Abstract
The phosphoinositide 3-kinase (PI3K) catalytic subunit p110δ is expressed in neutrophils and is thought to play a role in their accumulation at sites of inflammation by contributing to chemoattractant-directed migration. We report here that p110δ is present in endothelial cells and participates in neutrophil trafficking by modulating the proadhesive state of these cells in response to tumor necrosis factor α (TNFα). Specifically, administration of the selective inhibitor of PI3Kδ, IC87114, to animals reduced neutrophil tethering to and increased rolling velocities on cytokine-activated microvessels in a manner similar to that observed in mice deficient in p110δ. These results were confirmed in vitro as inhibition of this isoform in endothelium, but not neutrophils, diminished cell attachment in flow. A role for PI3Kδ in TNFα-induced signaling is demonstrated by a reduction in Akt-phosphorylation and phosphatidylinositol-dependent kinase 1 (PDK1) enzyme activity upon treatment of this cell type with IC87114. p110δ expressed in neutrophils also contributes to trafficking as demonstrated by the impaired movement of these cells across inflamed venules in animals in which this catalytic subunit was blocked or genetically deleted, results corroborated in transwell migration assays. Thus, PI3Kδ may be a reasonable therapeutic target in specific inflammatory conditions as blockade of its activity reduces neutrophil influx into tissues by diminishing their attachment to and migration across vascular endothelium. (Blood. 2004;103:3448-3456)
Introduction
The recruitment of neutrophils into inflamed tissues is dependent upon a series of sequential adhesive events that occur between these cells and the microvasculature.1,2 Tissue injury initiates this process by locally releasing mediators of inflammation such as histamine, tumor necrosis factor α (TNFα), and interleukin-1 (IL-1) that rapidly convert the endothelial cell surface to a proadhesive state. This includes up-regulation of P- and E-selectin on the luminal surface of blood vessels that can subsequently interact with constitutively expressed carbohydrate ligands on circulating neutrophils to promote rapid attachment and rolling of these cells in preparation for transendothelial migration. Selectin-mediated adhesion is critical to this process as it facilitates the engagement of secondary leukocyte adhesion receptors, such as the β2-integrins, with intracellular adhesion molecules (ICAMs) expressed on the surface of inflamed vascular endothelium. This event requires neutrophil stimulation by locally produced chemoattractants (ie, IL-8 and leukotriene B4 [LTB4]), which subsequently results in integrin-mediated stabilization of interactions between these cells and the vasculature. Neutrophils eventually transmigrate across the endothelial cell barrier toward inflammatory foci in response to a bacterial and/or host-derived chemoattractant(s).3 Failure to complete any of these steps will impede neutrophil accumulation in inflamed tissue, as evidenced by leukocyte adhesion deficiency syndromes I and II.4,5
Class I PI3Ks are known to play a pivotal role in the ability of neutrophils to undergo chemotaxis, as the lipid products they generate, such as phosphatidylinositol (3,4,5)-trisphosphate (PIP3), are critical for promoting asymmetric F-actin synthesis and thus cell polarization.6-9 Their function, however, is not limited to directed migration, as they are also required for phagocytosis and the ability of neutrophils to generate oxygen radicals in response to chemoattractants such as fMLP.10-14 The ability of class I PI3Ks to regulate these processes relies on PIP3-mediated recruitment of 2 lipid-binding protein kinases, PDK1 and protein kinase B/Akt, both of which can interact with this phosphoinositide derivative via their pleckstrin homology domains. Association of these kinases with PIP3 at the plasma membrane brings them into close proximity, facilitating the phosphorylation and activation of Akt by PDK1.15 These proteins are, in turn, responsible for many of the downstream signaling events associated with PI3K activity.
Structurally, class I PI3Ks exist as heterodimeric complexes, consisting of a p110 catalytic (classified α, β, γ, and δ) and a p55, p85, or p101 regulatory subunit.16,17 These enzymes can be further divided into 2 subclasses (Ia and Ib) on the basis of their mechanism of activation. The class Ia subgroup contains p110α, p110β, and p110δ, which associate with the p85 and p55 regulatory protein, and is activated by receptor tyrosine kinases.16,18,19 By contrast, class Ib consists solely of p110γ, which associates with p101 adaptor molecule, and is stimulated by G protein βγ subunits in response to chemoattractants. Neutrophils express all 4 members of class I PI3Ks and their involvement in cell migration is supported by the ability of nonselective class I PI3K inhibitors, such as LY294002 and wortmannin, to mitigate neutrophil chemotaxis. Moreover, demonstration that inhibition of a specific isoform of these lipid kinases may alter neutrophil extravasation into inflamed tissues has been suggested by the partial reduction in chemoattractant-directed migration of these cells in mice deficient in the p110γ catalytic subunit.12,20-23 Thus, these observations have triggered considerable interest in the development of specific small molecule inhibitors to class I PI3Ks that may prevent undesirable neutrophil accumulation in certain inflammatory disease states in humans.19
Recently, a newly developed class I small molecule inhibitor, IC87114, with specificity to the p110δ catalytic subunit has been identified.24 Importantly, this compound has been shown to block up to 65% of fMLP-induced PIP3 generation in human neutrophils as well as directed migration of these cells on surface-immobilized ICAM-1 in response to this N-formyl peptide. Moreover, it is speculated that selective blockade of the p110δ activity in vivo may be advantageous over inhibitors that target all class Ia PI3Ks, as its expression is considered to be largely restricted to circulating hematogenous cells and thus may limit undesirable side effects.25-27 Although the study suggests that PI3Kδ activity can be selectively targeted, it remains to be determined whether (1) the effect of IC87114 on neutrophil function is identical to that observed in animals deficient in this class Ia lipid kinase and (2) the sole function of this isoform is to participate in the directional migration of neutrophils in response to inflammatory stimuli.
We now demonstrate that PI3Kδ contributes to neutrophil accumulation in inflamed tissues by impeding not only chemoattractant-directed migration but also adhesive interactions between neutrophils and cytokine-stimulated endothelium. Importantly, selective blockade of this class Ia PI3K isoform with IC87114 resulted in identical alterations in neutrophil adhesion and chemotaxis as observed for animals deficient in the p110δ catalytic subunit, suggesting that inhibition of its activity may be of potential therapeutic utility in the treatment of specific immunologic disease states in humans.
Materials and methods
Reagents
Monoclonal antibodies (mAbs) and cell lines used in experiments included the ICAM-1 mAb R 1/1, fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 anti-mouse immunoglobulin (CALTAG Laboratories, Burlingame, CA), E-selectin mAb CL3 (American Type Culture Collection [ATCC], Manassas, VA), FITC-conjugated Gr-1 (BD PharMingen, Franklin Lakes, NJ), anti-Akt, PDK1, and p110δ (Santa Cruz Biotechnologies, Santa Cruz, CA), horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), and Chinese-hamster-ovary (CHO)-ICAM-1 cells (ATCC). Inflammatory agents and chemoattractants used included murine recombinant TNFα (PeproTech, Rocky Hill, NJ), human recombinant TNFα (R&D Systems, Minneapolis, MN), LTB4 (BIOMOL, Plymouth Meeting, PA), and fMLP (Sigma, St Louis, MO). The small molecule selective p110δ inhibitor, IC87114, as well as recombinant p110 proteins were synthesized and purified as described.24
Animals
Mice in which green fluorescent protein (GFP) was knocked into the lysozyme M locus or the p110δ catalytic subunit was deleted were generated as previously described.28,29 Subsequent matings were performed to yield mice that were heterozygous for GFP+/- but deficient in p110δ-/- (mixed 129/Sv-C57BL/6 background). All animals were handled in accordance with policies administered by institutional Animal Care and Use Committees.
Intravital microscopy studies
The surgical preparation of animals for all in vivo studies was performed using standard techniques.30 The cremaster muscle (CM) in GFP+/- or GFP+/-/p110δ-/- animals was pretreated with an intrascrotal injection of murine recombinant TNFα (20 ng/mouse) for 3 hours. The tissue was then surgically exposed and positioned over a custom-built stage for viewing. The stage was then placed on an intravital microscope (IV-500; Mikron Instruments, San Diego, CA) equipped with a silicon-intensified camera (VE1000SIT; Dage mti, Michigan City, IN), and the tissue was kept moist by superfusion with thermo-controlled (37°C) bicarbonate-buffered saline. GFP-expressing cells (neutrophils and monocytes) were visualized in the microcirculation through × 20 or × 40 water immersion objectives (Acroplan; Carl Zeiss, Thornwood, NY) by epifluorescence from a Xenon arc stroboscope (Chadwick Helmuth, El Monte, CA). Rolling fraction was defined as the percentage of cells that interact with a given venule in the total number of cells that enter that venule during the same time period. The sticking fraction was defined as the number of rolling cells that became stationary for more than 30 seconds after superfusion of the CM with LTB4 (0.1 μM). Venular shear rates were determined from optical doppler velocimeter measurements of centerline erythrocyte velocity. The extent of leukocyte transmigration was evaluated at 30 and 60 minutes after application of LTB4. Video images were recorded using a Hi8 VCR (Sony, Boston, MA) and analysis was performed using a personal computer-based image analysis system.31
Isolation of neutrophils from bone marrow
Mouse bone marrow (BM) polymorphonuclear cells (PMNs) were isolated from femurs and tibias obtained from p110δ-deficient mice and wild-type (WT) littermates by density centrifugation as previously described.32,33 Briefly, cells were flushed from the marrow using Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS; Sigma) supplemented with 0.2% bovine serum albumin (BSA), then washed, and neutrophils were isolated using a discontinuous Percoll (Pharmacia, Piscataway, NJ) gradient. Red cell depletion was performed using density centrifugation using Ficoll (density 1.119; 30 minutes at 1200g). The resulting cell populations in both genotypes were equivalent for expression of the granulocyte marker Gr-1 (79% to 84% positive).
P110δ Western blot analysis
Human umbilical vein endothelial cells (HUVECs, 3-4 passages; Cambrex, East Rutherford, NJ) were washed 3 times in ice-cold phosphate-buffered saline (PBS) and then lysed on ice in 50 mM Tris (tris(hydroxymethyl)aminomethane)-HCl (pH 7.4), 1% Triton X-100, 150 mM NaCl, 1 mM EDTA (ethylenediaminetetraacetic acid), and a cocktail of inhibitors to serine and cysteine proteases (Complete, Mini; Roche Applied Science, Indianapolis, IN). Lysates were harvested by scraping. The cell debris was removed by centrifugation at 12 000g for 15 minutes at 4°C. Recombinant p110α, β, γ, and δ proteins (20 ng/lane) and cell lysate (100 μg/lane) were electrophoresed in precast 8% polyacrylamide gels (Invitrogen Life Technologies, Carlsbad, CA), transferred electrophoretically to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Billerica, MA), and immunoblotted with primary and horseradish peroxidase-conjugated secondary antibodies.24 Bound antibody was detected by chemiluminescence using enhanced chemiluminescence (ECL) plus Western blot detection system according to the manufacturer's instructions (Amersham Biosciences, Piscataway, NJ).
Akt activation
Quiescent HUVECs were pretreated with IC87114 (2 or 5 μM) for 2 hours before stimulation with TNFα (5 ng/mL) for a further 45 minutes.34 Cell lysates were prepared as described for Western blot analysis except that the lysis buffer also contained phosphatase inhibitors, 2 μM microcystin LR, 10 mM NaF, 1 mM Na3VO4, and 1 mM β-glycerophosphate. Electroblots were analyzed for Akt activation by Western blot analysis of total and phosphorylated Akt using specific antibodies.
Measurement of PDK1 activity in HUVECs
HUVEC monolayers were subjected to partial fetal calf serum reduction (0.2%) and complete growth factor withdrawal for 18 hours. Cells were treated with either vehicle or IC87114 (2 or 10 μM) for 30 minutes at 37°C before the addition of TNFα (5 ng/mL). After an additional 10-minute incubation at 37°C, cells were washed twice in ice-cold PBS and then solubilized in 50 mM Tris, pH 7.5, 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA (ethylene glycol tetraacetic acid), 50 mM NaF, 10 mM sodium glycerophosphate, 5 mM Na4P2O7, 1 mM Na3VO4, 0.1% 2-mercaptoethanol, 2 μM microcystin LR, and a cocktail of inhibitors to serine and cysteine proteases (Complete, Mini; Roche Applied Science). Cell lysates were clarified by centrifugation at 5000g for 20 minutes and immunoprecipitated with PDK1 or isotype-matched antibodies. PDK1 activity was then measured in the immunoprecipitates using the Upstate Biotechnology kinase assay kits (Lake Placid, NY) according to the manufacturer's instructions.
Laminar flow assays
HUVECs grown on fibronectin-coated glass coverslips were pretreated with IC87114 (2 μM) or vehicle control for one hour prior to stimulation with TNFα (5 ng/mL, 4 hours). Peripheral blood neutrophils from healthy volunteers were isolated from whole blood by dextran sedimentation followed by density separation over Ficoll-Hypaque and hypotonic lysis. Approval was obtained from the Washington University institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. Neutrophils (1 × 106/mL; HBSS, 10 mM HEPES, 1 mM CaCl2, 0.5% human serum albumin [HSA], pH 7.4) were infused over the endothelial cell monolayer that was incorporated into a parallel plate flow chamber (GlycoTech, Rockville, MD) for 5 minutes at shear rates of 100 and 300 s-1. The percentage of neutrophils that attached to IC87114 versus control-treated TNFα-stimulated HUVECs was determined. For E-selectin blockade, HUVECs were incubated with mAb CL3 (50 μg/mL, 15 minutes) prior to adhesion assays.
For determining a role for p110δ activity in neutrophils in supporting selectin-mediated attachment to endothelium, purified PMNs (1 × 106/mL) from mouse BM were infused over a monolayer of TNFα-activated (5 ng/mL, 6 hours) bEND3.1 cells (ATCC) grown to confluence on fibronectin-coated glass coverslips. The number of interacting PMNs was determined after 5 minutes of flow (100 s-1) and expressed per unit area of the field of view.
For testing the effects of IC87114 on leukocyte integrin function, a 6-well tissue culture plate containing a confluent monolayer of CHO cells expressing ICAM-1 was assembled in a parallel plate laminar flow chamber. Purified neutrophils (2 × 106/mL in HBSS buffer containing 2 mM MgCl2) were activated with LTB4 (0.1 μM) and allowed to settle on CHO cells in stasis for 10 minutes. Controlled flow was applied at a shear force of 2 dyne cm-2 and 4 dyne cm-2. The percentage of cells that remained adherent after 20 seconds at each wall shear stress was determined by off-line video analysis.
Neutrophil transwell migration assay
Neutrophil chemotaxis was measured as described.35 Briefly, purified murine neutrophils were incubated with dimethyl sulfoxide (DMSO, 0.3% vol/vol) or IC87114 reconstituted in DMSO (0.3%) for 20 minutes at room temperature. Cells, added to bare filter inserts (Transwell, 3 μm pore size; Corning Costar, Cambridge, MA) that were placed into wells containing LTB4 or control medium, were incubated for one hour at 37°C in a 5% CO2 humidified environment. The number of neutrophils that migrated into the bottom well was determined by FACScan (Becton Dickinson, San Jose, CA). Results were expressed as percent neutrophil migration relative to the control (medium without inhibitor).
Measurement of PIP3 synthesis by activated mouse bone marrow PMNs
Isolated mouse bone marrow PMNs were labeled with [32P] orthophosphate as described by Sadhu et al.24 Labeled cells were then incubated with or without IC87114 (2 or 10 μM) at 37°C for 20 minutes before stimulation for 30 seconds with either 1 μM fMLP or control, DMSO. Phospholipids were extracted and resolved on thin layer chromatography (TLC) as previously described.36 The TLC-resolved radiolabeled phospholipids were detected and quantified by phosphorimaging (Storm; Molecular Dynamics, Sunnyvale, CA) and PIP3 was expressed as percent of total phospholipids.
LPS-induced lung inflammation
IC87114- or vehicle control (PEG-400)-treated Lewis rats were challenged with lipopolysaccharide (LPS).37 Briefly, the trachea was exposed by standard surgical procedures and instilled with 100 μL saline solution or saline containing LPS (Escherichia coli Serotype 0111:B4; Sigma). At 6 hours following the challenge, rats were killed and the bronchoalveolar lavage (BAL) fluid was collected for TNFα detection and cell differentials. Total white blood cell (WBC) and neutrophil counts were determined (Hemavet 850 FS cell counter). Cell populations were identified by morphologic examination of smears, prepared by cytocentrifugation. BAL samples were centrifuged and supernatant was collected and analyzed for TNFα by enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, MI).
IC87114 plasma level determination
Animals received a single oral dose of either IC87114 (25 mg/kg for mice and 20 or 40 mg/kg for rats) or vehicle (PEG-400). Blood samples were subsequently drawn at indicated time points and plasma concentration of the compound was determined after liquid-liquid extraction by liquid chromatography/mass spectroscopy. The lower quantification limit was 50 ng/mL. Plasma samples from control animals (vehicle alone) were used as the blank control.
Cytotoxicity analysis
The cytotoxic effects of IC87114 were assessed by monitoring relative adenosine triphosphate (ATP) levels compared with the vehicle-treated cells. Purified human neutrophils were incubated with DMSO (0.3% vol/vol) or IC87114 reconstituted in DMSO (0.3%) for 2 hours at 37°C in a 5% CO2 humidified environment. Neutrophils lysed with Triton X-100 (0.1% final) served as the control in this assay. ATP levels were measured by luminescent ATP detection assay kit (ATPLite-M; Packard BioScience Company, Meriden, CT) following the manufacturer's instructions. Cell viability was also determined by trypan blue exclusion studies.
Kinase/phosphatase assays
Epidermal growth factor receptor tyrosine kinase, insulin receptor tyrosine kinase, and CD45 tyrosine phosphatase activities were evaluated in LeadProfilingScreen by MDS Pharma Services (Bothell, WA). For the testing of PDK1, Lck, P70S6K, CDK2/cyclinA, and zeta-associated protein 70 (ZAP-70) activities the KinaseProfiler system of Upstate Biotechnology was used.24
Statistical analysis
A Student t test was used for statistical comparisons. Statistical significance was set at a P value of less than .05.
Results
The role of PI3Kδ in promoting neutrophil-endothelial interactions in vivo
To determine if PI3Kδ contributes to neutrophil accumulation in inflamed tissues as previously demonstrated for the gamma isoform, we first evaluated the ability of these cells to interact with TNFα-stimulated microvessels in the cremaster muscle of mice deficient in the p110δ catalytic subunit and/or treated with the delta selective inhibitor IC87114. Animals heterozygous for GFP expression under the murine lysozyme M locus control, which rendered neutrophils and other granulocytes visible by epifluorescence intravital microscopy, were used to quantitate their interactions with the vessel wall. Oral administration of IC87114 one hour prior to intrascrotal injection of the TNFα significantly impaired interactions between circulating granulocytes and venular endothelium compared with vehicle treatment alone (Figure 1A, panels i-ii). Demonstration that the effects of the compound were due to inhibition of PI3Kδ activity is suggested by the similar reduction in neutrophil attachment to inflamed venular endothelium in p110δ-deficient animals under identical conditions (Figure 1A, panel iii). Moreover, pharmacologic blockade and/or genetic deletion of this isoform in mice resulted in a significant decrease (> 50%) in the number of fluorescent cells that attached and rolled compared with vehicle-treated or WT-matched littermates, respectively (Figure 1B). The reduction in cell adhesion in these animals was not due to IC87114-induced leukopenia, as the number of circulating neutrophils was similar between the control and experimental groups (2857.3 ± and 2730.7 ± 1132.6 for control- and IC87114-treated animals, respectively). The absolute number of circulating neutrophils in animals deficient in p110δ was 2997.7 ± 776.1 (n = 8). Wall shear rates calculated for each vessel in vehicle- and IC87114-treated mice were comparable, thus negating alterations in hemodynamic flow as a potential mechanism for the observed differences in adhesion (Figure 2A). Furthermore, mean rolling velocities of neutrophils on inflamed venules in animals that received IC87114 were approximately 8-fold higher than the corresponding control group (40.5 ± 5 μm/s versus 4.9 ± 7.6 μm/s, respectively; Figure 2B) and were comparable with that observed in p110δ-deficient animals (35.7 ± 13.2 μm/s, n = 5). The administration of this compound to mice lacking PI3Kδ activity had no further impact on rolling velocities. Taken together, these data strongly suggest that the ability of neutrophils to initially form adhesive contact with the inflamed vessel wall is negatively impacted by selective inhibition or deletion of this catalytic subunit. Moreover, the lack of any additional effects of IC87114 on cell binding when administered to p110δ-deficient animals suggests its specificity toward this class Ia PI3K isoform.
The role of PI3Kδ in supporting neutrophil-endothelial cell interactions in vivo. (A) Representative intravital fluorescence micrograph depicting accumulation of GFP-expressing cells on inflamed venules in WT animals pretreated with either vehicle alone (i) or IC87114 (ii), or in GFP+/-/p110δ-/- mice (iii). (B) Percentage of cells attaching to and rolling on TNFα-stimulated microvessels per minute in the absence or presence of inhibitor. *P < .01 versus control. A total of 15 venules were analyzed in 5 animals for each control or experimental group. Data represent the mean ± SD.
The role of PI3Kδ in supporting neutrophil-endothelial cell interactions in vivo. (A) Representative intravital fluorescence micrograph depicting accumulation of GFP-expressing cells on inflamed venules in WT animals pretreated with either vehicle alone (i) or IC87114 (ii), or in GFP+/-/p110δ-/- mice (iii). (B) Percentage of cells attaching to and rolling on TNFα-stimulated microvessels per minute in the absence or presence of inhibitor. *P < .01 versus control. A total of 15 venules were analyzed in 5 animals for each control or experimental group. Data represent the mean ± SD.
The effect of IC87114 on rolling velocities of neutrophils and wall shear rate in vivo. (A) The impact of IC87114 on blood flow as a function of vessel diameter. (B) Mean rolling velocities for consecutive interacting cells (n = 20 per venule, 3 venules per animal for each experimental condition tested) in the absence or presence of inhibitor, or in p110δ-/- mice. *P < .01 versus control. Data represent the mean ± SD for 200 or more cells per experimental condition.
The effect of IC87114 on rolling velocities of neutrophils and wall shear rate in vivo. (A) The impact of IC87114 on blood flow as a function of vessel diameter. (B) Mean rolling velocities for consecutive interacting cells (n = 20 per venule, 3 venules per animal for each experimental condition tested) in the absence or presence of inhibitor, or in p110δ-/- mice. *P < .01 versus control. Data represent the mean ± SD for 200 or more cells per experimental condition.
PI3Kδ modulates the proadhesive state of TNFα-stimulated endothelium
The PI3K-Akt signal transduction pathway is involved in a number of cellular processes critical for the function of both neutrophils and vascular endothelium. However, p110δ protein expression and function have not been demonstrated in the latter cell type. Using an antibody specific for this isoform, we demonstrate by Western blotting that the p110δ catalytic subunit is present in cultured human endothelial cells (Figure 3A). Importantly, treatment of HUVECs with the selective p110δ inhibitor, IC87114 (2 μM), reduced TNFα-mediated signaling as demonstrated by a reduction in phosphorylation of Akt, a downstream substrate for class I PI3Ks (Figure 3B). Phosphorylation of this protein kinase has been widely used as an indirect measure of PI3K activity in multiple cell types including HUVECs. Moreover, broad inhibition of PI3Ks in endothelium with LY294002 has also been shown to reduce phosphorylation of this substrate in response to TNFα. Further evidence that suggests that IC87114 inhibits PI3Kδ function and not a downstream effector molecule involved in Akt phosphorylation is provided by direct measurement of the activity of PDK1 immunoprecipitated from TNFα-stimulated HUVECs pretreated with IC87114 or vehicle control. Incubation of intact HUVECs, but not their lysates, with this compound reduced the kinase activity of this pleckstrin homology domain-containing protein in response to TNFα (Figure 3C). This supports the concept that class I PI3K activity is required for PDK1 and Akt function in endothelium as described for neutrophils. Importantly, this compound does not inhibit additional intracellular signaling pathways (ie, p38 mitogen-activated protein kinase [MAPK] or insulin receptor tyrosine kinase) that are also critical for general cell function and survival (Table 1).24 Thus, impairment of PI3Kδ activity in either endothelium or neutrophils could potentially account for the observed reduction in adhesive interactions between these 2 cell types in vivo.
p110δ is present in venular endothelium and is activated by TNFα (A) Immunoblots of the p110δ catalytic subunit using recombinant proteins (panel 1) or lysates obtained from HUVECs or neutrophil (panel 2). (B) Representative photomicrograph (3 experiments performed in duplicate) demonstrating phosphorylation of Akt (Ser 243) in response to TNFα-induced activation of HUVECs and inhibition by IC87114. Total Akt present in cells is shown under all conditions tested. (C) Activity of PDK1 in response to TNFα-induced stimulation of vascular endothelium. Lysates from activated HUVECs, pretreated with either vehicle control or the p110δ inhibitor, were subjected to immunoprecipitation using beads coated with an antibody that recognizes PDK1. Enzyme activity was then assessed as described in “Materials and methods.” Relative kinase activity is the average ± SD of 3 experiments with duplicate immunoprecipitates. Activity of PDK1 from untreated cells was represented as 1. P < .01 versus vehicle control. ψIC87114 was added to immunoprecipitated PDK1 obtained from TNFα-stimulated HUVEC lysates to assess its ability to directly inhibit this kinase.
p110δ is present in venular endothelium and is activated by TNFα (A) Immunoblots of the p110δ catalytic subunit using recombinant proteins (panel 1) or lysates obtained from HUVECs or neutrophil (panel 2). (B) Representative photomicrograph (3 experiments performed in duplicate) demonstrating phosphorylation of Akt (Ser 243) in response to TNFα-induced activation of HUVECs and inhibition by IC87114. Total Akt present in cells is shown under all conditions tested. (C) Activity of PDK1 in response to TNFα-induced stimulation of vascular endothelium. Lysates from activated HUVECs, pretreated with either vehicle control or the p110δ inhibitor, were subjected to immunoprecipitation using beads coated with an antibody that recognizes PDK1. Enzyme activity was then assessed as described in “Materials and methods.” Relative kinase activity is the average ± SD of 3 experiments with duplicate immunoprecipitates. Activity of PDK1 from untreated cells was represented as 1. P < .01 versus vehicle control. ψIC87114 was added to immunoprecipitated PDK1 obtained from TNFα-stimulated HUVEC lysates to assess its ability to directly inhibit this kinase.
The effect of IC87114 on the activity of several protein kinases and a phosphatase
Enzyme . | Activity (% of control) ± SD . |
|---|---|
| EGF receptor tyrosine kinase | 102 ± 5.5 |
| Insulin receptor tyrosine kinase | 98 ± 6.2 |
| CD45 tyrosine phosphatase | 104 ± 2.2 |
| PKC-θ | 97 ± 5.5 |
| PDK1 | 91.5 ± 2.1 |
| Lck | 116.5 ± 9.2 |
| P70S6K | 98.5 ± 0.7 |
| CDK2/cyclinA | 92.5 ± 2.12 |
| ZAP-70 | 97.5 ± 13.4 |
| p38 MAPK | No inhibition* |
| DNA-PK | No inhibition* |
| CHK1 | No inhibition* |
| cSrc | No inhibition* |
| CK1 | No inhibition* |
| PKBα (Akt 1) | No inhibition* |
| PKCα | No inhibition* |
| PKCβII | No inhibition* |
Enzyme . | Activity (% of control) ± SD . |
|---|---|
| EGF receptor tyrosine kinase | 102 ± 5.5 |
| Insulin receptor tyrosine kinase | 98 ± 6.2 |
| CD45 tyrosine phosphatase | 104 ± 2.2 |
| PKC-θ | 97 ± 5.5 |
| PDK1 | 91.5 ± 2.1 |
| Lck | 116.5 ± 9.2 |
| P70S6K | 98.5 ± 0.7 |
| CDK2/cyclinA | 92.5 ± 2.12 |
| ZAP-70 | 97.5 ± 13.4 |
| p38 MAPK | No inhibition* |
| DNA-PK | No inhibition* |
| CHK1 | No inhibition* |
| cSrc | No inhibition* |
| CK1 | No inhibition* |
| PKBα (Akt 1) | No inhibition* |
| PKCα | No inhibition* |
| PKCβII | No inhibition* |
The selectivity of IC87114 (10 μM) was tested against several human protein kinases and a phosphatase. Protein kinase assays were performed in the presence of 100 μMATP.
EGF indicates epidermal growth factor; PKC, protein kinase C; PKB, protein kinase B; PK, protein kinase; CHK1, checkpoint kinase 1; and CK, casein kinase-1.
Sadhu et al.24
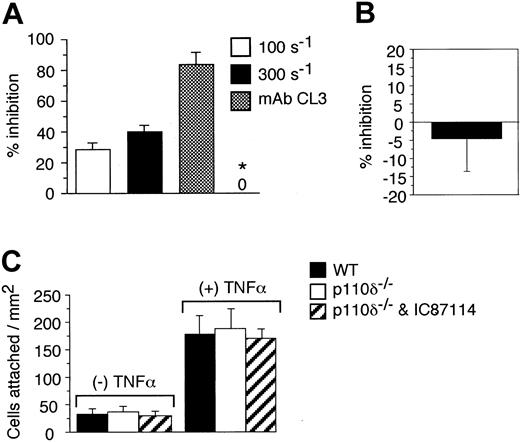
To determine the specific cell type in which PI3Kδ activity was essential for promoting attachment of neutrophils to endothelium in flow, we evaluated human and murine neutrophil binding to TNFα-activated HUVECs or bEND3.1 monolayer, respectively, using a parallel plate flow chamber apparatus. In these experiments, the HUVEC substrate, but not human neutrophils, was preincubated with IC87114 (2 μM) prior to being stimulated with TNFα. In comparison with endothelium treated with vehicle alone (0.3% DMSO), neutrophil attachment to endothelium preincubated with IC87114 was reduced by 28% and 40% at physiologic wall shear rates of 100 and 300 s-1, respectively (Figure 4A). By contrast, treatment of neutrophils with the identical concentration of inhibitor prior to their infusion over a HUVEC substrate preincubated with only TNFα did not perturb attachment (Figure 4B). Importantly, IC87114 did not directly inhibit neutrophil-endothelial cell interactions, as incubation of HUVECs following TNFα treatment with this reagent (2 μM) had no impact on attachment in flow. Moreover, no significant difference in binding was noted for WT versus p110δ-deficient neutrophils interacting with the murine endothelioma cell line under identical flow conditions (Figure 4C). These results are consistent with a previous study demonstrating that blockade of PI3K activity in this cell type with wortmannin or LY294002 does not alter their ability to attach to a selectin substrate in flow.38
The role of PI3Kδ in supporting neutrophil-endothelial cell interactions in vivo. (A) Percent reduction in neutrophil accumulation on HUVECs pretreated with IC87114 (2 μM) one hour prior to stimulation with TNFα. Results are expressed as the percentage of cells that bound to vehicle (0.3% DMSO)-treated endothelial cell substrate at wall shear rates of 100 and 300 s-1. The requirement for E-selectin is demonstrated by the ability of the function-blocking mAb CL3 to inhibit neutrophil attachment to cytokine-stimulated endothelium at a wall shear rate of 300 s-1. TNFα-stimulated HUVEC substrate was also incubated with IC87114 (2 μM for 15 minutes) to assess the ability of IC87114 to directly inhibit selectin-ligand interactions (*). All data represent the mean ± SD of 3 sets of experiments performed in duplicate. (B) Percent inhibition in attachment of human neutrophils that were pretreated with IC87114 (2 μM) to TNFα-activated HUVECs at a wall shear rate of 300 s-1. Results are expressed as the percentage of vehicle (0.3% DMSO)-treated cells that bound to cytokine-stimulated endothelium. (C) Attachment of WT versus p110δ-deficient neutrophils (with and without prior incubation with IC87114) to resting or TNFα-activated bend3.1 cells at a wall shear rate of 100 s-1. Results (A-C) represent the mean ± SD for 3 experiments performed in triplicate.
The role of PI3Kδ in supporting neutrophil-endothelial cell interactions in vivo. (A) Percent reduction in neutrophil accumulation on HUVECs pretreated with IC87114 (2 μM) one hour prior to stimulation with TNFα. Results are expressed as the percentage of cells that bound to vehicle (0.3% DMSO)-treated endothelial cell substrate at wall shear rates of 100 and 300 s-1. The requirement for E-selectin is demonstrated by the ability of the function-blocking mAb CL3 to inhibit neutrophil attachment to cytokine-stimulated endothelium at a wall shear rate of 300 s-1. TNFα-stimulated HUVEC substrate was also incubated with IC87114 (2 μM for 15 minutes) to assess the ability of IC87114 to directly inhibit selectin-ligand interactions (*). All data represent the mean ± SD of 3 sets of experiments performed in duplicate. (B) Percent inhibition in attachment of human neutrophils that were pretreated with IC87114 (2 μM) to TNFα-activated HUVECs at a wall shear rate of 300 s-1. Results are expressed as the percentage of vehicle (0.3% DMSO)-treated cells that bound to cytokine-stimulated endothelium. (C) Attachment of WT versus p110δ-deficient neutrophils (with and without prior incubation with IC87114) to resting or TNFα-activated bend3.1 cells at a wall shear rate of 100 s-1. Results (A-C) represent the mean ± SD for 3 experiments performed in triplicate.
The effect of p110δ inhibition on firm adhesion
The engagement of the leukocyte integrins with ICAMs expressed on venular endothelium is also essential for the ability of neutrophils to stably adhere to the vessel wall in order for transmigration to occur.39 To determine the role of PI3Kδ in this process, we investigated the ability of neutrophils rolling on inflamed venular endothelium in vivo to undergo integrin-mediated firm adhesion in response to an activating stimulus. By superfusing the TNFα-stimulated cremaster muscle with LTB4, we observed that rolling cells rapidly transitioned to firm adhesion despite the presence of the PI3Kδ inhibitor IC87114 (Figure 5A). Mean plasma level of the compound at the time of application of this chemoattractant was 12.8 ± 7 μM, a concentration known to predominantly inhibit p110δ activity. As this event requires participation of the β2-integrins (ie, Mac-1 and LFA-1) and endothelial cell ICAM-1, our data suggest that interactions between these receptor-ligand pairs are not significantly perturbed under these experimental conditions.
The effect of the PI3Kδ selective inhibitor on integrin-mediated firm adhesion of neutrophils. (A) Percentage of rolling cells that became stationary for more than 30 seconds upon LTB4 superfusion of CM in vehicle- versus IC87114-treated mice. Results are from 5 experiments for each condition tested. (B) Detachment in flow of resting versus LTB4-activated neutrophils incubated in stasis on CHO cells expressing ICAM-1 at the indicated wall shear stresses in the absence or presence of vehicle control (0.3% DMSO) or IC87114. Mean ± SD for 2 experiments performed in triplicate.
The effect of the PI3Kδ selective inhibitor on integrin-mediated firm adhesion of neutrophils. (A) Percentage of rolling cells that became stationary for more than 30 seconds upon LTB4 superfusion of CM in vehicle- versus IC87114-treated mice. Results are from 5 experiments for each condition tested. (B) Detachment in flow of resting versus LTB4-activated neutrophils incubated in stasis on CHO cells expressing ICAM-1 at the indicated wall shear stresses in the absence or presence of vehicle control (0.3% DMSO) or IC87114. Mean ± SD for 2 experiments performed in triplicate.
To confirm that the ability of the β2-integrins expressed on neutrophils to bind to ICAMs was not significantly altered in the presence of IC87114, we next evaluated LTB4-triggered firm adhesion to ICAM-1 in vitro. Neutrophils were first incubated with 2 μM IC87114 prior to adhesion assays, a concentration that primarily inhibits delta but not other class Ia or Ib PI3Ks. Treated cells were then stimulated with LTB4 and allowed to bind in stasis to CHO cells transfected with human ICAM-1 before subjecting them to physiologic wall shear stresses of 2 and 4 dyn cm-2. ICAM-1 expression on these cells was confirmed by flow cytometry using mAb R 1/1 (mean fluorescence intensity > 103, data not shown). In agreement with our in vivo experiments, PI3Kδ inhibition did not impair integrin-mediated firm adhesion, as more than 80% of LTB4-stimulated neutrophils were observed to remain bound to the ICAM-1 substrate in the presence or absence of IC87114 (Figure 5B).
The role of PI3Kδ in neutrophil chemotaxis
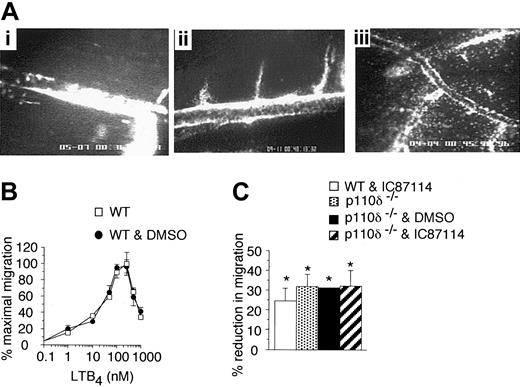
The final step required for accumulation of neutrophils in inflamed tissues relies upon chemoattractant-directed migration, an event that involves class I PI3Ks. A recent study has suggested that p110δ, in addition to the gamma isoform, contributed to this process as treatment of human neutrophils with IC87114 diminished fMLP-induced chemotaxis on an ICAM-1 substrate in vitro in the absence of hemodynamic forces.24 We confirmed that this isoform is indeed involved in chemotaxis, but its effect is not restricted to blockade of directed movement to this bacterial product; LTB4-induced migration of neutrophils across inflamed microvessels in vivo was also diminished in IC87114-treated animals as well as those deficient in p110δ. This occurred despite the continued accumulation of these cells on the luminal surface of the vessel wall (Figure 6A, panels i and ii, respectively). By contrast, extensive neutrophil transmigration was observed in vehicle-treated animals (Figure 6A, panel iii).
The effect of the PI3Kδ inhibition or deletion on neutrophil transmigration. (A) Representative micrographs depicting LTB4-induced transmigration of neutrophils in a WT mouse pretreated with IC87114 (i), an animal deficient in p110δ (ii), or WT animal pretreated with vehicle control (iii). Each micrograph represents an individual experiment one hour after continuous exposure to LTB4 as described in “Materials and methods.” (B) Chemoattractant dose response curve for murine neutrophil transwell migration to LTB4 in the absence or presence of DMSO (0.3%). (C) Neutrophil chemotaxis at a concentration of chemoattractant that supports half maximal transmigration (30 nM). Results are from 4 experiments performed in triplicate (mean ± SD). *P < .01 versus control.
The effect of the PI3Kδ inhibition or deletion on neutrophil transmigration. (A) Representative micrographs depicting LTB4-induced transmigration of neutrophils in a WT mouse pretreated with IC87114 (i), an animal deficient in p110δ (ii), or WT animal pretreated with vehicle control (iii). Each micrograph represents an individual experiment one hour after continuous exposure to LTB4 as described in “Materials and methods.” (B) Chemoattractant dose response curve for murine neutrophil transwell migration to LTB4 in the absence or presence of DMSO (0.3%). (C) Neutrophil chemotaxis at a concentration of chemoattractant that supports half maximal transmigration (30 nM). Results are from 4 experiments performed in triplicate (mean ± SD). *P < .01 versus control.
To further demonstrate that PI3Kδ does indeed participate in chemoattractant-directed migration of neutrophils, we next examined the effect of p110δ blockade and/or genetic deletion on the ability of these cells to undergo chemotaxis using a Transwell assay system. A dose response curve was generated to determine the concentration of LTB4 necessary to support half-maximal migration across a bare filter insert (Figure 6B). Maximal transmigration for neutrophils purified from mouse bone marrow occurred between 100 to 250 nM of LTB4 and is consistent with a previously published result.40 Treatment of WT neutrophils with 2 μM IC87114 diminished migration in response to LTB4 (30 nM) by approximately 30%, a value equivalent to that observed for PI3Kδ-deficient cells (Figure 6C). However, preincubation of cells lacking this PI3K isoform with the identical concentration of inhibitor had no further effects on chemotaxis, suggesting its specificity toward p110δ. These results also support the concept that PI3Kγ is not the only class I isoform that contributes to the directional migration of neutrophils.
PI3Kδ contributes to PIP3 production in activated neutrophils
The generation of PIP3 in neutrophils has been shown to be essential for directional movement of these cells in response to G-protein coupled receptor (GPCR) agonists such as fMLP. To demonstrate that PI3Kδ contributes to the production of this second messenger, neutrophils purified from WT or p110δ-/- murine bone marrow were pretreated with either IC87114 (10 μM) or vehicle control and then exposed to fMLP (1 μM) for 30 seconds to stimulate PIP3 synthesis as previously described for p110γ-deficient cells.22,23 Results indicate that PI3Kδ activity accounts for a significant amount of PIP3 generation in this cell type (∼ 64%) at a time point within the range tested for p110γ-/- neutrophils. IC87114 had no further impact on its production in combination with neutrophils deficient in the p110δ catalytic subunit (Figure 7A-B).
The impact of p110δ inhibition or deletion on PIP3 production in stimulated neutrophils. (A) Thin-layer chromatography of phospholipid extracts from neutrophils purified from WT or p110δ-deficient animals. Cells labeled with [32P] were pretreated with IC87114 (10 μM) or vehicle control prior to activation with fMLP (1 μM). PIP3 standard is shown for comparison. (B) PIP3 quantitation. Results are expressed as a percentage of total phospholipids and represent 2 independent experiments performed in duplicate.
The impact of p110δ inhibition or deletion on PIP3 production in stimulated neutrophils. (A) Thin-layer chromatography of phospholipid extracts from neutrophils purified from WT or p110δ-deficient animals. Cells labeled with [32P] were pretreated with IC87114 (10 μM) or vehicle control prior to activation with fMLP (1 μM). PIP3 standard is shown for comparison. (B) PIP3 quantitation. Results are expressed as a percentage of total phospholipids and represent 2 independent experiments performed in duplicate.
PI3Kδ activity contributes to neutrophil accumulation in a model of acute pulmonary inflammation
To determine if the effects of PI3Kδ blockade on neutrophil accumulation in inflamed tissues were limited to a specific vascular bed or for that matter a particular species, we next evaluated the contribution of this class Ia isoform in promoting their extravasation into inflamed lung. Instillation of LPS into the trachea of rats resulted in an approximately 100-fold increase in neutrophil counts in bronchoalveolar lavage (BAL) fluid 6 hours after challenge compared with PBS control (Figure 8A). Total leukocyte counts are shown for comparison. Treatment of animals orally with either 20 or 40 mg per kilogram of body weight of IC87114 one hour prior to LPS challenge resulted in approximately 60% to 80% reduction in the accumulation of neutrophils in BAL fluid, respectively. Importantly, plasma levels of the inhibitor were within the range that effectively blocked p110δ biochemical activity but not the other class I isoforms of PI3K that are expressed in neutrophils. Despite this reduction in neutrophil influx, TNFα, a cytokine essential for endothelial cell activation, was still detectable in BAL fluid of LPS-treated mice that received IC87114 (Figure 8B). In addition, this inhibitor does not appear to be toxic to cells, as neutrophils treated with IC87114 at concentrations as high as 50 μM remained more than 95% viable (Figure 8C).
The impact of IC87114 on endotoxin-induced acute lung injury. (A) Total WBC and neutrophil counts in BAL fluid obtained 6 hours after intratracheal instillation of LPS in the absence or presence of the PI3Kδ inhibitor. Mean number (± SD) of cells contained within BAL fluid as well as plasma levels of inhibitor are shown. Data represent 4 different experiments with 5 or 6 rats in each group. *P < .05 versus control. (B) Effect of IC87114 on LPS-induced TNFα release in BAL fluid. Data are mean ± SD of 5 animals in each group, representing 3 separate experiments. (C) Effect of IC87114 on neutrophil viability assessed by relative adenosine triphosphate (ATP) levels. Results represent the mean ± SD for 3 experiments performed in triplicate.
The impact of IC87114 on endotoxin-induced acute lung injury. (A) Total WBC and neutrophil counts in BAL fluid obtained 6 hours after intratracheal instillation of LPS in the absence or presence of the PI3Kδ inhibitor. Mean number (± SD) of cells contained within BAL fluid as well as plasma levels of inhibitor are shown. Data represent 4 different experiments with 5 or 6 rats in each group. *P < .05 versus control. (B) Effect of IC87114 on LPS-induced TNFα release in BAL fluid. Data are mean ± SD of 5 animals in each group, representing 3 separate experiments. (C) Effect of IC87114 on neutrophil viability assessed by relative adenosine triphosphate (ATP) levels. Results represent the mean ± SD for 3 experiments performed in triplicate.
Discussion
Class I PI3Ks are vital to chemoattractant-directed migration of neutrophils. This is evident by the partial reduction in chemotaxis of these cells in animals deficient in PI3Kγ. The present work sought to evaluate the role of PI3Kδ in contributing not only to this process but also to the adhesive interactions required for neutrophil accumulation in inflamed tissues. By using a newly identified selective inhibitor of PI3Kδ activity, IC87114, as well as animals lacking the p110δ catalytic subunit, we present evidence that this isoform plays a prominent role in leukocyte trafficking in animal models of inflammation by directly modulating the activity of both neutrophils and vascular endothelium.
Recently, it has been suggested that PI3Kδ participates in chemotaxis as treatment of human neutrophils with IC87114 reduced fMLP-mediated directional migration of these cells in an under-agarose assay system.24 Although this compound diminished both the number and distance that neutrophils were able to migrate toward this bacterial product in vitro, demonstration of its biospecificity has not been fully tested. By comparison to animals deficient in the p110δ catalytic subunit, oral administration of IC87114 to mice resulted in a similar and significant reduction in neutrophil extravasation into inflamed tissue. Moreover, the ability of neutrophils to undergo LTB4-induced migration in a Transwell assay system was diminished by more than 26% regardless of whether these cells were pretreated with the delta-specific inhibitor, lacked p110δ, or both. As PIP3 generation is a key event required for neutrophil polarization in response to GPCR agonists, then it is anticipated that treatment of cells with this inhibitor should also diminish its level of production to that observed in the absence of the p110 catalytic subunit. Indeed, an identical reduction in PIP3 production was observed in neutrophils incubated with IC87114 prior to fMLP stimulation compared with cells obtained from null animals. Moreover, treatment of neutrophils deficient in the p110δ with this compound had no further impact on the generation of this second messenger, which provides further evidence of specificity toward PI3Kδ. Our results also indicate that PI3Kγ is not the only class I isoform involved in neutrophil chemotaxis in response to stimulation of GPCRs, suggesting a dynamic interplay between class Ia and Ib isoforms in maintaining PIP3 levels in this cell type.
To date, a role for class I PI3Ks in modulating the ability of cytokine-stimulated vascular endothelium to promote adhesive interactions with neutrophils has not been demonstrated. In particular, previous reports suggest that p110δ expression is largely restricted to leukocytes. To our knowledge, we are the first to provide evidence that not only is this catalytic subunit present in venular endothelium, but that blockade of its activity in this cell type impedes neutrophil capturing at physiologic shear rates. This was evident by the significant reduction in neutrophil attachment in flow by (1) TNFα-activated microvasculature in animals pretreated with IC87114 or in those lacking the p110δ catalytic subunit and (2) HUVECs incubated with compound prior to cytokine-induced stimulation. Importantly, the observed alteration in capture efficiency appeared to be exclusively due to p110δ inhibition in endothelium as IC87114-induced blockade or genetic deletion of this catalytic subunit in neutrophils did not impair their ability to initiate contact in flow to TNFα-activated endothelium compared with vehicle-treated or WT cells. Evidence to suggest a potential perturbation in selectin- but not integrin-dependent adhesion was demonstrated by the ability of LTB4-activated neutrophils to stabilize adhesion to either vascular endothelium in animals deficient in p110δ or CHO cells expressing ICAM-1 in the presence of IC87114. The latter results are consistent with a previous report demonstrating that broad blockade of PI3K activity in neutrophils with the nonselective inhibitor LY294002 does not alter their ability to firmly adhere to ICAM-1 in vivo.38 Importantly, these data, in conjunction with the observed reduction in PDK-1 activity and Akt phosphorylation in IC87114-treated HUVECs, support the previously proposed concept that the PI3K signaling axis in endothelium is induced by TNFα.41,42
Overall, we have extended the role of PI3Kδ beyond its requirement for neutrophil directional migration to include regulation of the proadhesive state of cytokine-stimulated endothelium. Our results provide the first evidence that p110δ is present in endothelial cells and that it contributes to neutrophil accumulation in inflammation not only by participating in their migration to chemoattractants, but also in the ability of TNFα-stimulated endothelium to mediate effective capturing of these cells in flow. The impact of IC87114 on neutrophil attachment and migration suggests that blockade of PI3Kδ may be of therapeutic benefit in specific disease states. This is supported by the significant reduction in neutrophil accumulation in our LPS-induced model of acute lung injury. Importantly, blockade of p110δ function does not appear to alter biologic functions other than immune related, as mice deficient in this catalytic subunit are viable and fertile.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-05-1667.
Supported by the National Institutes of Health grant HL63244-01A1 (T.G.D.) and the Medical Research Council and the Biotechnology and Biological Sciences Research Council (M.T.).
Several of the authors (K.D.P., J.D., W.T.T., and J.S.H.) are employed by the company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ed Kesicki for the synthesis of IC87114, Wendy Wisdom for compound plasma level estimations, Guy Rosman for TNFα release assays, Esther Trueblood and Darcey Clark for bone marrow collection, and Sam Tran for cell differentials. Thanks to Drs Mike Gallatin and Don Staunton for their careful reading and critical appraisal of this article, and Elena Vigorito, Helen Reynolds, and Angela Janis for maintaining and genotyping mice.





![Figure 7. The impact of p110δ inhibition or deletion on PIP3 production in stimulated neutrophils. (A) Thin-layer chromatography of phospholipid extracts from neutrophils purified from WT or p110δ-deficient animals. Cells labeled with [32P] were pretreated with IC87114 (10 μM) or vehicle control prior to activation with fMLP (1 μM). PIP3 standard is shown for comparison. (B) PIP3 quantitation. Results are expressed as a percentage of total phospholipids and represent 2 independent experiments performed in duplicate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-05-1667/6/m_zh80090460640007.jpeg?Expires=1765886322&Signature=shFMrd~U8ijmMobjZwrAJdt6KNAYwHZSUlViXRhopqNed~hVdPXiG0Ec7Vny9k1Q8H59zqO~heZqKQfAZLZeKE0DyAc0pe0zPLK3YurBLt1-YnBHPFHT8Qslyq9nZF90kdn7jjpUVkx5jlMd7UXfDwzB~kFFBbks6C6bliBBBd2XY2BPXbT5kYRr6uJJMT-zGBDdZsj-dqjDJQoezXY9yEb-qX~z3Iik0G2W~bHs-O5zV810p1JoQT~XkI-XsvozvTlaf1729OpaOb8jwipHfkkrobPUIXC5FMecBkRXF1pa7sQUdCrkSDUQcR9PUkG12zv3OlFL-dy~cvIKnGMwbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal