Abstract
We report a novel case of gray platelet syndrome (GPS) where a severe deficiency of the platelet collagen receptor, glycoprotein (GP) VI, accompanies classical symptoms of a low platelet count and platelets lacking α-granules. Dense granules were normally present. Platelet aggregation with collagen was severely decreased, as was the response to convulxin (Cvx), a GPVI agonist. Quantitative analysis of GPVI using fluorescein isothiocyanate (FITC)–Cvx in flow cytometry showed its virtual absence on the patient's platelets. The GPVI deficiency was confirmed using monoclonal antibodies in Western blotting and in immunogold labeling on frozen thin sections where internal pools of GPVI were confirmed for normal platelets. The Fc receptor γ-chain, constitutively associated with GPVI in normal platelets, was present in subnormal amounts, and the phospholipase Cγ2–dependent activation pathway appeared to function normally. No autoantibodies to GPVI were found in the patient's serum using monoclonal antibody immobilization of platelet antigen (MAIPA). Sequencing of coding regions of the GPVI gene failed to show abnormalities, and mRNA for GPVI was present in the patient's platelets, pointing to a probable acquired defect in GPVI expression. Our results may provide a molecular explanation for the subgroup of patients with severely deficient collagen-induced platelet aggregation as previously described for GPS in the literature.
Introduction
The gray platelet syndrome (GPS) or α-platelet syndrome is a rare platelet disorder recognized in 1971 and defined by thrombocytopenia with an absence of α-granules.1,2 The number of cases reported in the literature has remained low. A frequent finding in GPS is the development of myelofibrosis thought to result from the spontaneous release of growth factors unable to be stored in granules but synthesized and secreted by megakaryocytes into the bone marrow.2-5 In some patients, partial α-granule deficiencies are also associated with abnormalities of platelet-dense granules in what has been called αδ-storage pool disease.6 Granule abnormalities in granulocytes have been reported in one family, indicating that the defect may not be restricted to platelets.7 Transmission in GPS is autosomal dominant in some cases but recessive in others, thereby suggesting heterogeneity in the origin of the defect.8
In this study, we report the molecular characterization of a severely modified platelet response to collagen in a previously unreported patient with GPS. From the literature, platelet aggregation defects are heterogeneous in GPS, with about 50% of patients showing an impaired platelet response to collagen (data reviewed by Hayward8 ). It is now accepted that a full response of platelets to collagen requires the coordinated action of both α2β1 and glycoprotein VI (GPVI) receptors.9-11 Nevertheless, signaling through GPVI is largely responsible for platelet activation. The recently cloned GPVI is homologous to immune receptors.12,13 It is noncovalently associated with the Fc receptor γ-chain (FcRγ), the latter mediating signaling through an immunoreceptor tyrosine-based activation motif (ITAM).14,15 Upon collagen ligation to GPVI, the FcRγ-chain becomes phosphorylated and in turn triggers a cascade of phosphorylation leading to the activation of phospholipase Cγ2.16-18 Other functional domains are present in the intracytoplasmic part of GPVI.19 For example, a membrane-proximal sequence of GPVI has been demonstrated to bind calmodulin.20 Thus, it has been concluded that GPVI-mediated signaling is essential for collagen-induced platelet aggregation.10 This is confirmed by the fact that human or mouse platelets specifically lacking GPVI, but expressing α2β1, fail to aggregate with collagen.10,21
We now show that the cause of the abnormal platelet collagen interaction in our patient is due to a severe GPVI deficiency. The FcRγ-chain was present, and the signaling pathway common to the GPVI-FcRγ complex and FcγRIIA was preserved. Nevertheless, no genetic abnormality of GPVI has yet been found in the patient's family, suggesting that the defect may be acquired. Although antibodies to GPVI can induce a durable loss of GPVI from platelets in a mouse model,22 serum autoantibody directed against GPVI was not detected, suggesting another cause. This is the first case of GPS where the defect in platelet aggregation to collagen has been linked to a deficiency of GPVI. Because GPS is a heterogeneous disorder, GPVI deficiency may be a characteristic of patients with a much reduced collagen response.
Patient, materials, and methods
Patient
The patient is a 55-year-old woman whose parents are first cousins. She presented with a lifelong bleeding syndrome featuring epistaxis, bleeding at the time of menarche, and hemorrhage during surgery. Hypersplenism is present. Recently, gastric bleeding was observed without detectable local lesions. She has been transfused with red cells and/or platelets on several occasions. Her HIV status is negative. Three years ago, the patient spontaneously developed deep vein thrombosis. She has 2 sons, and bleeding problems manifested during both deliveries. Her sons are in good health, and neither has a bleeding syndrome. All studies were performed with informed consent and in accordance with the Declaration of Helsinki. Platelet counts and hematologic examinations were by routine methods.
Platelet aggregation
Blood was taken into 3.8% sodium citrate and platelet-rich plasma (PRP) obtained by centrifuging for 5 minutes at 50g and then for a further 10 minutes at 120g. Platelet-poor plasma (PPP) was prepared by centrifuging the red blood cells for a further 20 minutes at 1000g at room temperature. Platelet aggregation was tested using 8 μM adenosine diphosphate (ADP) (Calbiochem, Fontenay sous Bois, France), 50 μM thrombin receptor activating peptide (TRAP) (NeoSystems, Strasbourg, France), 0.5 and 2 μg/mL Horm equine tendon collagen (Nycomed Pharma, Unterschleissheim, Germany), 2 and 20 μg/mL acid-insoluble fibrillar type I collagen (Sigma, Saint Quentin Fallavier, France), 2 μM epinephrine (Sigma), and 1.5 mg/mL ristocetin (Stago, Asnières-sur-Seine, France). Convulxin (Cvx), purified by one of us (M.J.-P.), was used at a concentration ranging between 0.4 and 4 nM.
To evaluate the signaling pathway through FcRγ, we took advantage of the fact that activation of the immunoglobulin receptor, FcγRIIA, also uses ITAM and the syk signaling cascade through phospholipase Cγ2 (PLCγ2).23 To test this pathway, we activated FcγRIIA through the binding of the nonactivating specific monoclonal antibody (MoAb) IV.3 (Medarex, Annandale, NJ) and its cross-linking by addition of F(ab′)2 fragments of a second antibody to mouse immunoglobulin G (IgG) (Silenus, Eurobio, Les Ulis, France).23 In these experiments we measured the aggregation of washed platelets suspended at a concentration of 100 000/μL in a modified Tyrode buffer prepared as described.24
The capacity of the patient's PPP to influence the function of normal platelets was also assessed. Here, washed platelets24 at a concentration of 250 000/μL were incubated for periods up to 90 minutes at 37° C with an equal volume of patient's or control PPP tested in parallel. Aggregation was tested with 0.4 nM Cvx.
Electron microscopy
PRP was prepared from citrated blood by centrifugation for 10 minutes at 80g, incubated for 20 minutes at 37° C, and fixed in 1.25% (vol/vol) glutaraldehyde diluted in 0.1 M phosphate buffer (pH 7.2) for 1 hour at room temperature. Samples were processed for standard electron microscopy (EM) and for uranaffin staining of dense granules as described.24,25 For immunogold labeling, washed pellets were infused with 2.3 M sucrose (Fluka, Buchs, Switzerland) before being frozen in propane and then in liquid nitrogen using a Reichert KF 80 freezing system (Leica, Vienna, Austria). Ultrathin sections were cut at –120° C with an Ultracut E ultramicrotome (Reichert, Villepinte, France) equipped with an FC 4E cryokit attachment and placed on collodion-coated nickel grids. The grids were first incubated for 10 minutes on drops of washing buffer consisting of phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin (BSA). Sections were then floated on buffer containing 5 μg/mL F5 MoAb prepared by immunizing mice with a soluble recombinant peptide representing an extracellular domain of GPVI (T.J.K., manuscript in preparation). After washing, bound MoAb was detected using goat anti-mouse IgG conjugated to 10 nm gold particles (Amersham, Orsay, France). The grids were then floated several times on PBS and then on water prior to staining the sections with uranyl acetate and osmium according to our standard procedures.24 Sections were embedded in a thin film of methylcellulose and observed with a Jeol JEM-1010 transmission electron microscope (Jeol, Croissy-sur-Seine, France) at 80 kV. Controls included the absence of primary antibody or its substitution with an irrelevant IgG of the same species and at the same concentration. The MoAb anti-CD56 (Dako, Trappes, France) served as the irrelevant antibody.
Flow cytometry
Platelet receptors αIIbβ3, GPIb-IX-V, and α2β1 were analyzed using PRP incubated with the MoAbs AP-2 (provided by T.J.K.), Bx-1 (prepared by the Bordeaux Laboratory), and Gi9 (Beckman Coulter, Marseille, France). Procedures were as previously described by us.26,27 Activation of αIIbβ3 was studied on platelets in whole blood incubated with 10 μM ADP and evaluated using PAC-1 (Becton Dickinson, Pont de Claix, France). Expression of P-selectin and granulophysin at the platelet surface was studied before and after activation of platelets by 50 μM TRAP using, respectively, the MoAbs VH10 prepared by the Bordeaux Laboratory and MoF11 kindly given by Dr P. Vincendeau (Bordeaux University, France). GPVI was initially analyzed using fluorescein isothiocyanate (FITC)–conjugated Cvx prepared as described by Jandrot-Perrus et al.12 Selected concentrations (see “Results”) diluted in 50 μL PBS, pH 7.2, were mixed with 10 μL PRP and incubated for increasing times at 37° C prior to being analyzed in the flow cytometer. Selected experiments were also performed using the anti-GPVI murine MoAb, 3J24.2 (5 μg/mL).28 Permeabilized unstimulated platelets fixed in 1% (wt/vol) paraformaldehyde (PFA)27 were used to assess the presence of the FcRγ-chain. Briefly, to permit access to the internal compartment, platelets were treated with 0.1% (vol/vol) Triton X-100 for 30 minutes, washed, and then incubated overnight at 4° C with 5 μg/mL purified IgG of a rabbit polyclonal antibody to FcRγ (Euromedex, Mundolsheim, France). After further washing, platelets were incubated with FITC-labeled F(ab′)2 fragments of a sheep antirabbit IgG (Silenus). For PAC-1 detection, 10 μL of a predetermined saturating concentration of dichlorotriazinyl amino fluorescein (DTAF)–conjugated affinity-purified F(ab′)2 fragments of donkey antimouse IgM (Jackson ImmunoResearch, West Grove, PA) was used. FITC-labeled F(ab′)2 fragments of a sheep antibody to mouse IgG (Silenus) were employed to detect murine MoAbs.26,27
Platelets were analyzed in a FACScan (Becton Dickinson). Gating to select most platelets was based on preliminary determinations of forward and wide-angle light scatter. Mean fluorescence intensity (MFI) was measured after passage through a 530 nm long-pass interference filter. Histograms were generated from measurements of 10 000 cells, and data were analyzed using LYSYS II software.
Dense granule contents
Dense granules were evaluated through their capacity to take up the fluorescent dye mepacrine.25 To do this, PRP was incubated with 50 μM mepacrine (DL-quinacrine HCl; ICN Pharmaceuticals, Orsay, France) for 10 minutes and platelets directly examined by flow cytometry.29 The number of dense granules was also evaluated by electron microscopy on sections stained with uranaffin25 as described under “Electron microscopy” above. The platelet pools of adenosine triphosphate (ATP) and ADP were measured after trichloroacetic acid treatment and ether extraction using the luciferin luciferase test as described.30
Analysis of selected α-granule proteins
Washed platelets prepared as described previously24,30 and resuspended at a concentration of 250 000/μL in Tyrode buffer, pH 7.2, were stored at –80° C. They were thawed in the presence of 1% Triton X-100 and 200 μg/mL leupeptin. Fibrinogen (Fg) and von Willebrand factor (VWF) contents were measured by enzyme-linked immunosorbent assay (ELISA) using commercial antibodies (Sigma and Stago, respectively). The results were expressed as a percentage of the amounts found for platelets of control donors or as micrograms per milligram of protein. ELISA was also used to measure the platelet-derived growth factor–AB (PDGF-AB) and transforming growth factor β1 (TGF-β1) content of the lysates using Quantikine Systems commercial kits as described by the manufacturer (R&D Systems, Abingdon, United Kingdom).
Western blotting
Washed platelets were lysed in buffer containing 10 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.0), 3 mM EDTA (ethylenediaminetetraacetic acid), 5 mM N-ethyl maleimide, and 2% sodium dodecyl sulfate (SDS). When performed, disulfide reduction was with dithiothreitol. Then, 10 μg or 20 μg protein was separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) on minigels and transferred electrophoretically onto nitrocellulose membrane using Trans-Blot Transfer Medium (Amersham Life Science, Bucks, United Kingdom). Nonspecific binding of protein was blocked by incubating the membrane for 1 hour in a solution of 5% fat-free milk in 20 mM Tris-NaCl, pH 8.2, containing 0.05% (vol/vol) Tween 20 (TB-T). Individual membrane strips were then incubated with MoAbs to GPVI (F5; 1 μg/mL), GPIbα (Bx-1; 1 μg/mL), αIIb (SZ22, Beckman Coulter; 0.5 μg/mL), or β3 (Y2/51, Dakopatts, Glostrup, Denmark; 0.5 μg/mL) for 12 minutes at room temperature. On the final occasion, we also used the anti-GPVI MoAbs, 3J24.228 and 9E18.2 (M.J.-P., unpublished data, 2004; see below) (5 μg/mL). After washing, the membrane was further incubated with a 1:10 000 dilution of horseradish peroxidase–conjugated antimouse IgG (Jackson ImmunoResearch, West Grove, PA) and bound antibody detected using a chemiluminescence procedure (Amersham Life Science).
MAIPA for antiplatelet antibodies
Testing for platelet-associated and serum antiplatelet antibodies was as described previously by us.31,32 For monoclonal antibody immobilization of platelet antigen (MAIPA), washed control platelets were resuspended at 2 × 109/mL in PBS buffer, pH 7.2, containing 2% (wt/vol) BSA (PBS-BSA). Aliquots (20 μL) were incubated at room temperature following the addition of 300 μL of decomplemented serum from the patient or control donors. After washing, platelets were incubated with 20 μL of a predetermined saturating concentration of one of a series of murine MoAbs to GPVI: F5 (5 μg/mL), 3J24.2 (5 μg/mL), 9E18.2 (5 μg/mL), 8M14.3 (5 μg/mL). The characterization of 9E18.2 and 8714.3 will be presented elsewhere (M.J.-P., manuscript in preparation). After washing, the presence of human IgG and IgM was assessed using alkaline phosphatase–conjugated goat antihuman IgM + IgG(H+L) (Jackson ImmunoResearch).
GPVI gene expression and genomic sequencing
Total RNA was extracted from washed platelets using TRIZOL Reagent (Invitrogen Life Technologies, Cergy Pontoise, France) at a ratio of 400 μL TRIZOL to 5 × 108 platelets. Total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) in 30 μL solution using a reverse transcriptase (RT) superscript (Invitrogen); 2 to 6 μL RT reaction was used for each polymerase chain reaction (PCR) amplification with specific primers designed from the GPVI cDNA sequence. A first fragment of 547 bp was amplified using the following primers: GPVI A (8-26) sense primer: CATCCCCGACCGCCCTCTT; and GPVI B (529-554) antisense primer: CTGGAGAAGCTGTAGCGGTAGGT. A second fragment of 540 bp was amplified using the following primers: GPVI C (472-495): GAGAGATGGTACCGGGCTAGTTTC; and GPVI D (992-1011): TAACCCGCTGTGAACAT. Amplification was performed using an Eppendorf Mastercycler for 35 cycles, with each cycle consisting of denaturation at 95° C for 30 seconds and synthesis at 70° C for 30 seconds. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
For genomic sequencing, leukocyte DNA was isolated from donors as described33 and used as a template for PCR amplification of the GPVI coding region. Primer pairs were designed to hybridize to intron sequences flanking each exon or pair of exons. The primer sequences were as follows: exon 1: forward (FOR) 5′ CAG GGA GTT TAT GGG AGC AC 3′ and reverse (REV) 5′ GGG GCT CTT ACA GGT TCC TT 3′; exons 2 and 3: FOR 5′ ATG CAT GCA AAT GTC TTA TCA CC 3′ and REV 5′ CTA GGC CAG TGC CTC GTT T 3′; exon 4: FOR 5′ GGG ACC TCC CCA GTC TCA G 3′ and REV 5′ CTG CCC TCC CAC TCC CTT 3′; exon 5: FOR 5′ CTC ATT TTT CCG GTC AGG AA 3′ and REV 5′ TTA GGA CAC CCA CCC TGT TT 3′; exon 6: FOR 5′ CAG TGT CTG TGC AGT GTG TCA 3′ and REV 5′ AAC GCT CCT CCT TCT GAA CC 3′; exon 7: FOR 5′ CTC CCC ATG TGT GTG TGT GT 3′ and REV 5′ CAC TGG AAC CCA AGA TCT GA 3′; exon 8: FOR 5′ GGC CAG TGT CTG TCT GTC TG 3′ and REV 5′ ATT TAT GGG GTG GAC AGC AA 3′.
PCR products were then directly sequenced. DNA was also tested for single-mutation polymorphisms of major platelet membrane glycoproteins including the α2 integrin subunit A alleles (A1, A2, and A3) as well as GPVI T and C alleles34 by allele-specific restriction enzyme analysis (ASRA) as previously described.35
Results
Initial platelet characterizations
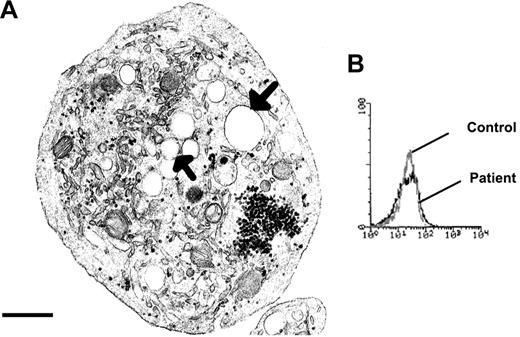
During the 2-year course of this study, the patient's platelet count ranged between 18 000/μL and 35 000/μL, with the lower count when the patient had bleeding problems. Whole blood smears stained with May-Grunwald Giemsa showed platelets with the characteristic gray color of GPS. Electron microscopy revealed that many of the platelets were spheroid and of large size, although their size increase was not comparable to that of congenital giant platelet syndromes (see Levy-Toledano et al2 ). The platelets lacked α-granules (Figure 1A). The surface-connected canalicular system (SCCS) was overdeveloped. Both of her sons possessed normal size platelets with a normal α-granule content.
Characteristics of the patient's platelets that are suggestive of GPS. (A) A typical large round platelet devoid of α-granules and with a well-developed SCCS (arrows), as viewed by transmission EM. Scale bar equals 0.5 μm. (B) Histograms showing mepacrine labeling of the patient's and control platelets as a measure of dense granule content by flow cytometry.
Characteristics of the patient's platelets that are suggestive of GPS. (A) A typical large round platelet devoid of α-granules and with a well-developed SCCS (arrows), as viewed by transmission EM. Scale bar equals 0.5 μm. (B) Histograms showing mepacrine labeling of the patient's and control platelets as a measure of dense granule content by flow cytometry.
Analysis of platelet granules
We assessed the content of 4 α-granule proteins in Triton X-100 lysates of the patient's platelets by ELISA. The contents of VWF, Fg, PDGF-AB, and TGF-β1 were all much decreased (Table 1). In contrast, dense granule content as assessed by mepacrine labeling and flow cytometry was normal (Figure 1B). The platelet content in ATP was 15.88 μmol/1011 platelets and, for ADP, 7.96 μmol/1011 platelets, which corresponds to normal values. The ATP/ADP ratio remained normal. Finally, the number of dense granules was evaluated by uranaffin cytochemical staining and electron microscopy and found to be 49 per 100 platelet sections for the patient and 52 for the corresponding control.
Results of ELISA for 4 α-granule proteins using detergent soluble lysates of platelets
. | Patient, 108 pl . | Control, 108 pl . |
|---|---|---|
| Fg, μg | 1.25 | 28 |
| VWF, % | 4 | 100 |
| PDGF-AB, ng | 0.34 | 5.4 |
| TGF-β1, ng | 1.15 | 8.6 |
. | Patient, 108 pl . | Control, 108 pl . |
|---|---|---|
| Fg, μg | 1.25 | 28 |
| VWF, % | 4 | 100 |
| PDGF-AB, ng | 0.34 | 5.4 |
| TGF-β1, ng | 1.15 | 8.6 |
Platelet aggregation
PRP prepared from the patient was tested on 4 occasions over a period of 2 years. A concentration of 8 μM ADP induced an irreversible aggregation with light transmission changes that reached 40% on the aggregometer scale despite the abnormal platelet morphology (Table 2). The platelets also responded well to 0.5 mg/mL arachidonic acid. With TRAP (50 μM), aggregation occurred but was lower than normal and reversible. No aggregation was found in response to 2 μM epinephrine. Agglutination with ristocetin was normal. With 2 μg/mL acid-insoluble fibrillar type I collagen (Sigma), there was no aggregation, and with a high dose such as 20 μg/mL only a delayed and much reduced residual response was observed (Table 2). Similar defective responses were confirmed using Horm collagen.
Platelet function testing
Agonist . | Patient . | Controls . |
|---|---|---|
| ADP, 8 μM | 40 | 75 ± 16 |
| TRAP, 50 μM | 20 | 77 ± 10 |
| Epinephrine, 2 μM | 0 | 41 ± 18 |
| Arachidonic acid, 0.5 mg/mL | 40 | 74 ± 12 |
| Collagen, 2 μg/mL | 0 | 40 ± 27 |
| Collagen, 20 μg/mL | 10 | 76 ± 11 |
| Ristocetin, 1.5 mg/mL | 60 | 79 ± 16 |
Agonist . | Patient . | Controls . |
|---|---|---|
| ADP, 8 μM | 40 | 75 ± 16 |
| TRAP, 50 μM | 20 | 77 ± 10 |
| Epinephrine, 2 μM | 0 | 41 ± 18 |
| Arachidonic acid, 0.5 mg/mL | 40 | 74 ± 12 |
| Collagen, 2 μg/mL | 0 | 40 ± 27 |
| Collagen, 20 μg/mL | 10 | 76 ± 11 |
| Ristocetin, 1.5 mg/mL | 60 | 79 ± 16 |
Platelet aggregation was studied using citrated PRP. Results are expressed as maximum light transmission changes. The platelet count for both the patient and controls (n = 6; ± SD) was 60 000/μL. The results for the patient are typical of those obtained on 4 different occasions. Collagen was from Sigma.
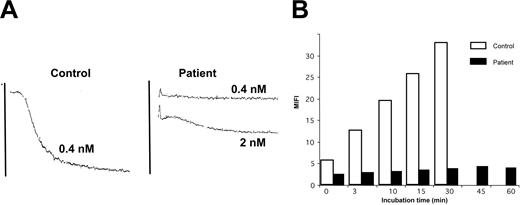
Convulxin was used at a concentration of 0.4 nmol/L, and this dose gave maximal aggregation for all the controls tested (n = 5). In contrast, no response was obtained for the patient. With a much increased dose of 4 nmol/L, only a small and irreversible (16%) residual aggregation was obtained (Figure 2). Platelets from both of the patient's sons responded normally to collagen and Cvx.
The patient's platelets show a much-decreased reactivity with convulxin. (A) The patient's platelets failed to react with 0.4 nM Cvx, whereas maximal aggregation was seen for control platelets. Only with much-increased amounts was a small residual aggregation seen for the patient. (B) Kinetics of the binding of FITC-Cvx to platelets as evaluated by flow cytometry. The histograms represent MFI values and show that increases were seen during a 30-minute incubation for the control, whereas for the patient the values remain close to baseline for even up to an hour.
The patient's platelets show a much-decreased reactivity with convulxin. (A) The patient's platelets failed to react with 0.4 nM Cvx, whereas maximal aggregation was seen for control platelets. Only with much-increased amounts was a small residual aggregation seen for the patient. (B) Kinetics of the binding of FITC-Cvx to platelets as evaluated by flow cytometry. The histograms represent MFI values and show that increases were seen during a 30-minute incubation for the control, whereas for the patient the values remain close to baseline for even up to an hour.
Flow cytometry
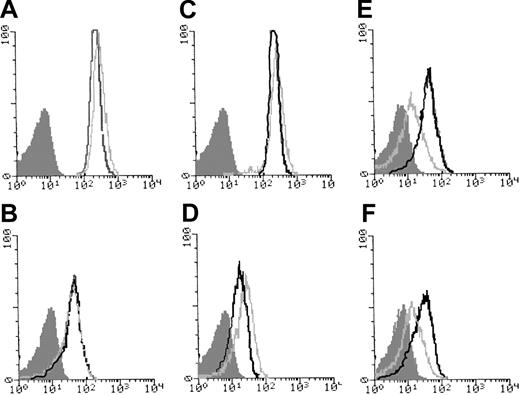
Cvx-FITC was used to evaluate the expression of GPVI in platelets (Figure 2). Kinetic studies showed that binding remained minimal to the patient's platelets over a 60-minute incubation. In contrast, Cvx binding to control platelets showed a time-dependent increase during 30 minutes after which it quickly reached saturation. Binding of the MoAb AP-2 indicated the normal presence of the αIIbβ3 integrin on the patient's platelets (Figure 3). GPIb was also normally expressed, as shown by the binding of Bx-1. When platelets were stimulated with TRAP, expression of P-selectin and granulophysin increased on the platelets of the patient, but the expression was much reduced compared with the control (Figure 3). A normal binding of PAC-1 after incubation of whole blood with ADP confirmed the normal activation of the αIIbβ3 integrin. Interestingly, the α2β1 integrin, a collagen receptor, was increased in comparison with the control.
Glycoprotein expression in platelets from the patient and a control donor as evaluated by flow cytometry. The histograms were obtained using citrated PRP incubated in unstirred suspensions. The MoAbs used against platelet surface glycoproteins were AP-2, directed against αIIbβ3 (A); Bx-1, against GPIbα (C); and Gi9, against α2β1 (D). The activation of αIIbβ3 was evaluated using PAC-1 in whole blood in the presence of 10 μM ADP (B). (E-F) Platelets in PRP were incubated with 50 μM TRAP. In panel E, P-selectin was evaluated using VH10 and, in panel F, granulophysin with MoF11. The light gray lines represent the results for the patient; the heavy lines, the control; and the shaded histograms are those for platelets of the patient incubated without primary antibody.
Glycoprotein expression in platelets from the patient and a control donor as evaluated by flow cytometry. The histograms were obtained using citrated PRP incubated in unstirred suspensions. The MoAbs used against platelet surface glycoproteins were AP-2, directed against αIIbβ3 (A); Bx-1, against GPIbα (C); and Gi9, against α2β1 (D). The activation of αIIbβ3 was evaluated using PAC-1 in whole blood in the presence of 10 μM ADP (B). (E-F) Platelets in PRP were incubated with 50 μM TRAP. In panel E, P-selectin was evaluated using VH10 and, in panel F, granulophysin with MoF11. The light gray lines represent the results for the patient; the heavy lines, the control; and the shaded histograms are those for platelets of the patient incubated without primary antibody.
Immunogold staining for GPVI
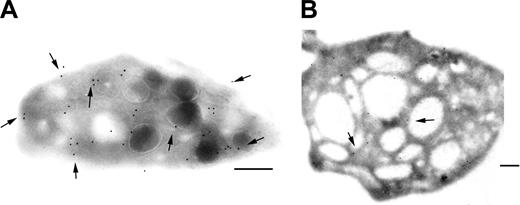
We studied the immunolocalization of GPVI on frozen ultrathin sections of platelets of the patient using the MoAb F5. As shown in Figure 4, GPVI was localized not only to the surface of normal platelets but also to internal membrane pools. These included the membranes of α-granules, thereby confirming a recently published study.36 For the patient, only occasional gold beads were found on the platelet sections, and these were associated with the membranes of the SCCS. A deficient surface pool of GPVI was therefore not due to internalization.
Immunogold labeling of GPVI on ultrathin frozen platelet sections. GPVI was detected using the MoAb F5, specific for an epitope on the extracellular domain. Bound antibody was detected using a second antibody specific for mouse IgG and adsorbed onto 10 nm gold particles. (A) For the control, gold particles were seen both on the surface and associated with internal membranes including those of granules. (B) For the patient's platelets, only an occasional labeling within the SCCS was seen. Bars = 2 μm.
Immunogold labeling of GPVI on ultrathin frozen platelet sections. GPVI was detected using the MoAb F5, specific for an epitope on the extracellular domain. Bound antibody was detected using a second antibody specific for mouse IgG and adsorbed onto 10 nm gold particles. (A) For the control, gold particles were seen both on the surface and associated with internal membranes including those of granules. (B) For the patient's platelets, only an occasional labeling within the SCCS was seen. Bars = 2 μm.
Western blotting
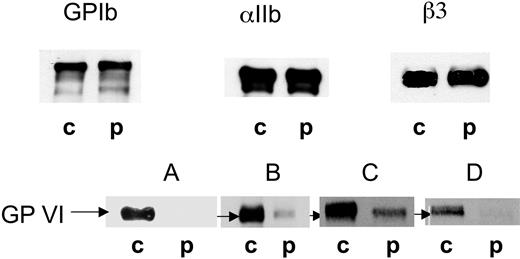
Western blotting was performed on SDS-soluble extracts of the patient's platelets using MoAbs to αIIb, β3, GPIbα, and GPVI. Typical results are shown in Figure 5. GPIb, αIIb, and β3 were all normally present both in terms of their intensity and their migration. There were no signs of unusual enzymatic degradation for the patient. When applying 10 μg protein, no band was detected in the position of GPVI for the patient, despite the MoAb F5 giving a strong band at 60 to 65 kDa in the control platelet sample. Increasing the amount of protein (20 μg) allowed the visualization of a weak band in the patient's sample. When identical samples were analyzed after disulfide reduction, a weak band of similar intensity and normal migration continued to be seen for the patient with F5, whereas the blot for control platelet GPVI remained strong. Similar results for GPVI were observed for platelets isolated from the patient on each of 3 occasions over a 2-year period. On the last occasion studied, blots were additionally performed using the anti-GPVI MoAbs 3J24.2 and 9E18.2. A weak band was seen for 3J24.2 for the patient whereas 9E18.2 remained negative while albeit binding more weakly than 3J24.2 to GPVI of normal platelets (Figure 5).
Western blotting of platelet receptors confirmed a GPVI deficiency. SDS-soluble extracts of platelets from the patient (p) and a control donor (c) were separated by SDS-PAGE without disulfide reduction and transferred to nitrocellulose membrane. The membranes were probed for GPIb (Bx-1), αIIb (SZ22), β3 (XIIF9), and GPVI using the MoAbs F5 (A,B), 3J24.2 (C), and 9E18.2 (D). Bound antibody was located using a chemiluminescence procedure. GPIb, αIIb, and β3 were assessed using 10 μg protein, while GPVI was analyzed using 10 μg (A) and 20 μg (B-D) protein.
Western blotting of platelet receptors confirmed a GPVI deficiency. SDS-soluble extracts of platelets from the patient (p) and a control donor (c) were separated by SDS-PAGE without disulfide reduction and transferred to nitrocellulose membrane. The membranes were probed for GPIb (Bx-1), αIIb (SZ22), β3 (XIIF9), and GPVI using the MoAbs F5 (A,B), 3J24.2 (C), and 9E18.2 (D). Bound antibody was located using a chemiluminescence procedure. GPIb, αIIb, and β3 were assessed using 10 μg protein, while GPVI was analyzed using 10 μg (A) and 20 μg (B-D) protein.
Evaluation of the of FcRγ-chain and of the ITAM-coupled signaling pathway
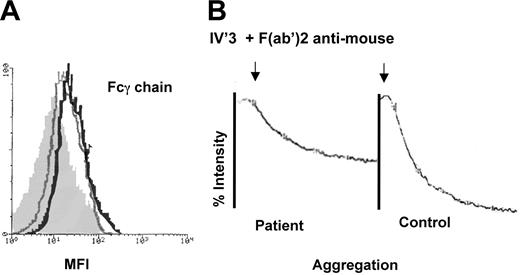
FcRγ is normally noncovalently associated with GPVI and mediates many of the signals transmitted following the interaction of GPVI with collagen. The presence of FcRγ was evaluated by flow cytometry using PFA-fixed and permeabilized platelets. Figure 6 shows typical histograms. Here, the MFI was 27.6 for the control and 14.7 for the patient after subtraction of the values for the isotype controls. Thus, FcRγ is present albeit in reduced amounts in the patient's platelets. To study the signaling pathway activated downstream of the GPVI-FcRγ complex, we took advantage of the fact that it is similar to the pathway coupled to the low-affinity receptor for the constant IgG fragment, FcγRIIA. Washed platelets were resuspended in Tyrode buffer and incubated first with the murine MoAb IV.3 (anti-FcγRIIA), followed by cross-linking bound IgG with F(ab′)2 fragments of antimouse IgG. On stirring, aggregation of the patient's platelets indirectly showed that signaling involving PLCγ2 was functional.
FcRγ is present in the patient's platelets, and the ITAM-coupled signaling pathway is functional. (A) Histograms obtained by flow cytometry of permeabilized platelets incubated with a rabbit anti–FcRγ-chain antibody. Results are shown for control platelets (black line), for the patient (gray line) and, for comparison, a negative control performed using the patient's platelets without primary antibody (shaded histogram). (B) Aggregation curves obtained using washed platelets from the patient and a control donor after incubation of platelets with the MoAb IV.3 (anti-FcRγII) followed by F(ab′)2 fragments of a sheep antimouse antibody. Note the positive aggregation response for the patient.
FcRγ is present in the patient's platelets, and the ITAM-coupled signaling pathway is functional. (A) Histograms obtained by flow cytometry of permeabilized platelets incubated with a rabbit anti–FcRγ-chain antibody. Results are shown for control platelets (black line), for the patient (gray line) and, for comparison, a negative control performed using the patient's platelets without primary antibody (shaded histogram). (B) Aggregation curves obtained using washed platelets from the patient and a control donor after incubation of platelets with the MoAb IV.3 (anti-FcRγII) followed by F(ab′)2 fragments of a sheep antimouse antibody. Note the positive aggregation response for the patient.
Testing for anti-GPVI activity in the patient's plasma
The search for antiplatelet antibodies on the surface of the patient's platelets by flow cytometry32 proved negative. Likewise, ELISA testing for antiplatelet serum IgG or IgM following the incubation of paraformaldehyde-fixed normal platelets with the patient's serum32 proved negative. We next used a series of murine MoAbs to GPVI in MAIPA (see “Patient, materials, and methods”). Again, no evidence for the presence of anti-GPVI antibodies was obtained. Finally, simultaneously incubating washed control platelets at room temperature with the patient's PPP or PPP from a control donor for intervals up to 90 minutes resulted in identical platelet aggregation responses to Cvx while surface GPVI continued to be detected on the normal platelets by flow cytometry using the MoAb 3J24.2.
GPVI gene and mRNA
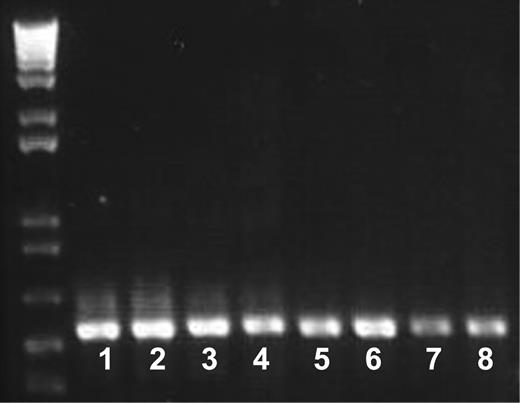
Genomic sequencing failed to show any potential mutations in the coding sequences or splice site junctions of the GPVI gene. By RT-PCR, 2 fragments covering the whole sequence encoding GPVI were obtained for the patient's and control donor cDNA. As shown in Figure 7, there were no differences in the abundance and migration of either fragment, thus indicating that the GPVI gene is normally expressed and transcribed in the patient's megakaryocytes. Polymorphisms against major platelet glycoproteins were evaluated by ASRA. The patient tested A1/A1 for the integrin α2 subunit and T/T for GPVI.
Messenger RNA encoding GPVI is present in the patient's platelets. Samples of mRNA isolated from the patient's platelets and those of a control donor were reverse transcribed. Two fragments of 547 bp and 540 bp, respectively, were amplified by PCR as described in “Materials and methods” and analyzed by electrophoresis in agarose gels. Lanes 1 to 4 and 5 to 8 correspond to the 8-554 and 472-1011 fragments, respectively, obtained with 2 μL (lanes 1, 3, 5, and 7) and 4 μL (lanes 2, 4, 6, and 8) samples. Control cDNA (lanes 1, 2, 5, and 6) and patient cDNA (lanes 3, 4, 7, and 8) are shown. Note the normal intensity and migration of the fragments corresponding to the patient's cDNA. Also shown are molecular size markers.
Messenger RNA encoding GPVI is present in the patient's platelets. Samples of mRNA isolated from the patient's platelets and those of a control donor were reverse transcribed. Two fragments of 547 bp and 540 bp, respectively, were amplified by PCR as described in “Materials and methods” and analyzed by electrophoresis in agarose gels. Lanes 1 to 4 and 5 to 8 correspond to the 8-554 and 472-1011 fragments, respectively, obtained with 2 μL (lanes 1, 3, 5, and 7) and 4 μL (lanes 2, 4, 6, and 8) samples. Control cDNA (lanes 1, 2, 5, and 6) and patient cDNA (lanes 3, 4, 7, and 8) are shown. Note the normal intensity and migration of the fragments corresponding to the patient's cDNA. Also shown are molecular size markers.
Discussion
We report a novel patient in whom a lifelong bleeding syndrome and absence of α-granules are linked to a much reduced expression of the collagen receptor, GPVI. There are few previous reports in the literature of defects in GPVI function in human beings.37-42 Although a Japanese patient had a mild bleeding syndrome suspected to be congenital in origin,37 GPVI functional defects are mostly associated with the presence of blocking autoantibodies, and the nature of any genetic defect has yet to be defined. Interestingly, a mouse model of GPVI gene deletion showed no signs of excessive bleeding when using tail bleeding time measurements.21 Nonetheless, genetic manipulation in mice associated with major reductions in GPVI levels severely modifies collagen-induced platelet responses.21,43 The characteristics from our patient included somewhat enlarged platelets, a clear absence of α-granules, a low platelet count, and the normal presence of dense granules. These are typical characteristics of GPS.2,4,8 The presence of a splenomegaly, previously seen in GPS,5 is in favor of the development of myelofibrosis, even if a bone marrow biopsy has not been performed for this patient. Marrow myelofibrosis is an often reported characteristic of GPS.2-4 The patient's sons have a normal platelet content of α-granules, and the platelets respond normally to collagen; there is no family history of bleeding. Autosomal dominant inheritance can therefore be ruled out, and a recessive transmission is more likely, as observed for most GPS patients.8 Final proof will require elucidation of the basic molecular genetic defect(s) in GPS.
Platelet aggregation testing showed that platelets from the patient responded poorly to collagen. Only at high concentrations was aggregation observed, and then it was delayed and with a much reduced intensity. An absence of aggregation with Cvx favored a defect in GPVI. Cvx is a snake venom protein known to stimulate platelets through GPVI.12,44,45 Other arguments in favor of a GPVI deficiency included (1) a virtual absence of Cvx binding to platelets in flow cytometry, (2) a much reduced detection by a series of MoAbs in Western blotting, and (3) a much reduced labeling with a mixture of anti-GPVI MoAbs on ultrathin cryosections in EM. Interestingly, it has recently been reported that Cvx binds to human GPIbα in transfected CHO cells, although it did not bind to mouse GPIb.46 However, the extent of Cvx binding to GPIb in normal human platelets is unknown, and for the patient it appeared to be minimal. Previous studies have not shown an interaction between Cvx and GPIb in human platelets,44,45 although an indirect influence of GPVI on this interaction cannot be ruled out. The extent of the deficiency of GPVI in the patient's platelets was important. In Western blotting, residual binding was seen only when a much increased amount of protein was applied to the gel. The migration of the residual GPVI was the same for control and patient, ruling out the synthesis of a truncated protein. Immunogold labeling for GPVI on ultrathin sections of normal platelets revealed not only a surface pool of the receptor but, also, appreciable amounts associated with internal membranes including those of α-granules. We have previously described a similar distribution for primary receptors of ADP (P2Y1) and thromboxane A2 (TXA2) (thromboxane-prostanoid-alpha receptor [TPα]).24 Our results for GPVI confirm those published recently for normal platelets by Susuki et al.36 The significance of this internal pool, also deficient in the patient's platelets, remains to be defined.
In an initial report, Croft et al34 showed that the GPVI gene has 2 common alleles, which have subsequently been shown to differ by 3 replacements in the glycosylated stem of GPVI and 2 in the cytoplasmic domain with allele “a” (equivalent to that termed T in the original study) encoding amino acids SKTQH and allele “b” (C) encoding PEALN.47 Flow cytometry has shown a significantly lower GPVI content in “bb” platelets when compared with “aa.” ASRA analysis showed that our patient belongs to the common aa (or T/T) group corresponding to the higher receptor density. Thus, the results obtained for the patient are unrelated to the presence of a specific low-density polymorphism. This is important because subjects homozygous for the bb allele have a significantly reduced platelet response to collagen.47 Furthermore, no mutations were detected in any of the 8 coding exons of the GPVI gene while her platelets were found to contain readily detectable amounts of mRNA coding GPVI. This latter study was performed using 2 pairs of cDNA primers and dividing the total cDNA into 2 equivalent parts, each PCR amplification allowing examination of half of the sequence. Overall, these results suggest that a normal synthesis of GPVI followed by an acquired loss of the receptor from the platelet membrane is a plausible explanation.
Nieswandt et al22 transfused mice with a rat MoAb reactive with mouse platelet GPVI and blocking its function. Surprisingly, they observed that GPVI was lost from the circulating platelets and that the loss continued for more than 2 weeks. More recently, these authors have shown that antigen loss was independent of the epitope recognized by the antibody on GPVI and that blocking GPVI's interaction with collagen was not necessary.48 For this reason, it was important to verify if antibodies directed against GPVI were present in the patient's serum. Neither in the MAIPA test performed with a series of MoAbs to GPVI nor in a series of other standard methods were serum antibodies to GPVI or platelets detected. Furthermore, neither platelet function testing nor flow cytometry analysis provided evidence for antibody-driven clearance or loss of GPVI when control platelets were incubated with the patient's plasma. So, while we are unable to totally exclude an antibody-induced loss of GPVI as explaining the patient's lack of response to collagen, we have found no evidence for it.
We evaluated the other major platelet receptors at the platelet surface but only GPVI was lacking, and both GPIb-IX-V and αIIbβ3 were normally present. In particular, a second collagen receptor, integrin α2β1, was readily detected by flow cytometry. In fact, it had a higher expression than the control performed in parallel. Considerable heterogeneity has been shown in α2β1 levels between individuals, with platelet expression varying up to 10-fold linked to polymorphisms within the gene encoding the α2 subunit.49 Our patient was A1/A1 for the α2 allele, which is in agreement with a high expression for this integrin. It is probable that the residual collagen-induced aggregation seen for the patient is linked to signaling through α2β1, a finding consistent with the results observed for the GPVI knock-out mouse.21
GPVI is present in the platelet membrane associated in a heterodimeric noncovalent complex with FcRγ. The expression of GPVI is severely decreased in mice deficient for FcRγ.10,50 This prompted us to determine if, in the absence of GPVI, FcRγ was still present in the patient's platelets. This chain is critical for PLCγ2-dependent platelet signaling through GPVI.10 Two approaches were used: (1) measuring FcRγ levels in permeabilized platelets by flow cytometry and then (2) analysis of the functionality of the coupled signaling pathway by stimulating FcγRIIA. FcRγ was somewhat reduced but readily detectable. Activation via cross-linking FcγRIIA by the antibody IV.3 efficiently induced aggregation of the patient's platelets. Notably, FcRγ levels were relatively unchanged in the GPVI knock-out mouse.21 These results make it unlikely that the GPVI deficiency in our patient's platelets is secondary to an FcRγ defect.
Recently, it has been demonstrated that GPVI can be cleaved during activation of platelets by agonists.51 The cleavage resulted from the action of a metalloprotease, and specific inhibitors of this family of enzymes prevented it. Cleavage leaves a platelet-bound 7-kDa fragment corresponding to the intracytoplasmic and transmembrane portion of GPVI while the extracellular domain is released to the medium. In our study, detection of GPVI was through a functional test such as platelet aggregation induced by collagen or Cvx, by FITC-Cvx binding, or by immunologic tests using MoAbs recognizing the extracellular domain of GPVI. Thus we would not detect a residual platelet-bound hydrolytic product. One explanation for our findings for our patient is that GPVI has been cleaved during megakaryocyte maturation or platelet production, resulting in loss of binding of collagen or Cvx. Notably, GPVI expression increases during normal megakaryocyte maturation.31 It will be interesting to determine whether a GPVI-derived extracellular domain can be detected in the plasma of the patient.
Intriguingly, analysis of the expression of a large number of genes for a patient with GPS using microarray technology showed that in fibroblast cultures a group of genes was overexpressed.52 These included those coding for collagen VI as well as fibronectin, thrombospondin-1, and metalloprotease-2 (MMP2), proteins that are potentially involved in the development of myelofibrosis. MMP2 is present in platelets as well as MMPs 1 and 9.53,54 These proteases are in an inactivated form but can became activated and exert a proteolytic activity not only on GPVI but also on GPIb at least during storage of platelets.54 For our patient, the levels of GPIb were normal, showing any degradation must be specific for GPVI. MMP2 has been shown to be present in platelets although its distribution was cytoplasmic rather than granular.53 The possible role of MMP2 in GPVI degradation will be a focus for further study as will its expression in marrow stromal and other cells in GPS.
In conclusion, we have shown that a defective production of α-granules in GPS can be accompanied by a severe deficiency of GPVI. The latter defect may be acquired and stable although its relationship to the defective production and/or maturation of α-granules remains to be defined. Because other GPS patients have been reported to have a major abnormality of collagen-induced platelet aggregation,8 it is possible that GPS is a heterogeneous syndrome and that GPVI deficiency will relate to a specific subgroup of patients. It is also interesting that the patient has a more severe bleeding syndrome than is generally reported for GPS. Although excessive bleeding was not reported in the GPVI knock-out mouse, a low expression of GPVI in human platelets is associated with reduced platelet functional responses and a somewhat prolonged PFA-100 closure time.47 In human disease, the effects of GPS and GPVI deficiency may prove to be cumulative.
Prepublished online as Blood First Edition Paper, March 9, 2004; DOI 10.1182/blood-2003-11-3842.
This work was performed in the context of the French network, “Rare Diseases of Platelet Function and Production,” financed by the GIS-Institut des Maladies Rares and INSERM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal