported a common odds ratio for VTE of 1.3. Importantly, most studies on which these meta-analyses are based are case-control studies. We could not reproduce the finding of an association between MTHFR homozygosity and any of these end points in our population-based prospective study. There are several possible explanations for this discrepancy, but differences in demographics and risk factor prevalence between previous studies and our study are likely contributors. Alternatively, differences in study design may account for the apparent discrepancy: clustering of additional risk factors among cases in case-control studies may introduce bias toward a higher risk estimate than in population based studies. Thus, subanalyses by Klerk et al11 demonstrated that the association between C677T homozygosity and the risk of IHD observed overall was due to associations observed in case-control studies rather than in nested case-control studies (which are conducted within prospective studies) (Table 6). However, the fact that we could not even reproduce an association between MTHFR C677T homozygosity and either IHD or ICVD in our case-control studies, which are by far the largest reported to date, suggest that design differences are not the only reason for discrepant results. Publication bias toward studies reporting positive associations between MTHFR C677T homozygosity and IHD and/or VTE also could contribute to the observed differences.
Meta-analyses of MTHFR C677T homozygosity versus noncarriers on ICD and VTE
Study . | Studies, no. . | Cases, no. . | Controls, no. . | End point . | OR (95% CI) . |
|---|---|---|---|---|---|
| ICD | |||||
| Brattström, 19989 | 17 | 4 654 | 5 224 | IHD | 1.1 (0.9-1.4) |
| Wald, 20022 | 46 | 12 193 | 11 945 | IHD | 1.2 (1.1-1.4) |
| Klerk, 200211 | 40 | 11 162 | 12 758 | IHD | 1.2 (1.1-1.3) |
| Case-control | 35 | 9 874 | 11 009 | IHD | 1.2 (1.1-1.3) |
| Nested case-control | 5 | 1 288 | 1 749 | IHD | 0.9 (0.7-1.1) |
| European | 22 | 6 207 | 8 343 | IHD | 1.1 (1.0-1.4) |
| North American | 10 | 3 146 | 2 532 | IHD | 0.9 (0.7-1.0) |
| Wald, 20022 | 7 | 1 217 | Not reported | ICVD | 1.3 (0.8-2.2) |
| Kelly, 20024 | 19 | 2 950 | 4 187 | ICVD | 1.2 (1.0-1.6) |
| VTE | |||||
| Brattström, 20008 | 15 | 2 683 | 3 306 | DVT/PE | 1.1 (0.9-1.3) |
| Wald, 20022 | 26 | 3 439 | 5 063 | DVT/PE | 1.3 (1.1-1.5) |
Study . | Studies, no. . | Cases, no. . | Controls, no. . | End point . | OR (95% CI) . |
|---|---|---|---|---|---|
| ICD | |||||
| Brattström, 19989 | 17 | 4 654 | 5 224 | IHD | 1.1 (0.9-1.4) |
| Wald, 20022 | 46 | 12 193 | 11 945 | IHD | 1.2 (1.1-1.4) |
| Klerk, 200211 | 40 | 11 162 | 12 758 | IHD | 1.2 (1.1-1.3) |
| Case-control | 35 | 9 874 | 11 009 | IHD | 1.2 (1.1-1.3) |
| Nested case-control | 5 | 1 288 | 1 749 | IHD | 0.9 (0.7-1.1) |
| European | 22 | 6 207 | 8 343 | IHD | 1.1 (1.0-1.4) |
| North American | 10 | 3 146 | 2 532 | IHD | 0.9 (0.7-1.0) |
| Wald, 20022 | 7 | 1 217 | Not reported | ICVD | 1.3 (0.8-2.2) |
| Kelly, 20024 | 19 | 2 950 | 4 187 | ICVD | 1.2 (1.0-1.6) |
| VTE | |||||
| Brattström, 20008 | 15 | 2 683 | 3 306 | DVT/PE | 1.1 (0.9-1.3) |
| Wald, 20022 | 26 | 3 439 | 5 063 | DVT/PE | 1.3 (1.1-1.5) |
OR indicates odds ratios; and CI, confidence interval.
Another important issue investigated by Klerk et al11 is effect modification of MTHFR C677T homozygosity by folate fortification of foods. Thus, stratification into European studies (where foods are not folate enriched) and North American studies (where foods are folate enriched) demonstrated that the overall association between C677T homozygosity and IHD was less pronounced in North American studies than in European studies (Table 6). However, despite the fact that our study was performed in Denmark where foods, like in other European countries, are not fortified, we could not demonstrate an association between MTHFR C677T homozygosity and ICD or VTE.
Limitations
The conclusions of our study apply to Danish white adults with a mean plasma homocysteine level of 10-15 μmol/L. Whether they also apply to populations with different ethnicity or with different homocysteine levels is unknown.
The fact that DNA samples were not obtained before the 1991-1994 examination is a source of potential bias. If the mortality were higher among MTHFR C677T homozygotes than among heterozygotes and noncarriers, our study would underestimate the association between MTHFR genotype and risk of ICD and/or VTE. However, the observed genotype frequency did not differ from that predicted by the Hardy-Weinberg equilibrium, suggesting that selective disappearance of C677T homozygotes from the cohort is unlikely. Additionally, genotype frequencies did not change with increasing age (MTHFR C677T genotype by 10-year age groups, chi2 -test, P = .29). Finally, hazard ratios of ICD and VTE in homozygotes versus noncarriers only from 1991-1994 onwards also did not differ from 1.0.
In conclusion, MTHFR C677T homozygosity with hyperhomocysteinemia is not associated with increased risk of ICD or VTE. Therefore, ICD and VTE may cause hyperhomocysteinemia, rather than vice versa. Alternatively, ICD/VTE and the elevated homocysteine levels in diseased compared to healthy individuals could both be consequences of a common underlying factor. In support of either of these 2 interpretations, the recently published Vitamin Intervention for Stroke Prevention trial showed that moderate reduction of total homocysteine level after ischemic stroke had no effect on vascular outcomes during 2 years of follow-up, but that elevated homocysteine levels predicted increased vascular risk.19
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-03-0897.
Supported by The Danish Medical Research Council, The Danish Heart Foundation, and the Chief Physician Johan Boserup and Lise Boserup's Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Helle Koldkjær and Nina Dahl Kjersgaard for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal