Rezvani et al have recently reported the presence of leukemia-associated antigen-specific memory CD8+ T cells in human leukocyte antigen (HLA)-A2–positive healthy individuals and chronic myeloid leukemia (CML) patients before and after stem cell transplantation (SCT).1 CD8+ cells specific for HLA-A2–restricted proteinase-3 (PR1) and Wilms tumor (WT1) peptides were undetectable using specific tetramers in all but 2 cases, both healthy individuals. Tetramers of HLA-A2 with BCR-ABL junctional peptide sequences were not used. Using quantitative polymerase chain reaction (qPCR) to measure interferon-gamma mRNA production in response to peptide-pulsed targets, Rezvani et al showed that CD8+ T cells with specificity for PR1 and WT1 peptides were present in both healthy individuals and CML patients. In contrast, T cells with specificity for the b3a2 BCR-ABL junctional peptide sequence GFKQSSKAL were identifiable only in the patient population.
Using tetramers of HLA-A3 and the BCR-ABL junctional sequence KQSSKALQR, we have previously reported that BCR-ABL–directed CD8+ cells are circulating in some HLA-A3–positive CML patients.2 We recently have extended this study to a larger population of both CML patients and healthy subjects3 (N.B. et al, manuscript submitted, 2003). Using this HLA-A3 tetramer and also a tetramer of HLA-B8 and the junctional sequence GFKQSSKAL, we found that 3 of 18 assessable HLA-A3– and/or HLA-B8–positive healthy subjects had circulating tetramer-positive CD8+ cells directed against BCR-ABL. Representative plots are given in Figure 1. These findings are in contrast to the findings of Rezvani et al.1 However, in line with their findings, we found that 9 of 13 HLA–A3- and/or HLA-B8–positive CML patients had evidence of a tetramer-positive CD8+ population, which in most cases was present on several occasions. These 9 cases included 2 HLA-A3–positive cases who had undergone allogeneic SCT several years previously and who were consistently PCR negative for BCR-ABL.
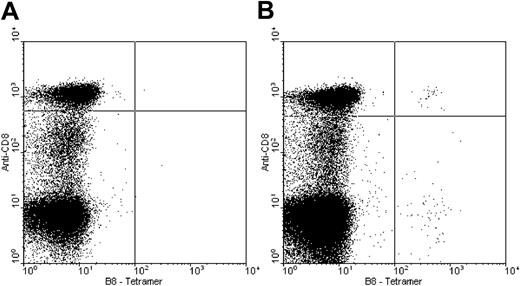
Peripheral blood mononuclear cells stained with a tetramer of HLA-B8-GFKQSSKAL. Panel A is from an HLA-B8–negative healthy donor, and panel B is from an HLA-B8–positive donor.
Peripheral blood mononuclear cells stained with a tetramer of HLA-B8-GFKQSSKAL. Panel A is from an HLA-B8–negative healthy donor, and panel B is from an HLA-B8–positive donor.
In an attempt to extend our observations to HLA-A2–positive patients and subjects, it has so far not been possible to make a tetramer of HLA-A2 with the BCR-ABL junctional sequence SSKALQRPV, which has been reported to bind well to HLA-A2.4 Interestingly, in a recent report, Cathcart and colleagues were unable to detect any CD8+ responses in 9 HLA-A2–positive patients immunized with SSKALQRPV, unlike their findings in HLA-A3–positive patients vaccinated with other junctional sequences.5 In contrast to the mass spectrometry findings with HLA-A3,2 it is not currently known whether HLA-A2–positive CML cells express a peptide derived from the BCR-ABL junction on the cell surface in association with HLA.
The observations of Rezvani et al make the useful point that qPCR may be more sensitive than tetramers in detecting low-frequency CD8+ populations and also go some way to defining the multi-epitope graft-versus-leukemia effect that prevails after allogeneic SCT in CML. We would, however, underline their caution against extrapolating their negative findings on CD8 responses against BCR-ABL in healthy individuals to HLA types other than HLA-A2, since the response against BCR-ABL may vary according to HLA type. Further studies on the HLA-specific response to BCR-ABL are required.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal