HEMOSTASIS, THROMBOSIS, AND VASCULAR BIOLOGY
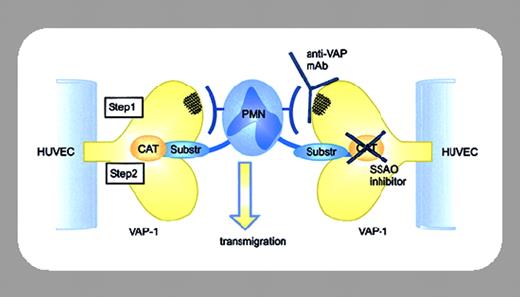
Polymorphonuclear leukocyte (PMN) migration through the endothelium to sites of tissue inflammation is regulated by a complex network of signals involving adhesion molecules and chemotactic cytokines. The first stage of the extravasation cascade involves leukocyte tethering to the endothelial surface, followed by rolling, events largely mediated via selectins. If additional activation signals are received, the leukocytes proceed to the second stage and firmly adhere. This phase is mediated by a variety of cell adhesion molecules. Leukocytes then transmigrate through the endothelial layer, and it is this third stage of the extravasation cascade that is perhaps the least well understood. In this issue of Blood, Koskinen and colleagues (page 3388) provide new insight into how leukocyte transmigration may be regulated. Vascular adhesion protein-1 (VAP-1) is unique amongst endothelial cell-expressed adhesion molecules because it possesses semicarbazide-sensitive amine oxidase (SSAO) activity. Until now it has not been clear whether the enzymatic activity of VAP-1 contributes to its actions as an adhesion molecule. Koskinen and colleagues provide the first evidence that VAP-1 SSAO activity is required for leukocyte rolling and transmigration through the endothelium.
The plethora of signals regulating the extravasation cascade of leukocytes can complicate assessment of the contributions made by individual molecules. The studies of Koskinen et al were facilitated by the fact that human umbilical vein endothelial cells (HUVECs) are normally VAP-1-negative, enabling expression of wild-type VAP-1 or catalytically inactive (Y471F) VAP-1 without the complication of endogenous VAP-1. The effects of SSAO inhibitors and anti-VAP-1 antibodies on rolling, firm adhesion, and transmigration of PMNs on the transduced HUVECs were quantified using a laminar shear flow capillary model designed to recapitulate the conditions of shear stress in postcapillary venules. BTT-2027, a newly described SSAO inhibitor, reduced leukocyte rolling on HUVECs expressing wild-type VAP-1, as did the prototypical SSAO inhibitors semicarbazide and hydroxylamine. Although the features of BTT-2027 have yet to be fully reported, this is the first evidence linking the enzymatic activity of VAP-1 to a role in mediating PMN migration. More striking and perhaps significant is the demonstration that VAP-1 SSAO activity is also required for leukocyte transmigration. Remarkably, BTT-2027 reduced transmigration through wild-type VAP-1-transduced HUVECs by almost 50%, quite surprising considering the wide range of cell adhesion molecule interactions occurring between PMNs and endothelial cells. The authors propose a sequential model for adhesion and SSAO activity in facilitating transmigration, but how the adhesive and enzymatic properties of VAP-1 collaborate to facilitate transmigration remains enigmatic. Clearly, further investigations will be required to determine whether one or more of the products of VAP-1 enzymatic activity are required to facilitate transmigration and, if so, their mechanism of action. Identification of molecules that interact with and activate VAP-1 are also important future goals. Critically for this study, preadministration of BTT-2027 reduced PMN extravasation in an in vivo model of acute inflammation. This “proof of principle” identifies VAP-1 SSAO activity as a potential new target for anti-inflammatory therapy, a particularly attractive prospect since VAP-1 is one of the few molecules currently known to possess SSAO activity in humans.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal