Abstract
Tissue factor pathway inhibitor (TFPI) is the major physiologic inhibitor of the extrinsic coagulation pathway. We have previously shown that TFPI is also a potent inhibitor of endothelial proliferation in vitro and of primary and metastatic tumor growth in vivo. Surprisingly, the antitumor activity of TFPI was demonstrated to be independent of its anticoagulant activity, suggesting a possible nonhemostatic mechanism of action for TFPI in these models. This antitumor mechanism may involve the very low density lipoprotein (VLDL) receptor because the in vitro antiproliferative activity of TFPI is mediated through interaction with the VLDL receptor. In the current study, we identify a 23-amino acid fragment of TFPI (TFPIc23) localized to the C-terminus, which mediates binding to the VLDL receptor. The TFPIc23 peptide inhibits endothelial cell proliferation through an apoptotic mechanism and blocks vessel outgrowth in the in vitro assays, and this activity is mediated through interaction with the VLDL receptor. In vivo, this peptide potently inhibits angiogenesis in Matrigel and chick chorioallantoic membrane models and also inhibits metastatic tumor growth. Our data demonstrate that this VLDL receptor-binding fragment of the TFPI molecule has apoptotic, antiangiogenic, and antitumor activity and suggests a possible mechanism whereby TFPI can regulate angiogenesis and tumor growth independently of its anticoagulant activity. (Blood. 2004;103: 3374-3380)
Introduction
The tissue factor (TF) or extrinsic coagulation pathway is activated when circulating factor VII (fVII) binds to TF on the surface of extravascular cells in the area of an injury.1 The resulting TF/fVIIa complex is proteolytically active and leads to the generation of a fibrin clot. In recent years, a significant amount of evidence has demonstrated that TF and the TF/fVIIa complex can promote tumor growth, metastasis, and angiogenesis.2-4 TF is up-regulated on endothelial cells within breast cancer5 and its elevated levels are correlated with an invasive carcinoma phenotype. In an experimental metastasis model, tumor cell-associated TF stimulates the formation of tumor cell/platelet/fibrin complexes, which greatly enhance tumor cell seeding in the lungs.4,6,7
The major physiologic inhibitor of the TF/fVIIa complex is the tissue factor pathway inhibitor (TFPI).1 TFPI is a multidomain protein consisting of 3 independently folded kunitz proteinase inhibitor (KPI) domains, and a highly basic carboxy-terminal tail.1,8 The first KPI domain specifically inhibits TF/fVIIa proteolytic activity, whereas the second KPI domain specifically inhibits fXa. TFPI inhibits coagulation by first blocking fXa activity and next forming a stable quaternary complex with TF/fVIIa. No inhibitory activity has been reported for the third KPI domain.1,8
Two classes of cell surface receptors have been described for TFPI, heparan sulfate proteoglycans (HSPGs)9-11 and several members of the low-density-lipoprotein (LDL) receptor family.12-14 Numerous reports have demonstrated through the use of truncated TFPI molecules that the C-terminal region of TFPI (amino acids 161-276), comprising the third KPI domain and the highly basic C-terminus, is required for TFPI binding to HSPGs,9 hepatoma15 and endothelial cells,16 the low-density-lipoprotein receptor-related protein (LRP),12 and the very low density lipoprotein (VLDL) receptor.14 Interestingly, this C-terminal domain is also required for optimal anticoagulant activity of TFPI.17,18
Recently, we showed that inhibitors of the TF/fVIIa complex, including TFPI, have potent antitumor and antiangiogenesis activity. Surprisingly this activity is independent of the thrombotic activity of TF/fVIIa.19 Previously, we reported that TFPI specifically inhibits the growth of endothelial cells in vitro, while having no inhibitory activity on several tumor cell lines tested.14 This growth inhibitory activity was also independent of its anticoagulant activity because TF is not expressed on quiescent human umbilical vein endothelial cells (HUVECs). These in vivo and in vitro data suggest that TFPI and the TF/fVIIa complex have additional nonhemostatic activities. In vitro, TFPI inhibited endothelial cell proliferation through a previously uncharacterized interaction with the VLDL receptor, and molecules that inhibited TFPI binding to the VLDL receptor blocked the antiproliferative activity of TFPI.14 To extend these findings, we sought to identify fragments of TFPI that mediate binding to the VLDL receptor and also mediate its antiproliferative and antitumor activity.
Here we describe the characterization of a 23-amino acid peptide corresponding to the basic carboxyl terminus of TFPI (TFPIc23), which inhibits endothelial cell proliferation through an apoptotic mechanism, but has no effect on tumor cell proliferation in vitro. As with TFPI, antibodies blocking TFPIc23 binding to the VLDL receptor also block the growth inhibitory activity of this peptide. We extended these findings by showing that TFPIc23 is also a potent inhibitor of angiogenesis in vivo. A smaller fragment of the TFPIc23 peptide retained VLDL-binding capacity but was devoid of growth inhibitory activity. Finally, we demonstrate that the TFPIc23 peptide inhibits metastatic tumor growth. These data demonstrate a novel way in which TFPI may control tumor growth and angiogenesis independent of its hemostatic activity. Additionally, the association between TFPI and the VLDL receptor may represent an important mechanism by which angiogenesis, and ultimately tumor growth, are regulated.
Materials and methods
Materials
TFPI carboxyl-terminal peptide, KTKRKRKKQRVKIAYEEIFVKNM, corresponding to residues 254 to 276 of human TFPI, a scrambled peptide, NFQRKEKREVIYKVKTKIKAKMR, and 2 hemipeptides, KTKRKRKKQRVK (TFPIc23A) and IAYEEIFVKNM (TFPIc23B), were synthesized by Infinity (Astor, PA). HUVECs, endothelial cell basal medium (EBM), and endothelial cell growth medium (EGM) were purchased from Clonetics (San Diego, CA). B16BL6 melanoma cells were obtained from the National Cancer Institute-Central Repository (Frederick, MD). Lewis lung carcinoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA). Dulbecco modified Eagle medium and additives were obtained from BioWhittaker (Walkerville, MD). Basic fibroblast growth factor (bFGF) was purchased from R&D Systems (Minneapolis, MN). The cell proliferation enzyme-linked immunosorbent assay (ELISA) bromodeoxyuridine (BrdU) kit was from Boehringer Mannheim (Indianapolis, IN). C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Cell proliferation assays
Proliferation studies were performed using a BrdU incorporation kit as described by the manufacturer. Briefly, quiescent HUVECs were stimulated with either bFGF (5 ng/mL) or vascular endothelial growth factor (VEGF; 10 ng/mL) and grown for 48 hours in the presence of various competitors. Tumor cells were cultured for 48 hours in serum-free medium, then stimulated by addition of culture medium plus 10% fetal bovine serum (FBS). Experiments to block the antiproliferative activity of the TFPI peptide were performed in the presence of 2 μM of the rabbit polyclonal anti-VLDLr IgG (R4522),14 which was preincubated with HUVECs for 1 to 2 hours at 37°C before addition of TFPIc23 peptide. Similar experiments were performed using vascular smooth muscle cells (vSMCs; Clonetics). In these studies, growth was stimulated with complete media supplemented with platelet-derived growth factor (PDGF; 10 ng/mL), to which was added TFPIc23, TFPIc23 scrambled peptide, or buffer control. Proliferation was quantified by counting cell number after 48 hours.
VLDL receptor-binding experiments
The interaction of the TFPIc23 peptide with the VLDL receptor was characterized by binding 125I-TFPIc23 peptide to immobilized bovine serum albumin (BSA) or sVLDLr1-8 (the ligand-binding region of the VLDL receptor characterized in Hembrough et al14 ) in the presence of increasing amounts of unlabeled peptide. sVLDLr1-8-coated wells were incubated overnight at 4°C with 1 μM 125I-TFPIc23 peptide in the absence or presence of increasing amounts of unlabeled peptide. After incubation and rinsing, bound 125I-TFPIc23 peptide was measured using a γ counter. In all solid-phase binding experiments the data represent triplicate determinations. Similar experiments were performed where sVLDLr1-8-coated plates were incubated with 1 nM receptor-associated protein (RAP) in the presence of increasing concentrations of TFPIc23 peptide. Control binding was determined in the absence of TFPIc23 peptide. Bound RAP was detected by ELISA, using a rabbit polyclonal antibody. Binding experiments were also performed using 125I-RAP in the presence of increasing concentrations of the TFPIc23 hemipeptides. In these experiments RAP binding was quantified, after washing, in a γ counter. Cellular binding of 125I-TFPIc23 was assessed on HUVECs and B16 melanoma cells by adding 50 μM 125I-TFPIc23 to cells in the presence of either 10 nM RAP or a 10 μM polyclonal mouse anti-VLDL receptor antibody. Cells were incubated for 1 hour at 37°C, then washed. Bound 125I-TFPIc23 was released from the cell surface by proteinase K-trypsin treatment and measured by counting in a γ counter.
Apoptosis assays
HUVECs were seeded at a density of 2.7 to 5.4 × 103 cells/cm2 in EGM and allowed to attach for at least 12 hours. The medium was aspirated, and then the cells were briefly rinsed with EBM-2 medium containing 0.1% BSA. Cells were then incubated in EBM-2/BSA alone, EBM-2/BSA supplemented with 5 ng/mL FGF-2, or EGM. Plates were then treated with 40 μM TFPIc23, 40 μM TFPIc23A, 40 μM TFPIc23B, or an equal volume of phosphate-buffered saline (PBS). After 24 or 48 hours of incubation, the detached, floating cells were collected and combined with the adherent cells recovered by trypsin treatment. The cells were pelleted, briefly washed with PBS, pelleted again, and then resuspended in PBS containing 25 μg/mL propidium iodide (Sigma, St Louis, MO), 0.3% saponin (Sigma), 5 mM EDTA (ethylenediaminetetraacetic acid), and 50 μg/mL DNase-free RNase (Sigma). Apoptosis was quantified by calculating the percentage of hypodiploid cells as measured by flow cytometry analysis. Apoptosis was also assessed by Western blot analysis of the levels of caspase-3 activation in cell lysates of HUVECs treated with increasing concentrations of the TFPIc23 peptide. Activated caspase-3 was blotted with a rabbit polyclonal antibody (Asp175) from Cell Signaling Technology (Beverly, MA).
Endothelial spheroid sprouting assay.
The endothelial spheroid described previously20,21 was modified as follows. Briefly, spheroidal endothelial cell aggregates suspended in neutralized collagen were layered on top of polymerized rat tail collagen in 96-well plates. After polymerization at 37°C for 1 hour, 2 to 8 spheroids are trapped in between the 2 collagen layers where they can be viewed in one focal plane. RPMI media containing 10% fetal calf serum (FCS), bFGF, and the experimental compounds are layered on top resulting in a final concentration of 30 ng/mL bFGF. The spheroids were incubated at 37°C at 5% CO2 and evaluated 24 hours later. Experiments were done in quadruplicate. For quantification, the average cumulative length of sprouts per spheroid was determined.
Chick CAM assay
Egg hammocks were fashioned by fixing a square of plastic wrap over an open plastic tube (8 cm diameter, 6 cm tall) with a ring. Fertilized eggs (3 days old) were cracked open, and the contents removed to the egg hammock and manipulated so the embryo was accessible on the surface of the egg. A Petri dish lid was placed on top of the hammock to maintain sterility. Embryos were maintained in a 37°C humidified incubator. Methyl cellulose disks were formed by drying 50 μL 2% methyl cellulose on a Petri dish overnight in a hood. Disks were peeled from the dish and placed over the chorioallantoic membrane (CAM), and then test compounds were added on the disks. CAM images were captured and quantified with Image-Pro-Plus system (Media Cybernetics, Carlsbad, CA).
Matrigel plug assay
Groups of 10 animals were injected with 0.5 mL Matrigel (Collaborative Research, Bedford, MA) to which bFGF (final concentration 2 μg/mL) was added. This mixture was then injected subcutaneously at the ventral midline, posterior to the xiphoid process. Animals were treated daily with TFPIc23 or PBS intraperitoneally. After 6 days, animals were humanely killed with CO2. The Matrigel plug was removed, weighed, and frozen after addition of 1 mL water. Angiogenesis was quantified by measuring hemoglobin within the plug. For this, the plug was homogenized and centrifuged at 20 000g for 20 minutes, and the amount of hemoglobin was quantified in the supernatant using the Sigma hemoglobin kit (527-A). Negative angiogenesis controls were assessed in animals given injections with Matrigel lacking bFGF.
Treatment of experimental pulmonary tumor metastasis
Groups of 5 C57BL/6J mice were inoculated with 5 × 104 Lewis lung carcinoma cells by intravenous injection through the tail vein and subsequently treated intraperitoneally with various concentrations of either the TFPI peptide, a scrambled peptide control, or equivalent volume of diluent. Treatment was initiated 3 days after tumor cell inoculation. After 11 days of treatment the mice were killed, autopsied, and the lungs removed and weighed. Results were analyzed for statistical significance using the Student t test.
Results
The carboxyl-terminal basic domain of TFPI inhibits proliferation of HUVECs
We have previously demonstrated that TFPI is a potent inhibitor of endothelial cell proliferation in vitro14 and that TFPI exhibits antitumor activity in vivo. To better define these activities, we sought to identify the domains within TFPI that were responsible for these activities. Based on numerous reports suggesting that the highly basic carboxyl terminus of TFPI mediates cell surface binding, we evaluated whether this fragment contained the domain responsible for the antiproliferative activity of TFPI.
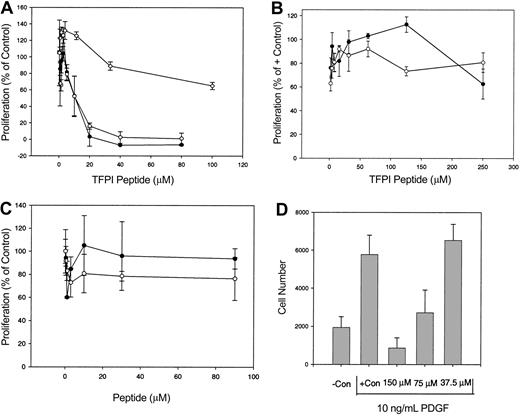
To characterize the C-terminal domain of TFPI, a synthetic peptide corresponding to the carboxy-terminal 23 amino acids (TFPIc23), was analyzed in a HUVEC proliferation assay. TFPIc23 was a potent inhibitor of HUVEC proliferation, exhibiting dose-dependent inhibitory activity in cells stimulated by either bFGF or VEGF (Figure 1A), as well as in cells grown in complete medium (data not shown). The concentration that inhibits 50% (IC50) for cells stimulated with these growth factors was 5 μM, and at 20 μM concentration or greater inhibition was complete. A randomly scrambled TFPIc23 peptide had no antiproliferative activity (Figure 1A). The growth inhibitory activity of the TFPIc23 peptide was also assessed on several other VLDL receptor-positive cell lines. On 2 of these, the tumor cell lines, B16 melanoma and EOMA hemangioendothelioma, TFPIc23 was unable to inhibit the cell proliferation at doses up to 250 μM (Figure 1B). A previous report demonstrated that TFPI can inhibit human vSMC proliferation.22 Because vSMCs also express the VLDL receptor, we next assessed whether the TFPIc23 peptide could inhibit vSMC proliferation. In these studies, we found that the proliferation of human aortic vSMCs stimulated with 10 ng/mL PDGF (Figure 1D) was inhibited by the TPFIc23 peptide (IC50 = 75 μM). Cells stimulated with complete medium were also inhibited, whereas cells were unaffected by the scrambled TFPIc23 peptide (data not shown).
Effect of TFPIc23 on cell proliferation. (A) Quiescent HUVECs were stimulated with either bFGF (○) or VEGF (•) in the presence of increasing amounts of the TFPIc23 peptide. BFGF-stimulated HUVECs were also treated with a scrambled TFPIc23 peptide (⋄). Cell proliferation was assessed by quantifying BrdU incorporation in triplicate for each point. Data, presented as percent of growth factor-stimulated HUVECs in the absence of inhibitor, are means ± SD. (B) EOMA hemangioendothelioma (•) and B16 melanoma (○) cells growing in the presence of FBS were treated with increasing amounts of TFPIc23. Cell proliferation was assessed 48 hours later by measuring BrdU incorporation in triplicate for each point. Data are presented as means ± SD. (C) Quiescent HUVECs were stimulated with 10 ng/mL bFGF in the presence of increasing amounts of TFPIc23A (○) and TFPIc23B (•). Cell proliferation was quantified 48 hours later by measuring BrdU incorporation in triplicate for each point. Data are presented as means ± SD. (D) Quiescent human aortic vSMCs were stimulated with 10 ng/mL PDGF, in the presence of increasing amounts of the TFPIc23 peptide. Cell proliferation was measured by cell counts 48 hours later. Data are presented as means ± SD.
Effect of TFPIc23 on cell proliferation. (A) Quiescent HUVECs were stimulated with either bFGF (○) or VEGF (•) in the presence of increasing amounts of the TFPIc23 peptide. BFGF-stimulated HUVECs were also treated with a scrambled TFPIc23 peptide (⋄). Cell proliferation was assessed by quantifying BrdU incorporation in triplicate for each point. Data, presented as percent of growth factor-stimulated HUVECs in the absence of inhibitor, are means ± SD. (B) EOMA hemangioendothelioma (•) and B16 melanoma (○) cells growing in the presence of FBS were treated with increasing amounts of TFPIc23. Cell proliferation was assessed 48 hours later by measuring BrdU incorporation in triplicate for each point. Data are presented as means ± SD. (C) Quiescent HUVECs were stimulated with 10 ng/mL bFGF in the presence of increasing amounts of TFPIc23A (○) and TFPIc23B (•). Cell proliferation was quantified 48 hours later by measuring BrdU incorporation in triplicate for each point. Data are presented as means ± SD. (D) Quiescent human aortic vSMCs were stimulated with 10 ng/mL PDGF, in the presence of increasing amounts of the TFPIc23 peptide. Cell proliferation was measured by cell counts 48 hours later. Data are presented as means ± SD.
Several reports have demonstrated that protamine, a short poly-basic peptide, has antiproliferative activity on HUVECs.23,24 Because the first 13 amino acids of TFPIc23 are highly basic, we also assessed the activity of 2 hemipeptides corresponding to amino acids 1 to 12 (TFPIc23A) and 13 to 23 (TFPIc23B) of TFPIc23. Neither of these peptides inhibited the growth of HUVECs stimulated with bFGF (Figure 1C) or serum (data not shown), suggesting that the activity of TFPIc23 is not solely mediated though its poly-basic region.
TFPIc23 is a ligand for the VLDL receptor
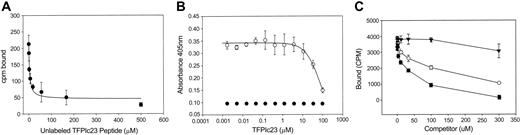
Because the antiproliferative activity of the parent protein, TFPI, is mediated by its interaction with the VLDL receptor, we next assessed whether the inhibitory activity of TFPIc23 also required interaction with the VLDL receptor. We first sought to determine whether TFPIc23 was a ligand for this receptor. In ligand-binding experiments, 125I-labeled TFPIc23 peptide is added to immobilized sVLDLr1-8 (the soluble ligand-binding domains of the VLDL receptor, described in Hembrough et al14 ), and binding is competed by addition of increasing amounts of cold TFPIc23 peptide. In this experiment (Figure 2A) excess unlabeled peptide inhibited the binding of 125I-labeled TFPIc23 peptide to immobilized sVLDLr1-8 and a Kd of 3.5 μM was calculated for this interaction. This binding was confirmed in a competition experiment (Figure 2B), where the TFPIc23 peptide was used to block the binding of RAP to the sVLDLr1-8. RAP is a high affinity (Kd < 1 nM) ligand for the VLDL receptor and most other members of the LDL receptor family.25,26 In this experiment, increasing concentrations of the TFPIc23 peptide blocked the binding of RAP to immobilized sVLDLr1-8 (Ki = 1.2 μM). To further define the domain responsible for the binding of this peptide, we assessed the VLDL receptor-binding activity of the 2 hemipeptides of TFPIc23. As shown in Figure 2C, the TFPIc23A hemipeptide was a ligand for the VLDL receptor, though with diminished affinity compared to the parental TFPIc23 peptide. In contrast, the TFPIc23B peptide did not bind to the VLDL receptor (Figure 2C).
Characterization of TFPIc23 binding to the VLDL receptor. (A) VLDL receptor-coated plates were incubated with 125I-labeled TFPIc23 peptide plus increasing concentrations of unlabeled TFPIc23. Samples were incubated overnight at 4°C and washed, and bound TFPIc23 was measured in a γ counter. Each point is the mean of 3 wells ± SEM. (B) VLDL receptor was immobilized in microtiter plates, and the binding of RAP was assessed in the presence of increasing concentrations of the TFPIc23 peptide (○). After rinsing, bound RAP was quantified by ELISA using a RAP-specific rabbit polyclonal antibody. Control binding of RAP to BSA-coated wells was negative (•). (C) VLDL receptor was immobilized in microtiter plates, and the binding of 125I-RAP was assessed in the presence of increasing concentrations of either TFPIc23 (•) or the hemipeptides TFPIc23A (○) and TFPIc23B (▾). RAP binding was quantified by using a γ counter.
Characterization of TFPIc23 binding to the VLDL receptor. (A) VLDL receptor-coated plates were incubated with 125I-labeled TFPIc23 peptide plus increasing concentrations of unlabeled TFPIc23. Samples were incubated overnight at 4°C and washed, and bound TFPIc23 was measured in a γ counter. Each point is the mean of 3 wells ± SEM. (B) VLDL receptor was immobilized in microtiter plates, and the binding of RAP was assessed in the presence of increasing concentrations of the TFPIc23 peptide (○). After rinsing, bound RAP was quantified by ELISA using a RAP-specific rabbit polyclonal antibody. Control binding of RAP to BSA-coated wells was negative (•). (C) VLDL receptor was immobilized in microtiter plates, and the binding of 125I-RAP was assessed in the presence of increasing concentrations of either TFPIc23 (•) or the hemipeptides TFPIc23A (○) and TFPIc23B (▾). RAP binding was quantified by using a γ counter.
We also confirmed that the TFPIc23 peptide was a ligand for cell surface VLDL receptor on several cell lines. In these studies, radioiodinated TFPIc23 was incubated with HUVECs and B16 melanoma cells in the presence and absence of RAP or polyclonal mouse anti-VLDL receptor antibody. VLDL receptor binding was defined as that component of binding that is blocked by coincubation with RAP or antibody. In these competition experiments, we found that cell surface binding of 125I-TFPIc23 is reduced 10% to 20% by the addition of either VLDL receptor-binding antagonist (data not shown). The remainder of the cell surface TFPIc23 binding is most likely mediated by cell surface proteoglycans.9-11 Therefore, we conclude that TFPIc23 is a ligand for cell surface-expressed VLDL receptor, even on cells such as B16 melanoma cells, which are insensitive to the growth inhibitory activity of TFPIc23.
VLDL receptor binding is required for TFPIc23 antiproliferative activity
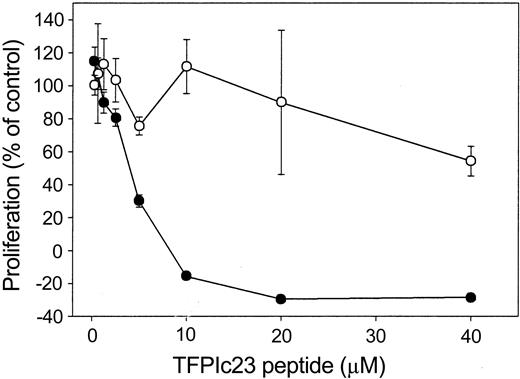
Because we demonstrated that the TFPIc23 peptide binds to the VLDL receptor, we next sought to determine whether this interaction with the VLDL receptor was necessary for its growth inhibitory activity. This was determined by using a specific antibody against the VLDL receptor (R4522) that blocks ligand binding.14 Preincubation of HUVECs with R4522 effectively inhibits the antiproliferative activity of TFPIc23, whereas the antibody alone had no effect on HUVEC proliferation, either as a stimulator or as an inhibitor (Figure 3). These data further suggest that the growth inhibitory activity of TFPIc23 is mediated through a VLDL receptor-dependent mechanism.
Effect of VLDL receptor antibody on TFPIc23 antiproliferative activity. Quiescent HUVECs were preincubated for 2 hours at 37°C with a VLDL receptor-specific polyclonal antibody (R4522; ○) or an equal volume of buffer (•). After incubation, 10 ng/mL bFGF was added with increasing concentrations of the TFPIc23 peptide. Cell proliferation was assessed 48 hours later by measuring BrdU incorporation in triplicate wells. Data are presented as means ± SEM.
Effect of VLDL receptor antibody on TFPIc23 antiproliferative activity. Quiescent HUVECs were preincubated for 2 hours at 37°C with a VLDL receptor-specific polyclonal antibody (R4522; ○) or an equal volume of buffer (•). After incubation, 10 ng/mL bFGF was added with increasing concentrations of the TFPIc23 peptide. Cell proliferation was assessed 48 hours later by measuring BrdU incorporation in triplicate wells. Data are presented as means ± SEM.
TFPIc23 induces apoptosis in HUVECs
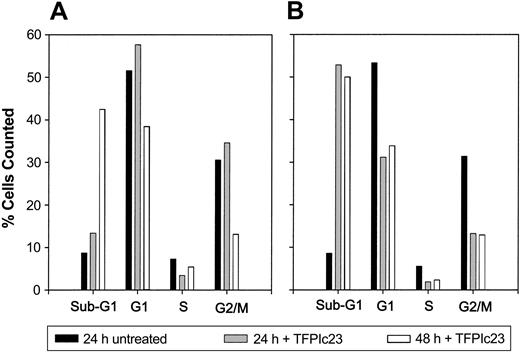
TFPI inhibits HUVEC proliferation and has also been shown to cause DNA fragmentation, indicative of apoptosis.27 Therefore, we sought to assess whether the antiproliferative activity of the TFPIc23 peptide on HUVECs was also the result of apoptosis. HUVECs stimulated with either bFGF or complete medium were treated with 10 μM TFPIc23 for various lengths of time and then analyzed. Cell cycle analysis in Figure 4 shows that treatment with TFPIc23 induces a large spike in sub-G1 cells, consistent with DNA fragmentation generated through apoptosis. Although similar results are obtained with bFGF and complete medium, the apoptotic effect is faster and more pronounced in cells growing in complete medium. At 24 hours the sub-G1 population is 15% with bFGF versus 51% for complete medium. When similar experiments were performed using the 2 TFPIc23 hemipeptides, neither of these peptides alone or in combination induced apoptosis (data not shown). Induction of apoptosis was confirmed by assessing the activation of caspase-3 by Western blotting. In these studies, incubation of HUVECs with the TFPIc23 peptide resulted in a dramatic increase in the amount of active of caspase-3 (data not shown). Together these data suggest that the antiproliferative activity of the TFPIc23 is mediated through an apoptotic pathway.
TFPIc23 induction of apoptosis in HUVECs. Quiescent HUVECs were stimulated with either 10 ng/mL bFGF (A) or complete serum (B) in the presence of 40 μM TFPIc23 or equal volume of PBS. After 24 or 48 hours, cells were fixed and sorted into sub-G1, G1, S, and G2/M groups on the basis of propidium iodide incorporation.
TFPIc23 induction of apoptosis in HUVECs. Quiescent HUVECs were stimulated with either 10 ng/mL bFGF (A) or complete serum (B) in the presence of 40 μM TFPIc23 or equal volume of PBS. After 24 or 48 hours, cells were fixed and sorted into sub-G1, G1, S, and G2/M groups on the basis of propidium iodide incorporation.
Characterization of TPFIc23 activity in sprouting, angiogenesis, and tumor models
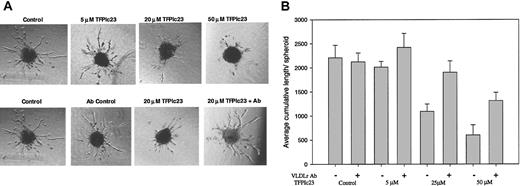
The apoptotic activity of the TFPIc23 peptide on cultured endothelial cells suggests that this peptide could act as an antiangiogenic agent. This was initially assessed in vitro by using a modified endothelial cell spheroid assay, in which endothelial cell spheroids treated with bFGF develop a large number of tubulelike sprouts emerging from its surface.19,20 In this assay, the TFPIc23 peptide exhibited a dose-dependent inhibitory activity (Figure 5A top panels) with nearly complete ablation of sprout outgrowth above 50 μM. A scrambled TFPIc23 peptide had no activity in this assay (data not shown). We next assessed whether the VLDL receptor played a role in the antisprouting activity by preincubating spheroids with 2 μM R4522. Pretreatment with R4522 blocks the inhibitory activity of the TFPIc23 in this assay (Figure 5A bottom panels), whereas the VLDL receptor antibody alone had no effect on tubule growth. The quantification of the data in Figure 5B demonstrates that there is dose dependency for TFPIc23 inhibitory activity. In addition, the data show that antibody R4522 completely abrogates the inhibitory activity (P < .01) of lower doses of the TFPIc23 peptide, and even at 50 μM TFPIc23, the highest dose tested, the antibody reduced TFPIc23 inhibition from 83% to 40% (Figure 5B).
Effect of TFPIc23 on endothelial spheroid vessel outgrowth. (A) Endothelial cell spheroids were suspended in collagen, and bFGF was added to induce vessel outgrowth. Increasing amounts of TFPIc23 were added with the bFGF, and vessel growth was assessed 24 hours later. Control spheroids and spheroids grown in the presence of 5 μM, 20 μM, and 50 μM TFPIc23 are shown in the top panels. The bottom panels show the effect of VLDL receptor antibody assessed by preincubating spheroids with R4522 and then treating with TFPIc23. Control spheroids were treated with bFGF and R4522 and bFGF alone. Original magnification × 100. (B) Quantification of spheroid tubule outgrowth. The effects of increasing amounts of TFPIc23 in the presence or absence of the VLDL receptor antibody R4522 were quantified by measuring the total length of all vessels from each spheroid (2-8 spheroids/well).
Effect of TFPIc23 on endothelial spheroid vessel outgrowth. (A) Endothelial cell spheroids were suspended in collagen, and bFGF was added to induce vessel outgrowth. Increasing amounts of TFPIc23 were added with the bFGF, and vessel growth was assessed 24 hours later. Control spheroids and spheroids grown in the presence of 5 μM, 20 μM, and 50 μM TFPIc23 are shown in the top panels. The bottom panels show the effect of VLDL receptor antibody assessed by preincubating spheroids with R4522 and then treating with TFPIc23. Control spheroids were treated with bFGF and R4522 and bFGF alone. Original magnification × 100. (B) Quantification of spheroid tubule outgrowth. The effects of increasing amounts of TFPIc23 in the presence or absence of the VLDL receptor antibody R4522 were quantified by measuring the total length of all vessels from each spheroid (2-8 spheroids/well).
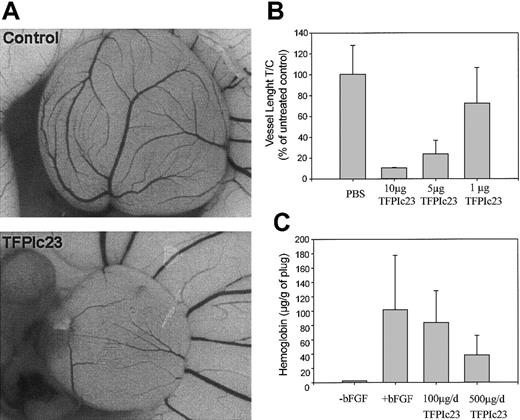
Because TFPIc23 exhibited inhibitory activity in 2 in vitro models of angiogenesis, we next assessed its activity in 2 in vivo angiogenesis models: the chick CAM assay28 and the mouse Matrigel plug assay.29 TFPIc23 significantly inhibited the growth of blood vessels in the chick CAM assay (Figure 6A). The TFPIc23-treated CAM is significantly smaller and less vascularized than the control. Quantitation of the total length of the vessels revealed that TFPIc23 inhibited blood vessel growth in a dose-dependent manner (Figure 6B), with an inhibition more than 90% at 10 μg/disk, which was the highest dose tested in this assay. Similar inhibition was seen in the mouse Matrigel plug assay after 6 days of treatment with 100 or 500 μg/d TFPIc23 (Figure 6C). As in all previous assays, TFPIc23 showed a dose-dependent response, with maximal inhibition of 65% at a daily dose of 500 μg (P < .05).
Inhibition of in vivo angiogenesis by TFPIc23. (A) Chick CAMs were treated with either PBS or TFPIc23 for 24 hours. TFPIc23 CAMs were uniformly smaller and less well developed than untreated control CAMs. Original magnification × 10. (B) Quantification of the effect of increasing doses of TFPIc23 on chick CAM development. Labeled doses of TFPIc23 were placed on a methyl cellulose disk on top of the CAM. Results are averages ± SD of 4 CAMs/group. (C) Inhibition of in vivo Matrigel plug angiogenesis by TFPIc23. Mice were implanted with Matrigel plugs and treated for 6 days. After treatment, Matrigel plugs were removed and hemoglobin content was measured. Results are average ± SD of 8 plugs.
Inhibition of in vivo angiogenesis by TFPIc23. (A) Chick CAMs were treated with either PBS or TFPIc23 for 24 hours. TFPIc23 CAMs were uniformly smaller and less well developed than untreated control CAMs. Original magnification × 10. (B) Quantification of the effect of increasing doses of TFPIc23 on chick CAM development. Labeled doses of TFPIc23 were placed on a methyl cellulose disk on top of the CAM. Results are averages ± SD of 4 CAMs/group. (C) Inhibition of in vivo Matrigel plug angiogenesis by TFPIc23. Mice were implanted with Matrigel plugs and treated for 6 days. After treatment, Matrigel plugs were removed and hemoglobin content was measured. Results are average ± SD of 8 plugs.
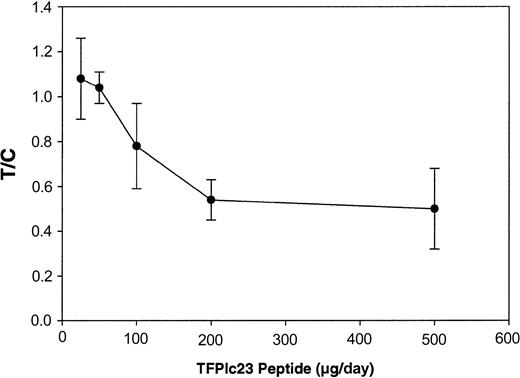
It is now well established that beyond a certain threshold in size, tumor growth is angiogenesis dependent, and many angiogenesis inhibitors exhibit antitumor activity.30-32 Because TFPIc23 demonstrated potent antiangiogenic activity, we next investigated its effects in the highly vascularized mouse Lewis lung carcinoma experimental metastasis model. Under our experimental conditions this model is a tumor growth model because treatment is initiated on the third day following intravenous inoculation, after tumor cells have homed to the lungs. In this model, treated animals show fewer and smaller metastatic foci, which are quantified by comparing the lung mass of treated animals with control animals. In this way we specifically assess the effect of TFPIc23 on the growth of the metastatic tumors in situ and not on the early coagulation-dependent steps of metastasis. TFPIc23 inhibits tumor growth in a dose-dependent manner, with maximal activity observed at 200 μg/d (P < .01; Figure 7). No further decrease in tumor growth was observed at 500 μg/d (P < .01), the highest dose tested. In similar studies, the scrambled TFPIc23 peptide demonstrated no antitumor activity at 500 μg/d (data not shown). In each of these in vivo studies, treatment of animals with the TFPIc23 peptide did not result in any overt hemostatic defects, and autopsy of treated animals confirmed this observation.
Inhibition of Lewis lung carcinoma metastatic tumor growth by TFPIc23. C57/BL6J mice injected with 5 × 104 tumor cells through the tail vein were treated with increasing doses of TFPIc23 intraperitoneally daily. After 11 days of treatment, tumor-bearing lungs were removed and weighed. Data are presented as the ratio of lung weight gain in animals treated with TFPIc23 (T) to the lung weight gain in control animals treated with PBS (C), ± standard error.
Inhibition of Lewis lung carcinoma metastatic tumor growth by TFPIc23. C57/BL6J mice injected with 5 × 104 tumor cells through the tail vein were treated with increasing doses of TFPIc23 intraperitoneally daily. After 11 days of treatment, tumor-bearing lungs were removed and weighed. Data are presented as the ratio of lung weight gain in animals treated with TFPIc23 (T) to the lung weight gain in control animals treated with PBS (C), ± standard error.
Discussion
The processes of coagulation and tumor growth are closely linked,33 because a significant percentage of patients with cancer develop hemostatic disorders, including deep vein thrombosis and migratory thromboemboli.34,35 Whether this hypercoagulable environment supports tumor growth or if it is the result of a response intended to restrict tumor growth is not completely understood. Our previous work demonstrated that inhibitors of the TF/fVIIa complex, including TFPI, are potent inhibitors of tumor growth and angiogenesis. Because inhibitors of fXa had no antitumor activity, we hypothesize that TFPI has additional important and novel biologic roles independent of its role as an anticoagulant. Our in vitro studies suggest that the interaction of TFPI with the VLDL receptor is part of the mechanism mediating its antiangiogenic and antitumor roles. Our characterization, in this report, of a VLDL receptor-binding domain of TFPI that mimics the antiproliferative and antitumor activity of TFPI further supports the concept of a new role and mechanism whereby TFPI can regulate angiogenesis and tumor growth independently of its established role in hemostasis.
We demonstrate, through the use of 2 different in vitro models, that the inhibitory activity of the TFPIc23 peptide requires interaction with the VLDL receptor. In addition, experiments with the 2 TFPIc23 hemipeptides show that although the TFPIc23A peptide binds to the VLDL receptor neither hemipeptide has any growth inhibitory or apoptotic activity, indicating that association with the VLDL receptor is not sufficient for activity. Moreover, cotreatment of cells with both hemipeptides did not inhibit endothelial proliferation or induce apoptosis, showing that this activity requires the intact TFPIc23 molecule. It should be noted that the IC50 for TFPIc23 (5 μM) is significantly greater than that of the parental TFPI molecule (250 nM). This discrepancy suggests that other TFPI sequences also play a role in mediating its apoptotic activity.
It is interesting that several cell lines that express the VLDL receptor are insensitive to the growth inhibitory activity of the TFPIc23 peptide. We have demonstrated through the use of radiolabeled TFPIc23 peptide that the VLDL receptor on these B16 melanoma cells is competent to bind the TFPIc23 peptide, even though the peptide has no activity on these cells. Thus, it appears that peptide binding to the VLDL receptor is not, in itself, sufficient to induce the apoptotic activities of the receptor. This is consistent with our data using the TFPIc23A hemipeptide, which avidly binds to the VLDL receptor in the in vitro assays, yet lacks growth inhibitory activity on HUVECs. Furthermore, other ligands of the VLDL receptor, including RAP, have no antiproliferative activity. These data suggest that there may be one or more associated proteins or cell surface receptors that are required for its apoptotic activity and whose expression may be more narrow than the expression of the VLDL receptor.
Although the LDL receptor family has traditionally been characterized as a group of endocytic receptors, many recent reports have demonstrated how LDL receptor family members could be also involved in cell signaling processes.36-38 A recent paper by Shirotani-Ikejama et al39 used DNA array technology to probe whether TFPI inhibition of endothelial proliferation was mediated through changes in gene expression and whether the VLDL receptor played a role in these changes. They reported that treatment of HUVECs with TFPI transiently induces mRNA expression of JUNB and GADD45B. Furthermore, they demonstrated that this gene induction is dependent on VLDL receptor binding, because blocking TFPI binding to the VLDL receptor by coincubation with RAP abrogates the response. It is interesting to note that the binding of RAP to the VLDL receptor does not stimulate gene expression. This result is consistent with our data showing that ligand binding to the VLDL receptor is not sufficient to induce antiproliferative effects.
In addition to binding the VLDL receptor, the C-terminal tail of TFPI is required for optimal anticoagulant activity.17,18 In the absence of this domain, TFPI has much lower affinity for fXa and lower activity against the TF/fVIIa complex. Interestingly, a recent report has demonstrated that thrombin proteolysis can inactivate the anticoagulant activity of TFPI, by liberating a 22-amino acid fragment from the extreme carboxyl terminus.40 Our preliminary studies show that this 22-mer, contained within TFPIc23, is also potent inhibitor of endothelial cell proliferation (data not shown).
Our previous work demonstrated that inhibitors of the TF/fVIIa complex, including TFPI, have antitumor and antiangiogenic activity. In this report, we demonstrate that an antiproliferative, antiangiogenic, and antitumor activity is localized in TFPI to a short, VLDL receptor-binding sequence found in its carboxyl terminus. This activity is independent of the hemostatic activity of TFPI and represents a previously unrecognized nonhemostatic mechanism whereby TFPI can regulate tumor growth and angiogenesis. Taken together our data suggest that TFPI regulates vascular biology through 2 separate mechanisms, first through inhibition of the TF/fVIIa complex, and second through its interaction with the VLDL receptor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-07-2234.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal