Abstract
We previously reported a transgenic mouse model expressing herpesvirus thymidine kinase (TK) gene under the control of a 2.3-kilobase fragment of the rat collagen α1 type I promoter (Col2.3ΔTK). This construct confers lineage-specific expression in developing osteoblasts, allowing the conditional ablation of osteoblast lineage after treatment with ganciclovir (GCV). After GCV treatment these mice have profound alterations on bone formation leading to a progressive bone loss. In addition, treated animals also lose bone marrow cellularity. In this report we characterized hematopoietic parameters in GCV-treated Col2.3ΔTK mice, and we show that after treatment transgenic animals lose lymphoid, erythroid, and myeloid progenitors in the bone marrow, followed by decreases in the number of hematopoietic stem cells (HSCs). Together with the decrease in bone marrow hematopoiesis, active extramedullary hematopoiesis was observed in the spleen and liver, as measured by an increase in peripheral HSCs and active primary in vitro hematopoiesis. After withdrawal of GCV, osteoblasts reappeared in the bone compartment together with a recovery of medullary and decrease in extramedullary hematopoiesis. These observations directly demonstrate the role of osteoblasts in hematopoiesis and provide a model to study the interactions between the mesenchymal and hematopoietic compartments in the marrow. (Blood. 2004; 103:3258-3264)
Introduction
Hematopoiesis is the process by which hematopoietic stem cells (HSCs) generate and replenish hematopoietic progenitors and all mature blood cells.1 During vertebrate ontogeny, hematopoiesis is established sequentially in different anatomic sites. Around birth time, hematopoiesis shifts from fetal liver to the bone marrow, which is the major site of hematopoiesis during adult life.2-5
The central cell in hematopoiesis is the HSC.6 This cell has the potential to self-renew and to clonally reconstitute all the blood components after adoptive transfer into lethally irradiated recipients. This activity is dependent on its migration to bone marrow and subsequent engraftment of progenitors.7,8 The initiation and maintenance of hematopoiesis is a complex process that depends on the participation of support cells, which generate the microenvironmental conditions that ensure the size of the stem cell pool and regulate the differentiation of HSCs into the required number of mature blood cells. In vitro methods have provided direct evidence that the bone marrow stroma layers are a necessary supporting matrix for the maintenance and differentiation of hematopoietic progenitors.9 In addition, direct observation and studies that track HSCs in vivo, after adoptive transfer, demonstrated a 3-dimensional matrix of stroma cells in tight contact with hematopoietic cells in the bone marrow. Interestingly, it was observed that early progenitors tend to localize in extravascular areas and concentrate in the subendosteal areas adjacent to the bone cortex.10-12 Stroma cells play a fundamental role in hematopoiesis by providing survival and/or differentiation signals through intimate contact with hematopoietic components and by secreting cytokines like interleukin-6 (IL-6), IL-7, cKit ligand, granulocyte-macrophage colony-stimulating factor (GM-CSF), and others.
Osteoblasts are one of the components of the stroma layers in primary bone marrow stroma.13 Developmentally, osteoblasts are derived from a multipotent cell, the mesenchymal stem cell (MSC), which can progress toward osteogenesis, chondrogenesis, adipogenesis, and myogenesis through a defined developmental program.14-16 It has been proposed that when MSCs commit to osteogenesis they differentiate to a putative committed osteoprogenitor that progresses to a preosteoblast, an osteoblast, and finally an osteocyte that mineralizes and forms bone.17 The signals responsible for driving this progression are poorly understood, as is the possible biologic significance of each of the proposed developmental stages.
The links between hematopoiesis and osteoblasts have not been easy to define, mostly because it is difficult to isolate the contribution of osteoblasts from those of cells that are part of the stroma and because there is a lack of osteoblast-specific cell surface markers.13 During development there is a correlation between the appearance of osteoblasts and the establishment of hematopoiesis in the bone marrow. In addition, as part of the bone remodeling system, osteoblasts regulate the development and activation of osteoclasts, which are hematopoietic in origin.18,19 Advances in the culture of mesenchymal cells have resulted in techniques that allow the generation of cultures enriched in osteoblast-like cells.20-22 These cells express alkaline phosphatase, a hallmark of osteoblast lineage, and genes like type I collagen, osteopontin, bone sialoprotein, osteocalcin, and others. These cultures have permitted the direct evaluation of the interactions between osteoblasts and hematopoietic precursors as well as defined the potential of osteoblasts to modulate hematopoiesis. From these studies it has become evident that osteoblasts have the capacity to produce a vast array of cytokines, which are important for several hematopoietic pathways, including the proliferation of progenitors. Osteoblast cultures produce or express granulocyte colony-stimulating factor (G-CSF),20 granulocyte-macrophage colony-stimulating factor (GM-CSF),23 interleukin-1 (IL-1),24 IL-6,25 tumor necrosis factor-α (TNF-α), tumor growth factor-β (TGF-β), and leukemia inhibitory factor (LIF).26 Many of these activities have also been observed in several osteoblast cell lines.22 These latter studies have shown that osteoblastic cell lines express several integrins that bind to hematopoietic progenitors.27,28
Interestingly, in several situations in which bone remodeling is compromised, hematopoiesis is also compromised.29-32 A dramatic phenotype was observed in mice deficient in Cbfa1, a transcription factor crucial for osteoblast progression. These animals do not develop osteoblasts, and they die at birth of respiratory failure. One of the major phenotypes in Cbfa1-deficient mice is the complete absence of bone marrow, indicating that osteoblasts are required to initiate bone marrow hematopoiesis.33,34 In addition, analysis of embryonic hematopoiesis in these mutants showed normal hematopoietic development in liver and spleen until day E17.5. However, at day E18.5 both organs exhibit signs of excessive extramedullary hematopoiesis.35 Recently, 2 reports have shown that osteoblast number defines the potential of animals to generate long-term hematopoietic stem cells and that osteoblasts probably are crucial for the generation of early hematopoietic niches in the bone marrow.36,37
We have generated a transgenic mouse line expressing herpesvirus thymidine kinase under the control of a 2.3-kilobase (kb) fragment of the rat collagen α1 type I promoter (Col2.3ΔTK).38 We found that, as predicted by the promoter activity, the transgene is expressed selectively in differentiated osteoblasts. After treatment with ganciclovir these mice suffered a progressive bone loss that is consistent with the ablation of osteoblasts. In addition, ganciclovir-treated Col2.3ΔTK mice present a dramatic loss of bone marrow cellularity, indicating that the presence of osteoblasts is important for the development of active hematopoiesis. In the present work we have analyzed the effect of osteoblast loss on hematopoiesis by phenotypic analysis of different hematopoietic compartments and by evaluation of in vitro hematopoietic activity during the ablation process. We have found that osteoblast ablation leads to a stop of differentiation in the bone marrow in conjunction with the establishment of an active process of extramedullary hematopoiesis. The presence of hematopoietic progenitors, evaluated by phenotypic and in vitro functional assays, also indicated that the absence of osteoblasts progressively affected the progenitor pool by blocking the progression of bone marrow hematopoiesis. Interestingly, this process was reversible, because the withdrawal of ganciclovir recovered osteoblasts in the bone and diminished the extramedullary hematopoietic process to basal levels.
Materials and methods
Mice
The generation and initial characterization of the Col2.3ΔTK transgenic mice has been described.38 All transgenic mice were bred and maintained at the Center for Laboratory Animal Care of the University of Connecticut Health Center. All the animals were used between 4 to 12 weeks of age. Animals were housed in sterile microisolators or in Thorens microisolators at no more than 5 mice per cage and given water and rodent chow ad libitum. All animal protocols used in this report were approved by the Animal Care Committee of the University of Connecticut Health Center.
Ganciclovir treatment
For all the treatments we used a dose of 8 mg/kg/d of ganciclovir (GCV) (Cytovene-IV; Roche Pharmaceutical, Nutley, NJ) in phosphate-buffered saline (PBS). This dose was defined previously and was administered by injecting 80 to 120 μL of a solution of 0.4 mg/mL GCV 2 times a day intraperitoneally, separated by an interval of 12 hours. Controls were nontransgenic animals injected with a similar dose of GCV, or transgenic animals injected with an equivalent volume of PBS were used.
Antibodies and flow cytometry
Lineage-specific monoclonal antibodies (mAbs) used in the dissection of bone marrow populations have been described39,40 ; these included anti-B-lineage cell mAb, anti-CD45R (B220) (RA3-6B2); antimonocyte-antimacrophage mAb, anti-Mac1 (M1/70); antigranulocyte mAb, anti-Gr1 (RB6-8C5); anti-T-cell lineage antibodies, mAbs anti-CD4 (GK1.5) and anti-CD8 (53.6.7); antierythroid progenitor monoclonal antibody (Ter119); antibodies to allelic determinants Thy1.1 (19XE5), Thy1.2 (53.2.1), CD45.1/Ly5.2 (104.2), CD45.2/Ly 5.1 (A20.1); anti-NK antibodies, NK1.1 (PK136); and antiprogenitor cells, Sca-1 (E13.161.7), anti-cKit/CD117 (2B8). All these antibodies are obtained directly conjugated to fluorochromes from commercial vendors including BD Pharmingen (San Jose, CA), e-Biosciences (San Diego, CA), and Caltag (Burlingame, CA).
Labeling of cells for cytofluorometric analysis or cell sorting was performed by standard staining procedures wherein directly conjugated or unconjugated plus second-step reagents were sequentially added to the cell preparation of interest. All antibodies were optimized in terms of their concentration and titration. All staining was done on ice to prevent quenching of signals. Dead cells were identified by their ability to incorporate propidium iodide. Flow cytometric analysis was performed in a FacsCalibur or a BD-LSR multicolor analyzer (BD Biosciences, San Jose, CA).
Histology
Tibias were fixed in 4% paraformaldehyde at 4°C for 7 days. After fixation, bones were decalcified in 15% EDTA (ethylenediaminetetraacetic acid) for 1 week, dehydrated in progressive concentrations of ethanol, cleared in xylene, and embedded in paraffin. The entire tibia was then sectioned longitudinally in 5 μm per section. Sections from the center of the tibia were used for histologic staining with hematoxylin and eosin.
Methylcellulose cultures
Cell suspensions were prepared from bone marrow, spleen, and liver in Iscove modified Dulbecco medium (IMDM) (Gibco, Grand Island, NY) supplemented with 2% fetal bovine serum. Aliquots were then plated in a methylcellulose medium with recombinant cytokines for colony assays of murine cells (Methocult M3434; StemCell Technologies, Vancouver, BC, Canada). This medium contained stem cell factor, IL-3, IL-6, and erythropoietin and allowed us to evaluate the activity of myeloerythroid progenitors. The plating was done according to the instructions of the manufacturer using between 2 × 104 and 1 × 105 cells per 35-mm tissue culture dish. Cultures were placed in a humidified chamber and incubated at 37°C with 5% CO2. The total number of colonies were counted between days 10 and 14 after plating.
Osteoclast progenitor assay
Total bone marrow suspensions from control and transgenic animals treated with GCV or PBS were cultured in α minimal essential medium (αMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) for 6 to 7 days. Culture media contained recombinant mouse M-CSF (rmM-CSF) (30 ng/mL; R&D Systems, Minneapolis, MN) and recombinant mouse receptor activator of nuclear factor-κB ligand (rmRANKL) (30 ng/mL; a gift from Immunex, Seattle, WA) and was changed every 3 days. To visualize osteoclasts, at the end of the experiment cells were fixed with 2.5% glutaraldehyde in PBS for 30 minutes at room temperature and then stained for tartrate-resistant acid phosphatase (TRAP). This last step was done using a commercial kit (Sigma-Aldrich, St Louis, MO). Osteoclasts were analyzed under an inverted microscope as TRAP-positive multinucleated cells, with at least 3 nuclei per cell.
Results
Col2.3ΔTK transgenic mice, a model for conditional ablation of osteoblasts
The Col1A1 promoter has proven to be useful for marking stages of osteoblast differentiation, because reporter genes under the control of this promoter localize predominantly to bone cells.41 A transgenic mouse model expressing thymidine kinase (TK) from herpesvirus, under the control of a 2.3 kb fragment of the Col1A1 promoter, was generated.38 This fragment was shown to direct the expression of gene products to osteoblasts. Thus, it was expected that treatment of these animals with the antiviral drug ganciclovir (GCV) would delete cells expressing TK. This prediction proved to be correct, and the main phenotype of these animals, under GCV treatment, was the progressive loss of bone due to the ablation of osteoblastic lineage cells. The depletion of osteoblasts was detected by histologic analysis as early as 7 days after daily injections of GCV at doses between 3 and 8 mg/kg per day. Interestingly, as depicted in Figure 1, this phenotype was accompanied by a drastic loss of bone marrow cellularity. This was unexpected, because previous studies that used a similar transgenic model employing the promoter for osteocalcin to drive the expression of TK did not show an appreciable loss of bone marrow cellularity.42 Because osteocalcin is expressed in more differentiated stages of the osteoblast lineage, one explanation for these discrepancies is that osteoblasts not only maintain homeostasis of bone but also regulate hematopoiesis in a developmentally regulated fashion.
Depletion of osteoblasts affects cellularity in the bone marrow. (A) Control wild-type littermates and Col2.3ΔTK mice were injected with GCV; at defined times they were killed, and their bones were subjected to histologic analysis. Tissue was placed in paraffin and stained with hematoxylin and eosin. Microphotographs were taken at 10 × magnification. (B) Absolute number of cells recovered from bone marrow of 4 long bones in control and Col2.3ΔTK mice after GCV treatment.
Depletion of osteoblasts affects cellularity in the bone marrow. (A) Control wild-type littermates and Col2.3ΔTK mice were injected with GCV; at defined times they were killed, and their bones were subjected to histologic analysis. Tissue was placed in paraffin and stained with hematoxylin and eosin. Microphotographs were taken at 10 × magnification. (B) Absolute number of cells recovered from bone marrow of 4 long bones in control and Col2.3ΔTK mice after GCV treatment.
Loss of bone marrow cellularity in the Col2.3ΔTK transgenic mice can be explained by a block in hematopoietic lineage progression
The loss in bone marrow cellularity could be explained by the direct elimination of hematopoietic cells after GCV treatment. The evidence against this possibility was that wild-type mice treated with a similar dose of ganciclovir did not suffer an appreciable loss of hematopoietic cells (Figure 1). Also, the initial analysis of these transgenic mice did not show TK expression in hematopoietic cells, as assessed by RNA analysis and immunoreactivity with anti-TK antibodies. The loss of marrow components drove us to investigate if this was due to the elimination of progenitors or to blockage of lineage commitment/progression. Both possibilities were feasible, because osteoblast cultures have shown the potential to produce factors involved in maintenance and self-renewal of HSCs as well as cytokines important for the progression and maintenance of various hematopoietic lineages.
To find the level at which hematopoiesis was compromised, we performed a phenotypic analysis by multiparameter flow cytometry using specific antibodies directed against different mature hematopoietic lineages. We initially evaluated B lymphocytes, T lymphocytes, natural killer (NK) cells, myeloid cells, and erythroid progenitors. We analyzed peripheral blood leukocytes plus cell suspensions from bone marrow, spleen, thymus, and liver. In agreement with our original hematologic analyses (not shown), we did not find appreciable changes in the hematopoietic compartment in peripheral blood leukocytes (PBLs). However, in other organs we noticed disturbances in the distribution of hematopoietic cells.
Figure 2 shows the analysis done in bone marrow, spleen, and liver cell suspensions using B-cell-specific markers (CD19 and B220), T-cell markers (CD3), myeloid markers (CD11b), and erythroid markers (Ter119). From the analysis of bone marrow it was evident that B-cell lymphopoiesis was drastically reduced; levels of B cells fell from 30% to 1% at day 25 of GCV treatment. A reduction in B cells was noticed as early as 8 days into GCV treatment, consistent with the reported disappearance of osteoblasts. Even when the depicted analysis seemed not to demonstrate major changes in the relative percent of myeloid lineage components, the number of cells in this compartment gradually disappeared. The cellularity at day 25 was at least 5 times lower in the transgenic treated group (Figure 1). The relative increase in myeloid components could be explained by longer half-life of the compartment. Interestingly, when peripheral organs were analyzed, a relative increase in myeloid components was observed consistent with the establishment of extramedullary hematopoiesis. A similar trend to the one seen for B cells was observed for erythroid progenitors, as assessed by staining with the antibody Ter119. Interestingly, both B cells and erythrocytes are primarily generated in bone marrow. No major changes were observed in the thymus when analyzed with antibodies against CD4, CD8, and CD3 (data not shown). These phenotypic changes were observed only in transgenic mice treated with GCV and not in transgenic mice injected with similar volumes of PBS (control group). This latter group had a phenotypic profile similar to wild-type CD1 mice injected either with PBS or GCV, which also were used as controls (not shown).
Osteoblast depletion alters the hematopoietic compartments. Two-color flow cytometric analysis of leukocyte suspensions from bone marrow, spleen, and liver isolated from Col2.3ΔTK mice injected for 25 days with PBS (control group) or with GCV. Cell suspensions were stained with antibodies against B cells (CD45R/B220 and CD19), T cells (CD3), myeloid cells (CD11b/Mac1), and erythroid progenitors (Ter119). Numbers in each region represent the proportion of cells in the total population.
Osteoblast depletion alters the hematopoietic compartments. Two-color flow cytometric analysis of leukocyte suspensions from bone marrow, spleen, and liver isolated from Col2.3ΔTK mice injected for 25 days with PBS (control group) or with GCV. Cell suspensions were stained with antibodies against B cells (CD45R/B220 and CD19), T cells (CD3), myeloid cells (CD11b/Mac1), and erythroid progenitors (Ter119). Numbers in each region represent the proportion of cells in the total population.
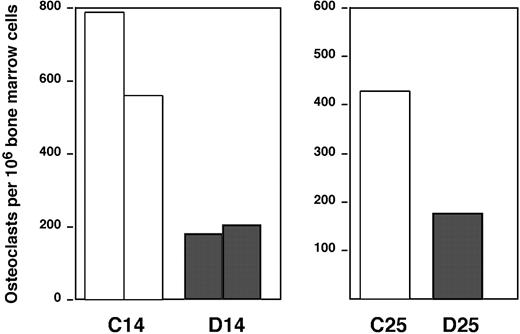
It is well documented that osteoclasts are regulated by osteoblasts during development and during bone remodeling processes after injuries.19,43 The analysis of this compartment by flow cytometry is impaired by the intimate association of these cells to bone tissue and the lack of specific surface markers. However, initial histologic analysis provided evidence that mature osteoclasts were decreased after elimination of osteoblasts by GCV. To evaluate if osteoclast progenitors were also affected, we quantified the ability to generate osteoclasts in vitro from total bone marrow cells cultured with M-CSF plus RANKL. As predicted, in Figure 3 we found that bone marrow from Col2.3ΔTK animals presents a lower number of osteoclast progenitors after GCV treatment.
Analysis of osteoclastogenesis after osteoblast depletion. Bone marrow suspensions were plated in media containing 30 ng/mL M-CSF plus 30 ng/mL RANKL. Five days later, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP). Osteoclast generation was evaluated by counting the number of TRAP-positive multinucleated cells. The groups considered are the following: Col2.3ΔTK mice treated with PBS (□) and Col2.3ΔTK mice treated with GCV (▦). The left graph shows the data from groups treated for 14 days (2 experiments); the right graph, data for mice treated for 25 days (1 experiment).
Analysis of osteoclastogenesis after osteoblast depletion. Bone marrow suspensions were plated in media containing 30 ng/mL M-CSF plus 30 ng/mL RANKL. Five days later, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP). Osteoclast generation was evaluated by counting the number of TRAP-positive multinucleated cells. The groups considered are the following: Col2.3ΔTK mice treated with PBS (□) and Col2.3ΔTK mice treated with GCV (▦). The left graph shows the data from groups treated for 14 days (2 experiments); the right graph, data for mice treated for 25 days (1 experiment).
Loss of functional hematopoiesis in the bone marrow induces a strong extramedullary hematopoiesis
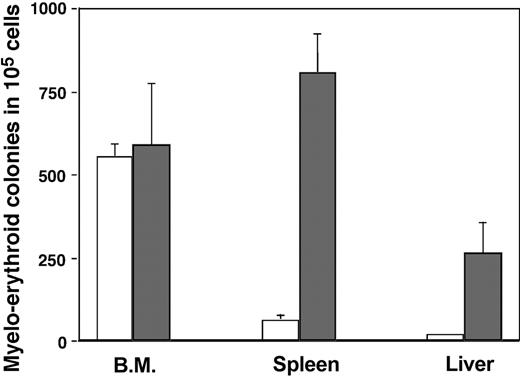
As mentioned, the phenotypic analysis indicated the possibility of extramedullary hematopoiesis. This is not uncommon and is observed in many types of myelodeficiency. In addition, several organs maintain low basal levels of hematopoietic progenitors. We explored this possibility by evaluating the presence of HSCs using phenotypic and functional assays. A limitation of our experimental system is the fact that the original transgenic mice were generated in an outbred mouse strain (CD1). Hence, we were not able to analyze HSC function by rescue and reconstitution of lethally irradiated recipients, because rejection was a certainty using outbred mice. Instead, we scored the potential of cell suspensions to differentiate into myeloerythroid components by plating them in methylcellulose supplemented with a cocktail of cytokines that drive early progenitors toward myeloerythroid development. Figure 4 demonstrates that no major differences exist between transgenic bone marrow cells isolated from animals treated with or without GCV for 25 days. In contrast, spleen and liver, organs with low basal colony-forming units, tremendously increase their potential to generate myeloerythroid colonies. This finding demonstrated clearly that extramedullary hematopoiesis was taking place when osteoblasts were eliminated.
Analysis of myeloerythroid progenitors after osteoblast depletion. Bone marrow, spleen, and liver suspensions from Col2.3ΔTK mice treated with PBS (□) or GCV (▦) were plated in methylcellulose media supplemented with CSF, IL-3, IL-6, and erythropoietin (Methocult M3434; StemCell Technologies). After 10 days of culture, the total number of colonies were counted as a representation of early myeloerythroid progenitors. The experiment was done after 25 days of treatment and includes 3 to 5 mice per experimental group.
Analysis of myeloerythroid progenitors after osteoblast depletion. Bone marrow, spleen, and liver suspensions from Col2.3ΔTK mice treated with PBS (□) or GCV (▦) were plated in methylcellulose media supplemented with CSF, IL-3, IL-6, and erythropoietin (Methocult M3434; StemCell Technologies). After 10 days of culture, the total number of colonies were counted as a representation of early myeloerythroid progenitors. The experiment was done after 25 days of treatment and includes 3 to 5 mice per experimental group.
Figure 5 shows flow cytometric analysis for evaluation of HSC phenotype from representative animals at day 25 after GCV treatment. In this assay, cells suspensions were stained with a cocktail of antibodies reacting against cell surface antigens expressed in mature hematopoietic cells from all the lineages. In addition, cells were also stained with 2 antibodies that react with antigens present on hematopoietic progenitors: Ly-6A/Sca-1 and cKit. The analysis of HSCs was done testing the progenitor markers in the context of cells negative for mature hematopoietic markers or lineage-negative (Lin-) cells. The population with the phenotype Lin- Sca-1+ cKit+ is highly enriched in HSCs.39 As expected, in control animals, these cells are readily detected in bone marrow but not in spleen or liver. Strikingly, transgenic mice treated with GCV showed unusually large proportions of cells with progenitor phenotype in peripheral organs, consistent with a process of extramedullary hematopoiesis. The persistence of cells bearing an HSC phenotype in bone marrow after GCV treatment indicates that these cells were not being eliminated nonspecifically as a result of drug treatment. This conclusion was also enforced by the observation that normal control animals treated with similar doses of GCV did not present deficiencies in hematopoietic parameters (data not shown). However, a progressive loss of early hematopoietic progenitors was evident after evaluation of cells with the phenotype Lin- Sca-1+ cKit+ within the total bone marrow population. After 25 days, in transgenic mice injected with PBS, these cells account for 0.047% ± 0.024% of the total population. In contrast, in the groups injected with GCV, they constitute 0.100% ± 0.054% of the total population. Considering that the cellularity of bone marrow in animals treated with GCV for 25 days was 5 to 10 times lower (Figure 1), then the absolute number of cells with the HSC phenotype decreased approximately 3- to 10-fold in animals treated with GCV compared with wild-type controls or transgenic animals injected with PBS.
Phenotypic analysis of early hematopoietic progenitors after osteoblast depletion. Cell suspensions from bone marrow, spleen, and liver were prepared from transgenic mice treated for 25 days with PBS (top contour plots) or GCV (bottom contour plots). Cells were stained with a cocktail of antibodies labeled with phycoerythrin and reactive against mature hematopoietic lineage components (lymphocytes, macrophages, granulocytes, erythrocytes). The cells were subsequentially stained with 2 antibodies reactive with early hematopoietic progenitors (Sca-1 coupled to fluorescein isothiocyanate [FITC] and cKit coupled to allophycocyanin). The figure shows representative contour profiles of the reactivity for progenitor markers in populations negative for lineage markers. The framed regions are the areas corresponding to cells with HSC phenotype, and the number shows the percent of cells contained within the Lin- population.
Phenotypic analysis of early hematopoietic progenitors after osteoblast depletion. Cell suspensions from bone marrow, spleen, and liver were prepared from transgenic mice treated for 25 days with PBS (top contour plots) or GCV (bottom contour plots). Cells were stained with a cocktail of antibodies labeled with phycoerythrin and reactive against mature hematopoietic lineage components (lymphocytes, macrophages, granulocytes, erythrocytes). The cells were subsequentially stained with 2 antibodies reactive with early hematopoietic progenitors (Sca-1 coupled to fluorescein isothiocyanate [FITC] and cKit coupled to allophycocyanin). The figure shows representative contour profiles of the reactivity for progenitor markers in populations negative for lineage markers. The framed regions are the areas corresponding to cells with HSC phenotype, and the number shows the percent of cells contained within the Lin- population.
The observed switch to active primary hematopoiesis in the periphery can be accounted for by the migration of HSCs from bone marrow compartments to spleen and liver. Alternatively, the low number of organ-resident HSCs could be the result of a reactivation by feedback mechanisms that sensed the deficiency of hematopoiesis in the bone marrow. To test if we could detect the migration of progenitors at early times of GCV treatment, we evaluated peripheral blood cells for their ability to generate myeloerythroid colonies in methylcellulose. These results are depicted in Figure 6, in which we compared kinetically the generation of methylcellulose colonies in different compartments. A very low number of colonies were generated from PBLs in normal conditions, and a modest increase was observed after day 7. We feel that this result is not conclusive, because this time is coincident with the increase of HSCs and colony-forming progenitor cells in all peripheral sources, and the observed increase could be also explained by leakage of newly generated progenitors from liver and spleen into the circulation.
Kinetics of medullarly and extramedullary hematopoiesis after GCV treatment. Organs and peripheral blood leukocyte suspensions were prepared from Col2.3ΔTK transgenic mice treated with PBS (□) or ganciclovir (•) at different time points. Cells were plated in methylcellulose semisolid media supplemented with cytokines that permit the development of myeloerythroid colonies. The colonies were evaluated as described in the legend to Figure 4.
Kinetics of medullarly and extramedullary hematopoiesis after GCV treatment. Organs and peripheral blood leukocyte suspensions were prepared from Col2.3ΔTK transgenic mice treated with PBS (□) or ganciclovir (•) at different time points. Cells were plated in methylcellulose semisolid media supplemented with cytokines that permit the development of myeloerythroid colonies. The colonies were evaluated as described in the legend to Figure 4.
The phenotype induced by the ablation of osteoblast upon treatment with GCV is partially reversible after withdrawal of the drug
One of the interesting observations reported in the initial characterization of this model is that the bone loss was reversed upon withdrawal of the GCV. This recovery was not completely normal, because an exacerbated formation of bone persists, probably as a result of the loss of coupling between osteoblast and osteoclasts.
Figure 7 shows the histologic analysis of bone marrow during the recovery phase. As shown, the bone marrow recovered slowly. The bone cavity is reconstituted, mostly with mineralized trabeculae, generating pockets of hematopoiesis instead of a continuous bone marrow compartment. Analysis of bone marrow hematopoiesis at this time has not been performed. However, analysis of extramedullary hematopoiesis indicated that after recovery this process returned to basal levels in liver and spleen. Interestingly, the recovery from extramedullary hematopoiesis to medullary hematopoiesis took longer compared with its initiation shortly after GCV treatment.
Analysis of bone marrow cellularity and extramedullary hematopoiesis after GCV withdrawal. (A) Col2.3ΔTK mice were treated with PBS or GCV for 45 days, after which the treatment was stopped. At different days after the withdrawal of treatment, animals were killed and their bones were evaluated histologically for their ability to reconstitute bone marrow cellularity. Paraffin section were stained with hematoxylin and eosin, and microphotographs were taken at an original magnification of 10 ×. (B) Animals were treated with ganciclovir or PBS as described. Spleen and liver suspensions were evaluated by their ability to generate myeloerythroid colonies in methylcellulose at different time points after withdrawal of treatment.
Analysis of bone marrow cellularity and extramedullary hematopoiesis after GCV withdrawal. (A) Col2.3ΔTK mice were treated with PBS or GCV for 45 days, after which the treatment was stopped. At different days after the withdrawal of treatment, animals were killed and their bones were evaluated histologically for their ability to reconstitute bone marrow cellularity. Paraffin section were stained with hematoxylin and eosin, and microphotographs were taken at an original magnification of 10 ×. (B) Animals were treated with ganciclovir or PBS as described. Spleen and liver suspensions were evaluated by their ability to generate myeloerythroid colonies in methylcellulose at different time points after withdrawal of treatment.
Discussion
Cumulative indirect evidence links osteoblasts to hematopoiesis. Osteoblastogenesis is temporarily linked to the initiation of bone marrow hematopoiesis,44,45 osteoblasts regulate the differentiation of osteoclasts,19 and osteoblast cultures have the potential to produce several hematopoietic cytokines.13,19-21 However, a direct study of the role that interactions between these 2 cell types have in vivo has been limited by the difficulties identifying and isolating osteoblasts.
The development of new technologies allowing us to isolate different stages of osteoblast development, and to delete this population on demand, has opened new ways to answer fundamental questions both in bone development and hematopoiesis. In this paper we provide one of the first in vivo demonstrations of the role that osteoblasts have on hematopoiesis. Using a transgenic model in which we can selectively eliminate osteoblasts after treatment with ganciclovir, we showed that loss of osteoblasts induces dramatic changes in the dynamics of hematopoiesis. Our initial observation showing that bone marrow loses cellularity as osteoblasts are being depleted suggested that hematopoiesis was being arrested after GCV treatment. Interestingly, this effect was not reported in a previous similar transgenic model that used the TK gene under the control of the osteocalcin promoter. It is likely that these differences develop because the osteocalcin promoter is expressed at later developmental time points than is the Col2.3 promoter utilized in this report.42 The finding that both mice presented similar bone phenotypes implies to us that the influence of osteoblasts in hematopoietic processes is developmentally regulated.
The presence of cells bearing an HSC phenotype, and the ability of bone marrow cells from GCV-treated transgenic mice to progress normally in methylcellulose cultures, suggests that osteoblasts are not essential for the maintenance of early progenitors. However, this needs to be further investigated, because our assays are short term and are limited to in vitro functional assays. The availability of transgenic mice in an inbred background will be essential to define these parameters by in vivo transplantation studies. It has been reported that GCV treatment can directly affect hematopoiesis; however, we did not observe any alteration in hematopoiesis in our control groups, which included normal mice treated with similar doses of the drug and transgenic mice treated with PBS.
The early defects observed on B lymphopoiesis and erythropoiesis, with persistence of active in vitro primary hematopoiesis, suggest that osteoblast deficiency arrests hematopoietic development in the bone marrow. This effect is coupled to a progressive loss of cells with an HSC phenotype in the bone marrow, which ultimately should lead to the death of the mice because of hematologic failure. However, animals survive the osteoblast depletion due to the establishment of an active extramedullary hematopoietic process. The mechanisms regulating this switch are not well defined. Most extramedullary hematopoietic development occurs because progenitors migrate to the periphery under conditions of stress in the bone marrow microenvironment.29-32 Our search for early hematopoietic progenitors in peripheral blood following GCV treatment is not conclusive, and more detailed analysis using transplantation of bone marrow progenitors will have to await the availability of the Col2.3ΔTK mice in a defined inbred background. Currently we are backcrossing the transgenic animals with C57BL/6 mice.
The reversibility of the bone phenotype, after GCV withdrawal, gave us the opportunity to test if hematopoiesis was also normalized in the bone marrow. It is clear from histologic analyses that after withdrawal of GCV the bone marrow began to repopulate its hematopoietic components. Interestingly, the process did not recover a normal bone marrow morphology, because mesenchymal progression proceeded in a disorganized fashion with increases in trabecular bone and adipogenesis. As indicated, one of the hematopoietic alterations induced by the osteoblast depletion is deficiency of bone marrow osteoclastogenesis. This alteration may produce the anomalous progression of trabecular bone generated de novo after GCV withdrawal because of a misbalance in the bone remodeling process.
Two recent reports have shown strong evidence for a role of osteoblasts in defining hematopoiesis.36,37 These studies showed clearly that increases in osteoblastic cells, achieved by 2 different genetic approaches, correlated with enhanced ability of stroma cultures to support early hematopoiesis, an increase in long-term reconstituting HSCs in vivo, and the establishment of new hematopoietic niches. Our report is complementary to those studies and provides the first in vivo experimental system showing that elimination of osteoblasts profoundly affects adult hematopoiesis.
It is still not completely known what drives the development of hematopoiesis in bone marrow, or which factors define the bone marrow microenvironment, and permits the engraftment of HSCs, their self-renewing properties, and their decisions to proliferate and go into lineage-committed pathways. The relationships between the bone marrow microenvironments and the microenvironment that develops in liver and spleen in situations in which extramedullary hematopoiesis is established are also unknown. If we achieve a complete knowledge of the factors that define hematopoiesis and the dynamics of the cellular interactions within the bone marrow microenvironment, we likely will be able to develop better ways to direct engraftment after bone marrow transplantation. Finally, the promise of mesenchymal progenitor cell transplantations to mediate regenerative therapies,46 including modification of hematopoietic microenvironments,47,48 relies on a better understanding of the relationships between mesenchymal components and other systems. The experimental approach delineated in this report provides a system in which we can begin to answer many of these questions.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-11-4011.
Supported by University of Connecticut Health Center Fund, National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant RO1 AI46708-01 (H.L.A.), NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) grant RO1 AR43457-06 (D.R.), and NIH/NIAMS grant RO1 AR4871401 (J.L.). Z.K. was supported by a fellowship from the Osteogenesis Imperfecta Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
D.V. and Z.K. contributed equally to this report.
An Inside Blood analysis of this article appears in the front of this issue.



![Figure 5. Phenotypic analysis of early hematopoietic progenitors after osteoblast depletion. Cell suspensions from bone marrow, spleen, and liver were prepared from transgenic mice treated for 25 days with PBS (top contour plots) or GCV (bottom contour plots). Cells were stained with a cocktail of antibodies labeled with phycoerythrin and reactive against mature hematopoietic lineage components (lymphocytes, macrophages, granulocytes, erythrocytes). The cells were subsequentially stained with 2 antibodies reactive with early hematopoietic progenitors (Sca-1 coupled to fluorescein isothiocyanate [FITC] and cKit coupled to allophycocyanin). The figure shows representative contour profiles of the reactivity for progenitor markers in populations negative for lineage markers. The framed regions are the areas corresponding to cells with HSC phenotype, and the number shows the percent of cells contained within the Lin- population.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-11-4011/6/m_zh80090460580005.jpeg?Expires=1765900497&Signature=birST8NwfeYxtCCggNG~71vqXn0jldDkqUGWqgszI0NQE8LPhA3IislQhBS1lajUGPTXkWQ51iI5Jf8qBAsFQ~52W-AKrnfAnOQGoE6PEmvL~Eycq2pIxLNJGy6bBrsCYmKpMZwc8wxcXyN9dYn20Ao8HlbGUjHhomALztgoJd9dyKzcnNff2uVhJRLWVQ~ekcmXhgtF7xf7PU1ABQACROO1LXmrNL6kUdIzqbI55DTbGswSYnkWEwd7dL3NDHTPvquxCvONJZSGayyCDt~~Sd1XJDRAspb-QCGsq37R1FXXSRYK067vitxOPVdN-~TZxTNnG8BLJ-ugVfsZgqJ5Qg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal