Abstract
The transcription factor TAL1 has major functions during embryonic hematopoiesis and in adult erythropoiesis and megakaryocytopoiesis. These functions rely on different TAL1 structural domains that are responsible for dimerization, transactivation, and DNA binding. Previous work, most often done in mice, has shown that some TAL1 functions do not require DNA binding. To study the role of TAL1 and the relevance of the TAL1 DNA-binding domain in human erythropoiesis, we developed an approach that allows an efficient enforced wild-type or mutant TAL1 protein expression in human hematopoietic CD34+ cells using a lentiviral vector. Differentiation capacities of the transduced cells were studied in a culture system that distinguishes early and late erythroid development. Results indicate that enforced TAL1 expression enhances long-term culture initiating cell (LTC-IC) potential and erythroid differentiation of human CD34+ cells as shown by increased βglobin and porphobilinogen deaminase (PBGD) gene expressions and erythroid colony-forming units (CFU-Es), erythroid burst-forming units (BFU-Es), and glycophorin A-positive (GPA+) cell productions. Enforced expression of a TAL1 protein deleted of its DNA-binding domain (named ΔbTAL1) mimicked most TAL1 effects except for the LTC-IC enhancement, the down-regulation of the CD34 surface marker, and the GPA+ cell production. These results provide the first functional indications of DNA-binding-dependent and -independent roles of TAL1 in human erythropoiesis. (Blood. 2004;103:3326-3335)
Introduction
Hematopoiesis is the process that leads to the generation of all mature blood cells. The cells that initiate this process lay in a small population, named hematopoietic stem cells (HSCs), that is subjected to various steps of differentiation, leading to the overall hematopoietic lineages. Several proteins are known to play a major role in the control of blood cell production, in particular the transcription factors.
The TAL1 (named also TCL-5 or SCL) protein belongs to the class II basic helix-loop-helix (bHLH) transcription factor family. These factors display a tissue-restricted expression and are known to play critical roles in regulating differentiation of many cell types,1 as shown for MYF5 or MYOD in muscles or neurogenin in the brain. TAL1 is expressed in the vascular and in the hematopoietic systems, especially in hematopoietic progenitors and in erythroid, megakaryocytic, and mast cell precursors,2,3 where it has been implicated at different steps of development and differentiation.1 TAL1 participates to the onset of primitive hematopoietic development. Tal1 null embryos fail to develop any hematopoietic cells4-6 and this failure can be rescued by expressing TAL1 using retroviral transduction before the onset of hematopoiesis.6 TAL1 is also important for the establishment of definitive hematopoiesis since Tal1 null mouse embryonic stem (ES) cells injected into blastocysts do not participate in any adult hematopoietic lineage, whereas their contribution to other tissues of the mouse body is normal.5,6 The role of TAL1 in the homeostasia of adult hematopoiesis has been recently investigated using a conditional TAL1 deletion model.7,8 Interestingly, deletion of Tal1 in adult bone marrow (BM) hematopoietic cells did not interfere with the reconstitution properties of hematopoietic stem cells and early progenitors such as colony-forming unit-spleen (CFU-S) in transplantation but impaired their erythrocytic and megakaryocytic differentiation capacities. These results strengthened previous findings on the major role that TAL1 plays during erythroid and megakaryocytic cell differentiation since both lineages were strongly impaired in the absence of TAL1 protein.7,8
The biologic activity of TAL1 relies on 2 important domains of the protein. The helix-loop-helix (HLH) domain is common to the HLH transcription factor family and allows its members to homo- or heterodimerize. Hence, this domain is crucial for the heterodimerization of TAL1 with the members of the ubiquitously expressed E2A proteins (ie, E47, E12, and HEB), which are main partners of TAL1.9 The basic domain is present in many HLH proteins where it confers binding to DNA on the E-box consensus sequence CANNTG and further transactivation of target genes.10 Mutations and deletions of these 2 domains and studies of the mutated TAL1 proteins in functional assays have indicated that the DNA-binding domain, but not the HLH dimerization domain, is dispensable for some TAL1 properties. The TAL1 protein lacking its capacity to bind to DNA can rescue some of the primitive and definitive hematopoietic potentials of Tal1-null mouse ES cells and of the zebrafish mutant cloche.11 Moreover, the same ΔbTAL1 mutant can induce T-cell neoplasia in transgenic mouse models.12
The molecular mechanisms of TAL1 functions have long been thought to be similar to the myogenic or neurogenic bHLH protein ones; that is, a direct positive (or negative) transcriptional regulation of target genes through DNA binding to consensus E-box sequences present in the regulating sequences of several genes.13 In vitro transcription assays have demonstrated that TAL1 can participate in large protein complexes that comprise E12, the LIM protein LMO-2, Ldb1, and GATA-1 or SP-1 in the erythroid differentiation or LMO-2 and E12/E47 or GATA-3 in T-cell acute lymphoblastic leukemia (T-ALL) cells. These complexes display transcriptional activity on promoters containing an E-box, an SP-1, or a GATA binding site.14-16 Finally, results showing that mutated TAL1 proteins have some functional activities have led to the emergence of a new concept of the molecular mechanisms by which TAL1 may act, such as the sequestration of a repressor or the titration of other proteins.11,12
The role of TAL1 during erythropoiesis is documented in the mouse,1 and recent experiments performed on human CD34+ cells have shown the major role of this transcription factor for human erythroid differentiation.16,17 However, these experiments used enforced TAL1 expression by oncoretroviral transduction and are limited by the levels of gene transfer obtained in the human primary hematopoietic cells using such vectors.
We have recently developed an approach to transduce efficiently the overall hierarchy of human hematopoietic cells using lentiviral TRIP vectors18,19 that avoid the selection of transduced cells. Using a 2-step culture system that reproduced the early and late erythroid differentiation,20 we found that enforced expression of TAL1 into CD34+ cord blood cells enhances erythroid differentiation, leading to 2 times more erythroid burst-forming units (BFU-Es), 10 to 20 times more erythroid colony-forming units (CFU-Es), and 2 times more glycophorin A-positive (GPA+) cells than in control cells. The long-term culture initiating cell (LTC-IC) compartment is also increased, with up to 3 times more clonogenic progenitors produced in the presence of high levels of TAL1. Part of this activity is independent of DNA binding since enforced expression of TAL1 deleted of its DNA-binding domain enhances CFU-E production and βglobin and erythroid porphobilinogen deaminase (ePBGD) expression at levels that are the same as those in the entire TAL1 protein. However, in the presence of ΔbTAL1, no increase in LTC-ICs is obtained, CD34+ cells are detected during an extended period of time in culture, and 2 times fewer GPA+ cells are produced in liquid culture compared with control cells. This indicates that DNA binding of TAL1 might be critical at some specific steps of human erythroid differentiation.
Materials and methods
Lentiviral vector
The lentiviral vector TRIPΔU3-EF1α-encoding enhanced green fluorescence protein (EGFP) has been described.21 The TAL1 and ΔbTAL1 coding sequences were cloned from the pBtKS-TAL1 and pBtKS-ΔbTAL1 plasmids into the pTRIPΔU3-EF1α vector plasmid where the EGFP cDNA has been taken off (Figure 1A).
Lentiviral constructs and enforced expression of TAL1 and ΔbTAL1 in human hematopoietic cells. (A) Structure of the lentiviral vector TRIPΔU3-EF1α encoding EGFP, TAL1, and ΔbTAL1 sequences under the control of the EF1α promoter. (B) Western blot analysis of TAL1 and ΔbTAL1 protein expression in Jurkat cell lines. Protein lysates were prepared from nontransduced Jurkat (JKT) cells (NT, lane 1), or JKT-L4 cells transduced with TRIP-EGFP (EGFP), TRIP-TAL1 (TAL1), or TRIP-ΔbTAL1 (ΔbTAL1) (lanes 2 to 5). Proteins were separated by SDS-PAGE and revealed with the anti-TAL1 2TL136 monoclonal Ab. (C) Gene transfer efficiency into cord blood-derived CD34+ cells. Transduced cells were assayed by flow cytometric analysis (histogram indicates percentage of EFGP-expressing cells; upper panel) or by PCR analysis for the detection of integrated TRIP-TAL1 provirus into genomic DNA extracted from individual colony-forming cell (CFC)-derived colonies (lower panel) recovered after 14 days in methylcellulose culture. Eight colonies are shown, of which one was negative for the vector integration. Controls are JKT-L4 cells nontransduced (-) or transduced (+) with TRIP-TAL1. (D) TAL1 and ΔbTAL1 expression into human CD34+ cells transduced with the TRIP vectors. Western blot was performed as described with proteins extracted from CD34+ cells after transduction. Blots were stripped and reprobed with anti-ERK1 polyclonal Ab as a control of the amount of proteins loaded onto the gel. Results are representative of 2 experiments.
Lentiviral constructs and enforced expression of TAL1 and ΔbTAL1 in human hematopoietic cells. (A) Structure of the lentiviral vector TRIPΔU3-EF1α encoding EGFP, TAL1, and ΔbTAL1 sequences under the control of the EF1α promoter. (B) Western blot analysis of TAL1 and ΔbTAL1 protein expression in Jurkat cell lines. Protein lysates were prepared from nontransduced Jurkat (JKT) cells (NT, lane 1), or JKT-L4 cells transduced with TRIP-EGFP (EGFP), TRIP-TAL1 (TAL1), or TRIP-ΔbTAL1 (ΔbTAL1) (lanes 2 to 5). Proteins were separated by SDS-PAGE and revealed with the anti-TAL1 2TL136 monoclonal Ab. (C) Gene transfer efficiency into cord blood-derived CD34+ cells. Transduced cells were assayed by flow cytometric analysis (histogram indicates percentage of EFGP-expressing cells; upper panel) or by PCR analysis for the detection of integrated TRIP-TAL1 provirus into genomic DNA extracted from individual colony-forming cell (CFC)-derived colonies (lower panel) recovered after 14 days in methylcellulose culture. Eight colonies are shown, of which one was negative for the vector integration. Controls are JKT-L4 cells nontransduced (-) or transduced (+) with TRIP-TAL1. (D) TAL1 and ΔbTAL1 expression into human CD34+ cells transduced with the TRIP vectors. Western blot was performed as described with proteins extracted from CD34+ cells after transduction. Blots were stripped and reprobed with anti-ERK1 polyclonal Ab as a control of the amount of proteins loaded onto the gel. Results are representative of 2 experiments.
Lentiviral vector supernatants
Vector particles were produced by transient calcium phosphate cotransfection of 293T cells.21 The viral titers measured on 293T cells were 2.6 × 1010/mL to 10 × 1010/mL using the TRIPΔU3-EF1α-encoding EGFP vector. This vector was further used as a transduction reference. P24 concentrations were 68 μg/mL to 1240 μg/mL for different vector-containing supernatant preparations. These resulted in multiplicities of infection (MOIs) of 1 to 12 for concentrations of the vector corresponding to 2500 ng viral P24/mL.
Collection and fractionation of hematopoietic CD34+ cells
Umbilical cord blood (UCB) samples were collected with the informed consent of the mothers, according to approved institutional guidelines. CD34+ cells were purified by immunomagnetic selection (Miltenyi Biotec, Paris, France).22 CD34+ cells (purity ≥ 80%) were used either immediately or after storage in liquid nitrogen.
Transduction protocols
Human CD34+ cells were plated at 1 × 106 cells/mL in serum-free medium (RMB00; Mabio, Tourcoing, France) in the presence of recombinant human (rhu) stem cell factor (SCF; 100 ng/mL; Amgen, Neuilly sur Seine, France), Flt3-ligand (FL; 100 ng/mL; Immunex, Seattle, WA), interleukin 3 (IL-3; 60 ng/mL; Novartis France, Rueil-Malmaison, France), and thrombopoietinmimetic peptide23 (TPOmp; 25 nM; Genosys Biotechnologies, St Quentin en Yvelines, France). Concentrated lentiviral vector particles were added at a concentration of 2500 ng/mL viral P24 twice at 24-hour intervals for a total of 72 hours as we recently described.19 Cells were then washed and cultured in conditions that support lymphoid and myeloid differentiation22 during 72 hours. EGFP expression in the CD34+ cell population was then analyzed by flow cytometry (FACScalibur; Becton Dickinson, Pont de Claix, France).
Jurkat-L4, a Jurkat subclone that expresses a mutant TAL1 protein deleted in its carboxy terminal domain24 , was transduced with the different vectors during 48 hours, in presence of a single dose of 350 ng/mL viral P24 in RPMI containing 10% fetal calf serum (FCS). Cells were then washed and established as cell lines in the same medium.
Western blot analysis
Western blot analysis was performed as previously described.24 Briefly, 5 × 105 transduced CD34+-derived cells, Jurkat, Jurkat-L4, or Jurkat-L4-transduced cells were pelleted and lysed into Laemmli buffer (60 mM Tris-HCL, pH 6.8; 5% 2 beta-mercaptoethanol; 2% sodium dodecyl sulfate [SDS]; 15% glycerol) and total protein extracts were run onto a 10% SDS-polyacrylamide gel electrophoresis (PAGE) gel. After protein transfer, the TAL1 and ΔbTAL1 proteins were revealed using the 2TL136 antibody kindly provided by D. Matthieu (Institut de Génétique Moléculaire, Montpellier, France). Blots were stripped and probed overnight with an anti-c-KIT (c-KIT, clone C-19) or with an anti-ERK1/2 (clone C-16) (all from TEBU, Santa Cruz Biotechnology, Le Perray en Yvelines, France). Quantification of TAL1 proteins within cells transduced with the different TRIP constructs was done by quantitative densitometry of TAL1 bands on Western blots (NIH Image 1.62 software).
Hematopoietic cell cultures
Colony-forming cells (CFCs) and LTC-ICs were assayed as described.25 Erythrocytic potential was assessed in specific culture conditions. Cells were cultured for 6 days in serum-free medium in the presence of IL-3 (10 ng/mL), IL-6 (10 ng/mL) and stem cell factor (SCF) (25 ng/mL) then for 4 additional days in the presence of the same cytokines supplemented by 2000 IU/L erythropoietin (Epo).20 During this culture, cells were phenotyped at different time points by FACS analysis for expression of differentiation markers using monoclonal antibodies (MoAbs), CD36-phycoerythrin (PE) (clone CB38; Pharmingen, Pont de Claix, France), GPA-PE (clone 11E4B-7-6), CD34-PECy5 (clone QBEnd10), and CD34-allophycocyanin (APC) (clone 581) (all from Immunotech, Villepinte-Roissy CDG, France).
In all the FACS analyses, nonspecific staining was measured using irrelevant labeled mouse immunoglobulin G1 (IgG1)-PE/PC5/APC (all from Immunotech) and IgM (Pharmingen) MoAbs.
PCR analysis
Integration of the TRIPΔU3-EF1α vector in the cellular genome was analyzed by polymerase chain reaction (PCR) analysis on genomic DNA extracted from individual CFC-derived colonies as previously described.26 Amplification of genomic DNA was performed on whole-cell extracts with primers that amplify part of the TRIP vector (5′ ATCCACTTTGGCTGATACCGC 3′ sense primer) and common parts of the vector-encoded TAL1 and ΔbTAL1 sequences (5′ GGTCATCCTGGGGCATATTT 3′ antisense primer) (Figure 1C). Amplification was performed for 35 cycles at an annealing temperature of 61°C, resulting in a 335-bp PCR product.
RNA extraction and cDNA synthesis
Sorted cells (105 cells) were lysed in 200 μL TRIzol (Invitrogen, Groningen, The Netherlands) and total RNA was purified as recommended by the manufacturer. Total RNA was then reverse transcribed using random hexamers and the Superscript RT kit (Invitrogen) according to manufacturer's instructions. The cDNA product was diluted 10-fold prior to PCR amplification.
Real-time quantification PCR
Real-time PCR was performed using a LightCycler rapid thermal cycler system (Roche Diagnostics, Lewes, United Kingdom) according to the manufacturer's instructions. Reactions were performed in a 10 μL volume with 0.5 μM primers, 3.5 mM MgCl2, and LightCycler-DNA Master SYBR Green I mix (Roche Diagnostics) including nucleotides Taq DNA polymerase and buffer. Typical PCR protocol consists of a TAQ polymerase activation step at 95°C for 10 minutes followed by 40 cycles with 60°C to 64°C annealing for 5 seconds, and 72°C elongation for 15 seconds. Primer sets were designed to span introns and were tested on dilution of cDNA from the UT7 cell line to ensure PCR efficiency and specificity. Primers used are as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sense 5′-GGGAAACTGTGGCGTGAT-3′, antisense 5′-GGAGGAGTGGGTGTCGCTGTT-3′; c-kit, sense 5′-TTCTTACCAGGTGGCAAAGG-3′, antisense 5′-AAATGCTTTCAGGTGCCATC-3′; βglobin, sense 5′-ACACAACTGTGTTCACTAGC-3′, antisense 5′-ACCGAGCACTTTCTTGCCAT-3′; ubiquitous PBGD (uPBGD), sense 5′-CCATGTCTGGTAACGGCAAT-3′; erythroid PBGD (ePBGD), sense 5′-TCGCCTCCCTCTAGTCTCTG-3′; common primer for uPBGD and ePBGD, antisense 5′TTCAATGTTGCCACCACACT-3′; CD34, sense 5′-GCAAGCCACCAGAGCTATTC-3′, antisense 5′-TGCATGTGCAGACTCCTT-3′. The GAPDH and uPBGD housekeeping genes are used as an internal standard to normalized cDNA input. Experiments were performed twice for each point.
Statistical analyses
Statistical analyses were done using a Student t test (paired, 2-sided). Data were considered statistically significant when P was less than .05.
Results
Lentiviral transduction and expression of TAL1 and ΔbTAL1 in human hematopoietic cells
The human TAL1 and ΔbTAL1 coding sequences were cloned into the lentiviral vector TRIPΔU3-EF1α (Figure 1A). To study the TAL1 and ΔbTAL1 protein production by these lentiviral vectors, Jurkat/L4 cells were transduced with TRIPDU3-EF1α-TAL1 (hereafter called TRIP-TAL1), TRIP-ΔbTAL1, and control TRIP-EGFP vectors. The expression of the TAL1 proteins was studied by Western blot using the monoclonal 2TL136 antibody that recognizes an epitope located between Met 160 and Met 176 of TAL1 and thus does not recognize the carboxy terminal truncated form of TAL1 expressed by Jurkat/L4 cells. Several bands corresponding to the expected molecular weight of the phosphorylated and nonphosphorylated forms of TAL1 and ΔbTAL1 were detected in Jurkat/L4 cells transduced with the corresponding vectors. The intensities of these bands were comparable to the ones obtained with wild-type Jurkat cells (Figure 1B), indicating that the TRIP-TAL1 and TRIP-ΔbTAL1 vectors were functional for the production of the transgenic proteins.
We have recently defined conditions that reproducibly allow high-efficiency transduction and expression of the lentiviral vector TRIP-EGFP in human CD34+ cells.19 To determine the gene transfer efficiency of the TRIP-TAL1 and TRIP-ΔbTAL1 vectors into hematopoietic progenitor cells, UCB CD34+ cells transduced in these conditions were seeded into clonogenic progenitor (CFC) assays for 2 weeks (n = 4). PCR analysis performed on genomic DNA extracted from individual colonies indicated that 85% ± 13% (90 colonies analyzed) and 71% ± 16% (74 colonies analyzed) of the clonogenic progenitors had been transduced with the TRIP-TAL1 and TRIP-ΔbTAL1 vectors, respectively. This transduction efficiency correlated with the expression of EGFP measured in the CD34+ cells transduced in parallel with the TRIP-EGFP control vector (80% ± 14%; Figure 1C). Based on these results, we used the TRIP-EGFP vector as a transduction efficiency reference for the TRIP-TAL1/ΔbTAL1 vectors.
The amount of transgenic TAL1 and ΔbTAL1 proteins produced in transduced CD34+ cells was studied by Western blot analysis of proteins extracted from CD34+ cells at the end of the transduction procedure (Figure 1D). Quantification of the TAL1 protein contents in the different cell populations showed an increase in cells transduced with TRIP-TAL1 (×7 ± 3) and TRIP-ΔbTAL1 (×6 ± 2) vectors compared with TRIP-EGFP-transduced cells.
Altogether these results indicate that TAL1 and ΔbTAL1 transgenes are efficiently transduced and expressed in hematopoietic cells using the TRIP vector.
Function of TAL1 in early differentiation of human CD34+ cells into erythroid cells
To study the effect of TAL1 and ΔbTAL1 overexpression during human erythroid differentiation, UCB CD34+ cells were transduced with the TRIP vectors and cultured for 6 days in the presence of rhu-SCF, rhu-IL-3, and rhu-IL-6. Epo was then added for 4 supplementary days. These conditions define a first period of expansion of the erythroid progenitors (-Epo, early erythroid differentiation) prior to terminal differentiation (+Epo, late erythroid differentiation).20 The early erythroid differentiation was monitored by the expression of the CD34 and CD36 surface markers and 2 progenitor populations, CD34+CD36- and CD34+CD36+, that include multipotential and erythroid-restricted progenitors, respectively (Figure 2), were distinguished.
Early erythroid differentiation of human CD34+UCB cells. A proposed schematical relation between the surface expression of the CD34 and CD36 markers on the cells produced in erythroid differentiation culture system and their functional properties.
Early erythroid differentiation of human CD34+UCB cells. A proposed schematical relation between the surface expression of the CD34 and CD36 markers on the cells produced in erythroid differentiation culture system and their functional properties.
After transduction, more than 90% of the cells were CD34+, or whatever TRIP vector was used (Figure 3A). From that time, cells that overexpressed TAL1 displayed a similar differentiation profile to EGFP+ control cells (Figure 3A-B) with a gradual but rapid decrease of the proportion of CD34+ cells; at day 5, less than 20% of cells were CD34+ (Figure 3A). On the contrary, cells that expressed ΔbTAL1 maintained a CD34+ phenotype during this early erythroid differentiation (Figure 3A-B). CD34+ cells represented 68.2% ± 22% and 52.3% ± 11% of the population that expressed ΔbTAL1 compared with 53.3% ± 19% and 11.8% ± 8% in the EGFP+ cells at day 3 (n = 3) and day 5 (n = 4, P < .01), respectively (Figure 3A). As proliferation was similar during this first period of culture for cells expressing ΔbTAL1 or EGFP (data not shown), the absolute number of CD34+ cells remained high with, in 4 experiments, 4.3 ± 2 times more CD34+ cells in the presence of ΔbTAL1 than in control EGFP+ cells. The CD34+ cell population was still detectable at day 7, one day after the addition of Epo, where it represented 27% ± 14% of the cells transduced with TRIP-ΔbTAL1 compared with 7% ± 3% in the control EGFP+ cells (n = 6, P < .01). Interestingly, the detection of the CD34 antigen on CD36- or CD36+ cells was enhanced when ΔbTAL1 was present (mean fluorescence intensity [MFI]: ×2.2 ± 0.5 and ×3 ± 1.2, respectively; n = 4; Figure 3B-C) compared with the TAL1- or EGFP-transduced cells. This result was obtained with 2 different clones of anti-CD34 antibodies, and it correlated with high CD34 mRNA levels especially on the CD34+CD36+ cells (inset in Figure 3C).
Early erythroid differentiation of human CD34+ cells transduced with TRIP-TAL1 and TRIP-ΔbTAL1. (A) CD34+ cells transduced with TRIP-EGFP, TRIP-TAL1, and TRIP-ΔbTAL1 were cultured for 7 days in serum-free medium with SCF, IL-3, and IL-6. At day 6, Epo was added to allow terminal erythroid differentiation. Cells were labeled every day with MoAbs directed against CD34 and CD36 cell surface markers. Results are expressed as percent of CD34+ cells (mean ± standard deviation [SD], n ≥ 2). (B) Phenotypic analysis of human hematopoietic cells transduced with the TRIP vectors cultured during 3 days as in panel A. Quadrants were set according to isotype controls. Percent of cells for each quadrant is indicated. Boxed numbers indicate the MFI of the CD34 labeling in CD34+CD36- (upper left) or CD34+CD36+ (upper right) populations. Results are representative of 4 different experiments. (C) High expression of CD34 surface marker on cells with enforced expression of ΔbTAL1, but not of TAL1, compared with control EGFP. Shown are results obtained with CD34+CD36- (▪) and CD34+CD36+ (▧) cells. Results (mean ± SD; n = 4) are compared with those of EGFP+ control cells, referred to as 100%. Inserted in the figure are results of CD34 mRNA expression in both CD34+CD36- and CD34+CD36+ populations obtained using quantitative RT-PCR analysis (representative of 2 experiments). Statistical analyses were performed using the Student t test (paired, 2-sided). *P < .05.
Early erythroid differentiation of human CD34+ cells transduced with TRIP-TAL1 and TRIP-ΔbTAL1. (A) CD34+ cells transduced with TRIP-EGFP, TRIP-TAL1, and TRIP-ΔbTAL1 were cultured for 7 days in serum-free medium with SCF, IL-3, and IL-6. At day 6, Epo was added to allow terminal erythroid differentiation. Cells were labeled every day with MoAbs directed against CD34 and CD36 cell surface markers. Results are expressed as percent of CD34+ cells (mean ± standard deviation [SD], n ≥ 2). (B) Phenotypic analysis of human hematopoietic cells transduced with the TRIP vectors cultured during 3 days as in panel A. Quadrants were set according to isotype controls. Percent of cells for each quadrant is indicated. Boxed numbers indicate the MFI of the CD34 labeling in CD34+CD36- (upper left) or CD34+CD36+ (upper right) populations. Results are representative of 4 different experiments. (C) High expression of CD34 surface marker on cells with enforced expression of ΔbTAL1, but not of TAL1, compared with control EGFP. Shown are results obtained with CD34+CD36- (▪) and CD34+CD36+ (▧) cells. Results (mean ± SD; n = 4) are compared with those of EGFP+ control cells, referred to as 100%. Inserted in the figure are results of CD34 mRNA expression in both CD34+CD36- and CD34+CD36+ populations obtained using quantitative RT-PCR analysis (representative of 2 experiments). Statistical analyses were performed using the Student t test (paired, 2-sided). *P < .05.
These results show that overexpression of TAL1 does not affect the first steps of erythroid differentiation in liquid culture. However, TAL1 without its DNA-binding domain maintains cells that express the CD34 antigen. This suggests that TAL1 DNA binding plays an important role for efficient progression through erythroid differentiation or that the CD34 gene expression is dependent on bHLH proteins.
Enforced TAL1 expression enhances CFU-E production independently of DNA binding
We next studied the progenitor compartments after transduction of CD34+ cells with the TRIP-TAL1 and TRIP-ΔbTAL1 vectors. As previously described,16,17 the enforced expression of TAL1 had a small effect on the absolute number of CFCs after the transduction step. The cloning efficiency and the total number of colonies were increased 1.8- ± 0.3-fold (n = 5) compared with EGFP+ cells and no significant difference in the size of the colonies and the number of GPA+ cells produced per colony was observed (not shown). Surprisingly, no major effect on the CFCs was observed with cells transduced with TRIP-ΔbTAL1 although in 5 of 7 experiments, an increased proportion and number of CFU-Es were detected compared with EGFP+ cells (not shown).
To discriminate between a functional impact of ΔbTAL1 on the potentials of cells and a regulation of the expression of the cell surface CD34 protein by TAL1, CD34+CD36-, and CD34+CD36+ cells were isolated by cell sorting after transduction with the TRIP vectors and 2 to 3 days of culture in the presence of SCF, IL-3, and IL-6. The sorted cell populations were studied for their clonogenic progenitor content. Whatever vector used for transduction, sorted CD34+CD36- cells had the same clonogenic potential, with the same proportion of granulocytes and macrophage colony-forming units (CFU-GM) and BFU-E (Table 1).
Effect of enforced TAL1 and ΔbTAL1 expression on human CFCs
Cells and transgenes . | Percent of cells* . | No. of cells × 106† . | CFCs/1000 cells . | Total CFCs‡× 103 . | Percent of progenitors (absolute no., × 103) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CFU-Es . | BFU-Es . | CFU-GMs . | ||
| CD34+CD36−§ | |||||||||
| EGFP | 40 | 2.2 | 174 | 383 | 1 | 51 | 48 | ||
| TAL1 | 46 | 2.7 | 176 | 475 | 2 | 50 | 48 | ||
| ΔbTAL1 | 49 | 2 | 177 | 354 | 3 | 48 | 49 | ||
| CD34+CD36+§ | |||||||||
| EGFP | 20 | 1.1 | 162 | 178 | 2.5 (4.5) | 92 (163) | 5.5 | ||
| TAL1 | 45 | 2.7 | 304 | 821 | 14.5 (126) | 81.5 (669) | 4 | ||
| ΔbTAL1 | 42 | 1.7 | 194 | 330 | 30.5 (101) | 65.5 (216) | 4 | ||
| CD34+CD36−∥ | |||||||||
| EGFP | 29 | 1.7 | 170 | 292 | 0 | 42 | 58 | ||
| TAL1 | 31 | 2 | 150 | 307 | 1 | 45 | 55 | ||
| ΔbTAL1 | 45 | 2.8 | 130 | 363 | 0 | 27 | 74 | ||
| CD34+CD36+∥ | |||||||||
| EGFP | 24 | 1.4 | 302 | 423 | 3 (13) | 91 (384) | 5 | ||
| TAL1 | 36 | 2.4 | 321 | 761 | 20 (152) | 75 (570) | 5 | ||
| ΔbTAL1 | 43 | 2.7 | 325 | 865 | 18.5 (160) | 78 (674) | 3 | ||
Cells and transgenes . | Percent of cells* . | No. of cells × 106† . | CFCs/1000 cells . | Total CFCs‡× 103 . | Percent of progenitors (absolute no., × 103) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | CFU-Es . | BFU-Es . | CFU-GMs . | ||
| CD34+CD36−§ | |||||||||
| EGFP | 40 | 2.2 | 174 | 383 | 1 | 51 | 48 | ||
| TAL1 | 46 | 2.7 | 176 | 475 | 2 | 50 | 48 | ||
| ΔbTAL1 | 49 | 2 | 177 | 354 | 3 | 48 | 49 | ||
| CD34+CD36+§ | |||||||||
| EGFP | 20 | 1.1 | 162 | 178 | 2.5 (4.5) | 92 (163) | 5.5 | ||
| TAL1 | 45 | 2.7 | 304 | 821 | 14.5 (126) | 81.5 (669) | 4 | ||
| ΔbTAL1 | 42 | 1.7 | 194 | 330 | 30.5 (101) | 65.5 (216) | 4 | ||
| CD34+CD36−∥ | |||||||||
| EGFP | 29 | 1.7 | 170 | 292 | 0 | 42 | 58 | ||
| TAL1 | 31 | 2 | 150 | 307 | 1 | 45 | 55 | ||
| ΔbTAL1 | 45 | 2.8 | 130 | 363 | 0 | 27 | 74 | ||
| CD34+CD36+∥ | |||||||||
| EGFP | 24 | 1.4 | 302 | 423 | 3 (13) | 91 (384) | 5 | ||
| TAL1 | 36 | 2.4 | 321 | 761 | 20 (152) | 75 (570) | 5 | ||
| ΔbTAL1 | 43 | 2.7 | 325 | 865 | 18.5 (160) | 78 (674) | 3 | ||
Percent evaluated by flow cytometry.
Calculated based on the FACS data and the proliferation rate in the culture.
Number of CFCs generated during the erythroid culture.
Experiment 1 cells were sorted after 2 days of culture in presence of SCF, IL-3, and IL-6.
Experiment 2 cells were sorted after 3 days of culture in presence of SCF, IL-3, and IL-6.
The transduced CD34+CD36+ cell population that mainly contained erythroid progenitors had similar cloning efficiencies with whatever TRIP vector had been used. However, the enforced expression of TAL1 and ΔbTAL1 modified the nature of the colonies. The proportions of BFU-Es and CFU-Es generated from TAL1-transduced (78% ± 5% and 17% ± 4%, respectively) and ΔbTAL1-transduced (70% ± 7% and 24% ± 8%) cells were similar but different than control EGFP-transduced cells (91% ± 0.7% and 3% ± 0.3%). Thus, enforced expression of TAL1 or ΔbTAL1 increased CFU-Es and decreased BFU-Es, suggesting an acceleration of the erythroid differentiation when these transgenes were expressed.
In both experiments, TAL1 and ΔbTAL1 expression also enhanced the number of CD34+CD36+ cells generated from transduced CD34+ cells that resulted in 2 to 4.6 times more colonies (ie, TRIP-TAL1: ×3.2 ± 2 and TRIP-ΔbTAL1: ×1.9 ± 0.1) compared with control EGFP+ cells (Table 1). Taking into account the proliferation rate and the proportion of CFU-Es and BFU-Es, an increase of more than 10 times in the absolute numbers of CFU-Es was observed in cells transduced with the TRIP-TAL1 (×26 and ×12; n = 2) or the TRIP-ΔbTAL1 (×22.5 and ×12.6; n = 2) vector whereas the numbers of BFU-Es were less affected (×1.3 to ×4; Table 1).
Altogether, these results indicate that enforced expression of TAL1, with or without its DNA-binding domain, enhances erythroid differentiation through the production of erythroid progenitors, mainly CFU-Es.
Enforced expression of TAL1 but not ΔbTAL1 protein increases the LTC-IC compartment
TAL1 is known to play a role during erythroid differentiation and in the development of hematopoiesis in the mouse.1 This effect has been described to be independent of the DNA-binding capacity of TAL1.11 As cells transduced with the TRIP-ΔbTAL1 vector maintained a high level of surface CD34+ expression, a phenotype of immature cells, we studied the impact of enforced expression of TAL1 and ΔbTAL1 for the function of primitive cells in humans.
Cells recovered after transduction or CD34+CD36- cells, sorted after 2 to 3 days in the presence of SCF, IL-3, and IL-6, were tested for their LTC-IC potentials. Results indicate that enforced TAL1 expression increases the number of colonies generated after 5 weeks in LTC up to 3-fold (×2.2 ± 0.8; n = 4) in cells immediately after transduction, and 2.4 and 3 times (n = 2) in the transduced CD34+CD36- sorted cells (Table 2). This increase is not related to the effect of TAL1 on BFU-Es, since more than 90% of the colonies were CFU-GMs. ΔbTAL1 had only a small effect on the generation of CFCs from LTC-ICs with a diminution (×0.7 ± 0.25; n = 4) or no effect on the number of colonies obtained from the cells at, respectively, the end of the transduction process or after 2 and 3 days of culture with cytokines (Table 2).
Effect of enforced expression of TAL1 and ΔbTAL1 on the LTC-IC compartment
Transgene . | Percent of CD34+ cells* . | No. of cells × 106† . | Total CFCs/well . | Total CFCs × 103 . |
|---|---|---|---|---|
| EGFP‡ | 60 | 3.3 | 416 | 306 |
| TAL1‡ | 91 | 5.4 | 823 | 739 (×2.4)∥ |
| ΔbTAL1‡ | 91 | 3.7 | 482 | 307 |
| EGFP§ | 53 | 3.1 | 124 | 43 |
| TAL1§ | 67 | 4.4 | 321 | 132 (×3)∥ |
| ΔbTAL1§ | 88 | 5.5 | 80 | 45 |
Transgene . | Percent of CD34+ cells* . | No. of cells × 106† . | Total CFCs/well . | Total CFCs × 103 . |
|---|---|---|---|---|
| EGFP‡ | 60 | 3.3 | 416 | 306 |
| TAL1‡ | 91 | 5.4 | 823 | 739 (×2.4)∥ |
| ΔbTAL1‡ | 91 | 3.7 | 482 | 307 |
| EGFP§ | 53 | 3.1 | 124 | 43 |
| TAL1§ | 67 | 4.4 | 321 | 132 (×3)∥ |
| ΔbTAL1§ | 88 | 5.5 | 80 | 45 |
These results indicate that expression of TAL1 without its DNA-binding domain in CD34+ cells has little impact on the human immature LTC-IC compartment and are in agreement with the results obtained with the conditional knockout of TAL1 in adult murine HSCs.7,8 In addition, we show that enforced expression of the wild-type TAL1 protein has a positive effect on the development of LTC-ICs. Altogether, these results suggest that this effect requires binding to DNA.
The effect of enforced expression of TAL1 on progenitor cells is not mediated by an increase of c-KIT expression
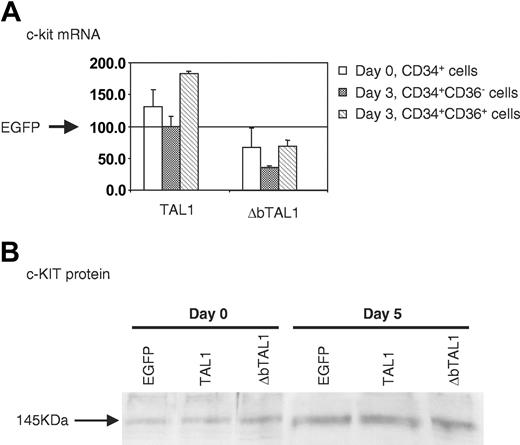
As TAL1 and ΔbTAL1 expressions strongly increased the CFU-E compartment, we studied the expression of c-KIT, whose role in the BFU-E/CFU-E transition has been shown.27 First, c-kit mRNA was quantitatively measured in the different TRIP vector-transduced cells. When cells were studied after transduction, c-kit mRNAs were slightly increased in the presence of enforced TAL1 expression whereas expression of ΔbTAL1 decreased the amount of c-kit mRNA compared with EGFP+ cells (Figure 4A). When c-kit mRNA levels were tested in transduced CD34+CD36+/- populations sorted after 3 and 4 days of culture (Figure 4A, representative of 2 experiments), variations were observed that correlated with the differentiation stage. In the presence of TAL1, no increase in c-kit mRNA levels was observed in the CD34+CD36- population whereas its levels were increased in the CD34+CD36+ cells. In cells expressing ΔbTAL1, the c-kit mRNA levels were always diminished. These results indicate a possible TAL1 regulation of the c-kit gene expression dependent on DNA binding.
c-kit gene expression in human hematopoietic cells transduced with the TRIP vectors. (A) Quantitative measurement of c-kit mRNA levels in CD34+ cells after transduction (day 0, CD34+ cells) and in transduced CD34+CD36+/- cells sorted after 3 days of serum-free culture in the presence of SCF, IL-3, and IL-6. Results of TRIP-TAL1/ΔbTAL1-transduced cells are expressed as a proportion of the results obtained with the EGFP+ cells. Each measurement was made 3 times. (B) c-KIT protein levels in human hematopoietic cells recovered after transduction (day 0; all cells are CD34+) and 5 days after the end of the transduction, following a culture period in conditions described in “Materials and methods.” Blot was reprobed with the anti-c-KIT Ab (clone C19, TEBU) after a first probing with the 2TL136 anti-TAL1 Ab (Figure 1D). All data are representative of 2 experiments.
c-kit gene expression in human hematopoietic cells transduced with the TRIP vectors. (A) Quantitative measurement of c-kit mRNA levels in CD34+ cells after transduction (day 0, CD34+ cells) and in transduced CD34+CD36+/- cells sorted after 3 days of serum-free culture in the presence of SCF, IL-3, and IL-6. Results of TRIP-TAL1/ΔbTAL1-transduced cells are expressed as a proportion of the results obtained with the EGFP+ cells. Each measurement was made 3 times. (B) c-KIT protein levels in human hematopoietic cells recovered after transduction (day 0; all cells are CD34+) and 5 days after the end of the transduction, following a culture period in conditions described in “Materials and methods.” Blot was reprobed with the anti-c-KIT Ab (clone C19, TEBU) after a first probing with the 2TL136 anti-TAL1 Ab (Figure 1D). All data are representative of 2 experiments.
Finally, we performed a Western blot analysis on transduced CD34+ cells to measure c-KIT protein levels at day 0 and day 5 of the erythroid culture. The results of 2 experiments indicated that enforced expression of TAL1 and ΔbTAL1 did not modify the expression of c-KIT during the culture. Indeed, similar results were obtained for cells transduced with TRIP-TAL1/ΔbTAL1 and TRIP-EGFP (Figure 4B) whereas the amount of total TAL1 protein was increased in TRIP-TAL1/ΔbTAL1-transduced cells compared with controls for the same blot (Figure 1D).
These results highlight modulations of the c-kit mRNA levels when TAL1 and ΔbTAL1 expression was enforced. However, these variations in c-kit mRNA levels did not correlate with modifications of the expression of the c-KIT protein in TRIP-TAL1/ΔbTAL1-transduced CD34+-derived cells.
Positive role of TAL1 on erythroid differentiation depends on DNA binding
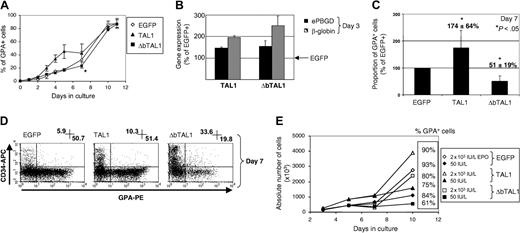
To study the effect of enforced TAL1 expression during the erythroid differentiation, the onset of the GPA marker was followed by flow cytometry during the erythroid liquid culture. When cells transduced with TRIP-TAL1 were cultured in the presence of SCF, IL-3, and IL-6, but without Epo, a major effect was seen in the GPA+ population, which was raised to 19.8% ± 5% (n = 4) at day 3 and 45% ± 11% (n = 2) at day 5 compared with 7.3% ± 3% and 17.6% ± 1.9% in the EGFP+ cells (Figure 5A; P < .05). This result correlated with a 3- to 4-fold increase in the absolute number of GPA+ cells in the TRIP-TAL1-transduced cells compared with the EGFP+ cells at day 3 to day 5. Moreover, analysis of the βglobin and ePBGD mRNA levels at 3 to 4 days of culture indicated, respectively, a 2- and 1.5-fold increase in the CD34-CD36+ cells that overexpressed TAL1 compared with EGFP+ cells (Figure 5B). These results suggest that enforced TAL1 expression improves the erythrocytic potentials of progenitors in the absence of Epo.
Effect of TAL1 and ΔbTAL1 enforced expression on the generation of GPA+ cells during erythroid culture. CD34+ cells transduced with TRIP-EGFP, TAL1, and ΔbTAL1 vectors were cultured for 6 days in serum-free medium with SCF, IL-3, and IL-6. Epo was added at day 6 for 5 additional days. (A) Percent of GPA+ cells generated from EGFP- (⋄), TAL1- (▴), and ΔbTAL1-transduced (▪) CD34+ cells (n ≥ 2). (B) Quantification of βglobin (n = 2) and erythroid PBGD (n = 2) mRNA expressions in CD34-CD36+ cells sorted 3 days after the end of the transduction using quantitative PCR analysis. Results obtained in the cells transduced with TRIP-TAL1 and TRIP-ΔbTAL1 are expressed as percent of gene expression compared with cells transduced with TRIP-EGFP. (C) Absolute number of GPA+ cells generated from transduced cells after 7 days of culture (n = 6). Results are expressed as a proportion of the control EGFP+ cells. (D) Flow cytometry analysis of CD34+ and GPA+ cells after 7 days of culture. Quadrants were set as in Figure 2. Shown are percent of CD34+ cells (upper left) and GPA+ (lower right) cells. (E) Growth curves of transduced cells in response to 2 concentrations of Epo (50 IU/L and 2 × 103 IU/L). Shown are cumulative cell numbers and percent of GPA+ cells measured by flow cytometry after 10 days of culture. Error bars (A-C) represent standard deviation.
Effect of TAL1 and ΔbTAL1 enforced expression on the generation of GPA+ cells during erythroid culture. CD34+ cells transduced with TRIP-EGFP, TAL1, and ΔbTAL1 vectors were cultured for 6 days in serum-free medium with SCF, IL-3, and IL-6. Epo was added at day 6 for 5 additional days. (A) Percent of GPA+ cells generated from EGFP- (⋄), TAL1- (▴), and ΔbTAL1-transduced (▪) CD34+ cells (n ≥ 2). (B) Quantification of βglobin (n = 2) and erythroid PBGD (n = 2) mRNA expressions in CD34-CD36+ cells sorted 3 days after the end of the transduction using quantitative PCR analysis. Results obtained in the cells transduced with TRIP-TAL1 and TRIP-ΔbTAL1 are expressed as percent of gene expression compared with cells transduced with TRIP-EGFP. (C) Absolute number of GPA+ cells generated from transduced cells after 7 days of culture (n = 6). Results are expressed as a proportion of the control EGFP+ cells. (D) Flow cytometry analysis of CD34+ and GPA+ cells after 7 days of culture. Quadrants were set as in Figure 2. Shown are percent of CD34+ cells (upper left) and GPA+ (lower right) cells. (E) Growth curves of transduced cells in response to 2 concentrations of Epo (50 IU/L and 2 × 103 IU/L). Shown are cumulative cell numbers and percent of GPA+ cells measured by flow cytometry after 10 days of culture. Error bars (A-C) represent standard deviation.
The increase in the percent and numbers of GPA+ cells with TAL1 overexpression progressively disappeared upon addition of Epo to the culture. At day 7, the difference in proportion and absolute cell numbers between TRIP-TAL1 and TRIP-EGFP cells was still significantly different (Figure 5A,C; P < .05) whereas at day 10 both populations were identical (Figure 5A). The effects of enforced TAL1 expression on the production of GPA+ cells are not mediated through an increased sensitivity to Epo since a low dose (50 IU/L) of Epo leads to a low production of cells, including GPA+ cells, with whatever TRIP (EGFP/TAL1/ΔbTAL1) vectors were used to transduce the CD34+ cells (Figure 5E).
Results were different with cells transduced with TRIP-ΔbTAL1. First, the CD34-CD36+ cells showed a delay in the erythroid differentiation as the proportions of this cell population were always lower in the presence of ΔbTAL1 compared with EGFP+ cells (Figure 3D and not shown). When βglobin and ePBGD mRNA expressions were measured in the CD34-CD36+ cells at day 3 or day 4, their levels were as high (×2.5 ± 0.4 and ×1.54 ± 0.3; n = 2) as in the cells overexpressing TAL1 (Figure 5B), suggesting common features in these populations independently of the presence of the DNA-binding domain of TAL1. Although the levels of GPA+ cells were not significantly altered by the presence of ΔbTAL1 at early time points of culture, their production measured after Epo addition at day 7 was reduced (Figure 5A,C,D). The percent of GPA+ cells was decreased to 22% ± 10% with ΔbTAL1 compared with 34% ± 12% in control cells (n = 7; P < .05; Figure 5A). At this time point, the absolute number of GPA+ cells was reduced 2-fold (×0.51 ± 0.2; n = 7; P < .005; Figure 5C) compared with control cells, due to the lower proportion of cells and a lower cell number.
These results show that enforced TAL1 expression allows an early wave of differentiation of CD34+ cells into mature GPA+ erythroid cells independently of the presence of Epo in the culture system. This effect is dependent on the presence of the DNA-binding domain. Moreover, overexpression of wild-type or mutant TAL1 protein transiently pertubates GPA+ cell production in the presence of Epo but independently of its dose.
Discussion
In this study, we show that enforced TAL1 expression enhances human erythroid differentiation and that some of these effects need DNA binding.
We previously developed and described TRIP lentiviral vectors to transduce the whole hierarchy of human hematopoietic cells with a high efficiency.18,19 To our knowledge, this is the first description of the use of a lentiviral vector to study the role of enforced protein expression in primary human hematopoietic progenitor cells. Interpretation of previous works of enforced TAL1 expression in human CD34+ cells is limited by the low levels of gene transfer obtained with oncoretroviral vectors and by the type of cells targeted by this gene transfer, mainly mature clonogenic progenitors. Consequently, the effect of such enforced expression was studied on transduced cells selected on the resistance to antibiotics.16,17 Our approach offers a new way of studying the effect of overexpressing transgenic proteins in a high proportion of human hematopoietic progenitors, mature and immature, avoiding bias of selection and allowing expression of transgenes to be measured at the protein level. In these conditions, levels of TAL1 and ΔbTAL1 transgenic proteins obtained at the end of the transduction protocol were 5- to 6-fold higher than levels in the endogenous TAL1 protein.
A major role for TAL1 during erythropoiesis has been suggested by previous reports performed in cell lines and primary hematopoietic cells.1 Our results definitively show the function of TAL1 as a positive regulator of human erythroid differentiation. TAL1 overexpression increases erythroid differentiation based on (1) an increased βglobin and ePBGD gene expression at early time points of culture, (2) a high proportion of CFU-Es compared to BFU-Es, and (3) an enhanced GPA+ cell production. Moreover, this study uses a 2-step (-Epo/+Epo) culture system20 that allows us to differentially explore the role of TAL1 during the early and late erythroid differentiation. In these conditions, we observed a first wave of GPA+ cells from cells transduced with TRIP-TAL1 that was independent of the presence of Epo, the further addition of Epo abolishing the difference detected between the controls and the TRIP-TAL1-transduced cells. The mechanism that allows GPA+ cells to develop from TRIP-TAL1-transduced cells is unknown. In the conditions we used, low levels of GPA+ cells are produced from control CD34+ UCB cells. One can speculate that enforced TAL1 expression does not bypass the requirement of Epo but allows the GPA+ cells to proliferate or to survive and thus accumulate during the early period of the culture. This hypothesis could be applied to the high proportion and absolute numbers of CFU-Es versus BFU-Es that we detected when TAL1 expression was enforced. Indeed, when cells were plated directly in clonogenic assays, a 2-fold increase of BFU-E numbers was observed, comparable with previous reports.16,17 When transduced cells were allowed to proliferate and differentiate in the presence of cytokines, we observed a bias in erythroid progenitors toward small CFU-Es, although the number of BFU-Es remained relatively stable. These results can thus be explained by (1) an acceleration of differentiation with BFU-Es becoming CFU-Es in the presence of high levels of TAL1, (2) an increase of proliferation, or (3) an improved survival of the overall erythroid progenitors, especially of CFU-Es. To investigate these hypotheses, we measured the size of the erythroid colonies and we measured apoptosis using annexin V staining (data not shown). The fact that both aspects (ie, proliferation and survival [data not shown]) were not significantly modified in the presence of enforced TAL1 expression, argue for an effect of enforced TAL1 expression in enhancing red cell differentiation. Interestingly, our results indicate that this effect is not mediated by an increase of sensitivity to Epo.
The absence of a negative effect of enforced TAL1 expression on CFU-GM production (Table 1) and on the production of macrophages or granulocytes in liquid cultures (E.R., D.R., M.T., et al, unpublished observations, December 2002) was unexpected. Down-regulation of TAL1 expression has been reported to occur in early myeloid differentiation and to be necessary for proper monocytic and granulocytic differentiation.29,30 Constitutive expression of TAL1 in cell lines such has TF-1, M1, HL-60, and 32D, further cultured in conditions that allow myeloid differentiation, interfered with the expected monocytic and granulocytic differentiation.28-30 When enforced TAL1 expression was obtained in primary human hematopoietic cells, contradictory results were reported that seemed to be dependent on the culture conditions.16,17 Our results on CFU-GMs suggest that the positive effect we observed on progenitors generated from LTC is related to an increase of the LTC-IC compartment rather than a direct effect on the CFU-GM progenitors. Enforced expression of TAL1 increased the number of progenitors detected after 5 weeks, progenitors that were mainly CFU-GMs as in controls. Of importance, BFU-Es were detected in these cultures only when TAL1 was overexpressed (not shown), suggesting that TAL1 can interfere with the potential of LTC-ICs. Whether these BFU-Es are derived from LTC-ICs or are clonogenic progenitors that survive during the LTC is presently not known. The study of the effect of enforced TAL1 expression, especially on primitive progenitors that require long-term experiments, is limited by the fact that TAL1 is constitutively expressed during the overall culture. As a first approach, we developed a system of conditional TAL1 function using a fusion between the cDNA of TAL1 and that of the mutated estrogen receptor ERt2. However, as recently described,31 adverse effects of ERt2 alone or of tamoxifen, the ERt2 ligand, on human hematopoiesis were detected (E.R., D.R., M.T., et al, unpublished results, November 2001).
Several studies have shown that some TAL1 functions do not need DNA binding, implying a different molecular mechanism for some activities of TAL1 than direct binding to an E-box present in its target genes.11,12,15,32 In this study, we distinguish DNA-binding-dependent and -independent functions of TAL1 during human erythropoiesis. The increase of βglobin and ePBGD expressions, the increase in total erythroid progenitors and in the CFU-E/BFU-E ratio when cells were cultured for several days, was identical in the presence of enforced TAL1 and ΔbTAL1 expression. However, ΔbTAL1 appeared as a negative factor for other aspects. Increased production of GPA+ cells and down-regulation of the CD34 surface marker expression were not reproduced with ΔbTAL1. These results suggest that complete erythroid differentiation requires TAL1 DNA binding or that ΔbTAL1 interferes with normal expression of surface markers such as CD34 and GPA. This latter hypothesis would be in agreement with the work of Lahlil et al,33 which shows that the transcription of GPA is regulated by TAL1 and that it requires its DNA-binding domain. Furthermore, (1) βglobin and ePBGD gene expression are not altered by the presence of ΔbTAL1, (2) the CD36 marker is up-regulated as in controls, and (3) BFU-Es and CFU-Es are produced equally well by TAL1- and ΔbTAL1-transduced cells. Altogether, this suggests that the initiation of erythropoiesis is conserved and the erythroid progenitor cells generated with TAL1 or ΔbTAL1 share common features. On the other hand, these data could also agree with the results obtained in the rescue of hematopoieisis in Tal1 null murine ES cells with ΔbTAL1.11 In these experiments, primitive erythropoiesis could be restored and definitive hematopoiesis was initiated in the fetal liver of chimeric animals but terminal erythroid differentiation was incomplete, with only few enucleated cells.11 Finally, enforced expression of TAL1 into human CD34+ cells improves the LTC-IC compartment whereas ΔbTAL1 does not. This implies that, in the limits of the tests we used, there is no direct correlation between the high levels of cell surface CD34 protein and the function of the cells. A possible explanation would be that the regulation of the CD34 expression during red blood cell differentiation is bHLH dependent. ΔbTAL1 could interact with and titrate an unknown bHLH protein that is required for normal down-regulation of CD34. Finally, the lack of effect of enforced ΔbTAL1 expression on the LTC-IC compartment can also be considered an extension of the recent reports in the mouse to human cells.7,8 Indeed, conditional deletion of TAL1 in adult murine hematopoiesis has no effect on the reconstitutive potential of primitive progenitor cells, such as HSC and CFU-S,7,8 suggesting that compensatory mechanisms may exist that can replace TAL1 function in adult HSCs. According to our data, enforced TAL1 protein expression into human hematopoietic progenitor cells increases their primitive potentials, indicating collaborative functions for TAL1 and the other effector proteins. These data are now confirmed in vivo in nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice (D.R., E.R., M.T., et al, submitted manuscript, January 2004).
The molecular mechanisms that underlie TAL1 functions are still a matter of debate, as very few target genes are known. TAL1 has been reproducibly shown to interfere with apoptosis,24,28 and this effect could be mediated by cytokines and/or by their receptors. The main candidate that was described to regulate or be regulated by TAL1 is c-KIT, the SCF receptor,32,34-36 although contradictory data exist.37 Correlation between c-KIT and TAL1 expressions has been documented, as has interference of c-KIT expression, and thus survival of cells in response to SCF, when antisense TAL1 or ΔbTAL1 are expressed.36 Moreover, it has been recently shown that c-KIT expression was up-regulated by TAL1 and that this regulation is independent of the DNA-binding property of TAL1.32,36 As the transition BFU-E/CFU-E is dependent on c-KIT expression,27,38 we investigated whether enforced TAL1 or ΔbTAL1 expression in CD34+ cells could increase the level of c-KIT expression and thus explain the enhancement of CFU-E we observed. Our results do not support this hypothesis since c-KIT protein levels were not affected by the amount of transgenic TAL1 or ΔbTAL1. Moreover, enforced expression of ΔbTAL1 decreased the c-kit mRNA levels, and this result was observed at the end of the transduction time and after 3 to 4 days of erythroid culture, in 2 different cell populations. This result indicated a TAL1 or bHLH regulation of c-kit transcription since in primitive progenitors as in erythroid cells, c-kit gene expression is quantitatively important,39 and thus an increase would be difficult to detect. Regulation of c-kit gene expression by enforced TAL1 expression in B cells that normally express low levels of c-kit mRNA32 might be easier to measure, from a sensitivity point of view.
In conclusion, this study gives new insights into the functions of TAL1 in human erythropoiesis. Specifically, we show that specific steps of erythroid differentiation that are regulated by TAL1 do not depend on DNA binding whereas others do. Whether these results suggest a dynamic turnover of the protein complexes in which TAL1 participates during erythroid differentiation or the regulation of the expression of some proteins (CD34, GPA) that are key markers for normal erythropoiesis is yet unknown. Future experiments will be designed to understand the molecular nature of the complexes that include TAL1.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-05-1689.
Supported by INSERM and by grants from Association Française contre les Myopathies (AFM/ATG 1999), Association pour la Recherche sur le Cancer (ARC 7551), and Fondation de France (Engt 2002 004534). E.R. was supported by fellowships from the Ministère de la Recherche et des Technologies, Fondation pour la Recherche Médicale (FRM), and Société Française d'Hématologie. D.R. was supported by fellowships from the Ligue Nationale contre le Cancer and ARC.
E.R. and D.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Danielle Matthieu for providing the 2TL136 anti-TAL1 antibody, Leila Maouche-Chrétien and Nicolas Goardon for helpful discussions, Sophie Amsellem-Bosc for the production of the first TRIP-TAL1 vector preparations and for humorous and stimulating discussions. We wish to thank particularly the midwives and Dr Y Rouquet from the Clinique des Noriets, Vitry-sur-Seine for providing the numerous cord blood samples, and B. Canque, R. Haddad, and M. Yagello from EMI0013 INSERM and the Department of Common Facilities in the Institut Cochin, for cell sorting.


![Figure 3. Early erythroid differentiation of human CD34+ cells transduced with TRIP-TAL1 and TRIP-ΔbTAL1. (A) CD34+ cells transduced with TRIP-EGFP, TRIP-TAL1, and TRIP-ΔbTAL1 were cultured for 7 days in serum-free medium with SCF, IL-3, and IL-6. At day 6, Epo was added to allow terminal erythroid differentiation. Cells were labeled every day with MoAbs directed against CD34 and CD36 cell surface markers. Results are expressed as percent of CD34+ cells (mean ± standard deviation [SD], n ≥ 2). (B) Phenotypic analysis of human hematopoietic cells transduced with the TRIP vectors cultured during 3 days as in panel A. Quadrants were set according to isotype controls. Percent of cells for each quadrant is indicated. Boxed numbers indicate the MFI of the CD34 labeling in CD34+CD36- (upper left) or CD34+CD36+ (upper right) populations. Results are representative of 4 different experiments. (C) High expression of CD34 surface marker on cells with enforced expression of ΔbTAL1, but not of TAL1, compared with control EGFP. Shown are results obtained with CD34+CD36- (▪) and CD34+CD36+ (▧) cells. Results (mean ± SD; n = 4) are compared with those of EGFP+ control cells, referred to as 100%. Inserted in the figure are results of CD34 mRNA expression in both CD34+CD36- and CD34+CD36+ populations obtained using quantitative RT-PCR analysis (representative of 2 experiments). Statistical analyses were performed using the Student t test (paired, 2-sided). * P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-05-1689/6/m_zh80090460710003.jpeg?Expires=1767706021&Signature=YWurQsD6qQbqv44v6B4lYBrUrDJrYsAfxyv-J2H2~yZMc~CuFLE9CSB~dBF2~rew-KvdimJfCOXoJnUjeUSqMZDEBU9MMAvB1Yt1EQYRTTiK1QT~g6sc-byEcg~LAEFR~ZMMfNPz7sjyzERDheJycYl46BoDPd7Id7KAkNZ0SWYO3XSgbBMpNwHT~Eq~QB9iqEoeR8uH2D9UFZoY6pDzSzjpID95w-QA1Bq6tNrvs1CFvGxzTQ-jhx2RfezajuDNQCBRZOhmQiTN0RkVCpBGpcABeVHD0Xrll6KMFy6rCHNO7EBVohp70pm81AGHH6LacsFST8h85r9gdB~41~LcIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal