Abstract
The recent description of an early T-lineage progenitor (ETP) population in adult mouse thymus implies the presence of a bone marrow predecessor that has not yet been identified. Here we describe a LinNeg Sca-1Pos c-kitHi Thy-1.1Neg L-selectinPos adult mouse bone marrow population that resembles the thymic ETP in both antigen expression phenotype and posttransplantation lineage potential. These cells produce wavelike kinetics of thymic seeding and reconstitute the irradiated thymus with kinetics comparable to a thymocyte graft after intravenous transplantation. Transient B-lineage reconstitution is also observed, but little myeloid potential can be detected in transplant experiments. A second subset of progenitors is L-selectinNeg and is highly enriched for rapid and persistent T- and B-lineage potential, as well as some myeloid potential. L-selectin (CD62L) is therefore an effective marker for separating lymphoid progenitors from myeloid progenitors and hematopoietic stem cells in mouse bone marrow. (Blood. 2004;103: 2990-2996)
Introduction
The identity of T- and B-lymphoid progenitors in the hematopoietic environment of the bone marrow has been a topic of intense investigation for many years. Of particular interest has been the question of whether commitment to the T lineage occurs prior to or after homing of bone marrow–derived cells to the thymus.1 The direct approach to addressing this question has been to isolate progenitor populations from bone marrow and to evaluate their lineage potential in culture and transplant experiments. Using this approach, several groups have identified bone marrow progenitor cell subsets that are capable of both T- and B-lineage development, but lack significant levels of myeloid progenitor activity (common lymphoid progenitor [CLP]).2,3 Precursor cells committed to T-lineage differentiation (pre-T cells) have been identified in the thymus4 and fetal blood5 but not the bone marrow, suggesting that during adult life progenitor cells capable of both T- and B-lineage development commit to the T lineage only after entry into the thymus. This interpretation is supported by analysis of primitive mouse thymocyte populations, which has demonstrated the presence of intrathymic progenitors with T, B, natural killer (NK), myeloid, and dendritic cell potential.6-9 In human studies, common progenitors for the T and NK lineages have been identified,10 and the role of basic helix-loop-helix factors in regulating early human T-cell development has been reported.11 Recovery and retransplantation of early thymic migrants support the conclusion that although many cell types initially enter an irradiated thymus, commitment to lymphoid development occurs relatively rapidly.12 A recently described thymic population, termed the early T-lineage progenitor (ETP),13 is consistent with the concept of multipotent but lymphoid-biased progenitor cells as the most primitive cells in the thymus. However, the thymic ETP arises independently of previously described CLP cells in the bone marrow,2,3 suggesting that additional pathways of T-lymphoid development may exist in the bone marrow.1 If multiple pathways for T-lineage development do indeed exist, it is of considerable interest to determine the degree to which each pathway contributes to the mature T-cell pool. Considerations for such an analysis must include thymic homing, intrathymic expansion, and degree of contribution to peripheral T-cell pools.
We have recently described isolation of an adult mouse bone marrow progenitor population that is capable of repopulating an irradiated thymus within 2 weeks following intravenous transfer.14 Levels of donor cell engraftment were equivalent to those seen after hematopoietic stem cell (HSC) transplantation, but reached peak levels 9 days more rapidly4,14-16 and persisted for at least 2 months. These cells are identified phenotypically as LinNeg Sca-1Pos c-kitHi Thy-1.1Neg (hereafter referred to as the Thy-1.1Neg population). In addition to T-lineage potential, the Thy-1.1Neg population harbors notable B lymphocyte but very limited myeloid potential in intravenous transplantation experiments.14 In the absence of single-cell studies, which are difficult to perform in an intravenous transplantation model, it is unknown whether the Thy-1.1Neg population includes a mixture of separate precursors for T, B, and myeloid lineages, or if the separate lineages derive from multipotent progenitors that are biased toward lymphoid differentiation. Addressing the clonality question in culture models introduces several concerns, including the loss of a requirement for thymic homing and the possibility that lineage commitment may be redirected by culture conditions.17
We hypothesize that the Thy-1.1Neg population includes a subset comprising the immediate bone marrow precursor of the thymic ETP. To determine if such a progenitor exists within the Thy-1.1Neg population, we screened Thy-1.1Neg cells for markers that are also expressed by primitive thymocytes. We found that separation of the Thy-1.1Neg population based on L-selectin (CD62L) expression resolved 2 significant populations. The smaller L-selectinNeg fraction harbors a very high degree of B-lineage potential, along with high T-cell potential and notable myeloid potential. In contrast, the L-selectinPos fraction contains robust T-lineage progenitor activity, with minor B-lineage and myeloid potential similar to the thymic ETP. Furthermore, in vivo analysis showed that Thy-1.1Neg L-selectinPos progenitors colonize the thymus with wavelike kinetics reminiscent of normal thymic seeding.18,19 Accordingly, we conclude that the L-selectinPos population includes a bone marrow analog to the thymic ETP and likely includes the population from which the thymic ETP originates.
Materials and methods
Mouse strains
C57BL/6 (B6) and B6-Thy-1.1-Ly-5.1 mice were bred and maintained at the Animal Resource Center facility of the University of Utah. Mice used were between 4 and 24 weeks of age and were maintained on autoclaved, acidified water (pH 2.5) and autoclaved chow.
Antibodies
Monoclonal antibodies against CD8 (53-6.7), CD11b (M1/70), erythrocytes (TER119), Ly-6G (RB6-8C5), CD3 (KT3-1.1), CD5 (53-7.3), CD2 (Rm2.2), CD45R (B220; RA3-6B2), Thy-1.1 (19XE5), CD19 (1D3), L-selectin (Mel-14), and Ly-5.1 (A20) were purified from media of cultured hybridoma cell lines and were conjugated with biotin, phycoerythrin (PE), or fluorescein (F) in our laboratory. Biotinylated antibodies were secondarily stained with either PE-streptavidin (PE-SAv; Biomeda, Foster City, CA) or F-SAv (Biomeda). PE-conjugated monoclonal antibodies to Sca-1 and CD19, and allophycocyanin-conjugated c-kit (APC-c-kit) antibody were purchased from PharMingen (San Diego, CA).
Isolation of hematopoietic stem and progenitor cell populations
Bone marrow cells were isolated as previously described20 and incubated in a cocktail of antibodies to CD2, CD3, CD5, CD8, CD11b, Ly-6G, TER119, CD45R, and CD19. Lineage depletion was carried out by 2 successive incubations in sheep antirat Ig-coupled magnetic beads (Dynal, Oslo, Norway). LinNeg cells were stained with PE-Sca-1 and sorted using a FACS Vantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) in enrichment mode to collect all cells with PE emission above background, excluding dead cells based on forward scatter and propidium iodide (PI; Molecular Probes, Eugene, OR) staining. The sorted LinNeg Sca-1Pos cells were stained and resorted in normal mode into specific populations as noted in “Results.” For all experiments, cells were sorted directly onto 20 μL 100% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT) contained in the wells of a round-bottomed 96-well microtiter plate.
Methylcellulose assays
Cell populations were cultured in methylcellulose at a plating density of approximately 50 cells/35-mm culture dish. Each milliliter of culture media contained α-minimal essential medium (α-MEM; Gibco BRL, Gaithersburg, MD), 1.2% methylcellulose (Shinetsu, Tokyo, Japan), 30% FCS, 1% deionized bovine serum albumin (Sigma Chemical, St Louis, MO), 0.1 mM 2-mercaptoethanol (Mallinckrodt Chemical, Chesterfield, MO) supplemented with optimized concentrations of the cytokines listed (see “Cytokines”). Colonies were counted using an inverted microscope, and 4 to 8 plates were scored for each population tested. Individual colonies were plucked from the methylcellulose culture media with a 200-μL pipette, washed in Hanks balanced salt solution with 5% FCS (H5F), and labeled with F-labeled anti–Ly-6G and PE-labeled CD19 antibodies.
Cytokines
Steel factor (SF) and granulocyte colony-stimulating factor (G-CSF) were a kind gift from Gemini Science (San Diego, CA), a subsidiary of Kirin Pharmaceuticals. Flt3 ligand (Flt3L) and interleukin 6 (IL-6) were kindly provided by Immunex (Seattle, WA). Recombinant human erythropoietin (EPO) was purchased from Ortho Pharmaceuticals (Raritan, NJ). Recombinant murine IL-3 and IL-7 were purchased from Peprotech (Rocky Hill, NJ). The cytokines were used at the following concentrations: SF, 100 ng/mL; G-CSF, 10 ng/mL; Flt3L, 75 ng/mL; IL-6, 20 ng/mL; EPO, 5 U/mL; IL-3, 10 ng/mL; and IL-7, 10 ng/mL.
In vivo assessment of progenitor potential
All recipient mice were exposed to a single 6.5-Gy dose of radiation from a 137Cs source (Mark I gamma irradiator; J. L. Shepherd and Associates, Glendale, CA). Several hours later, mice were anesthetized with isofluorane (Isosol; Vedco, St Joseph, MO) and the purified cell population was infused intravenously via the retro-orbital sinus. Host animals were 6- to 24-week-old B6 mice; donor cells were obtained from 4-to 8-week-old B6-Thy-1.1-Ly-5.1 mice. Recipient animals were maintained on oral neomycin sulfate (Biosol, 2 mg/mL; Upjohn, Kalamazoo, MI) for 2 weeks after irradiation and transplantation. At the time points indicated, the recipient B6 mice were killed and each thymic lobe and the marrow from one femur were collected separately. Spleens were also collected in experiments where indicated in “Results.” Donor-derived cells were identified and phenotyped by flow cytometry using a FACScan analyzer (Becton Dickinson).
To assess the ability of cell populations to rescue lethally irradiated mice, host mice were given a second 6.5-Gy radiation dose 3 hours after the first dose and prior to intravenous injection of the purified cells to be tested.
Results
L-selectin is expressed by bone marrow progenitors and primitive thymocytes
Previous studies have indicated that thymocyte progenitor activity in mouse bone marrow is confined to the Sca-1Pos LinNeg compartment.14 Prothymocyte potential can be further enriched from marrow by selecting for bright expression of CD117 (c-kitHi),21 whereas negative selection for Thy-1.1 (Thy-1.1Neg) excludes HSCs.22 LinNeg Sca-1Pos c-kitHi Thy-1.1Neg cells largely lack CD127 (IL-7Rα), outcompete endogenous thymocyte recovery in mildly irradiated mice, and repopulate the thymus after intravenous transfer with kinetics more than 1 week faster than parallel grafts specifically enriched for HSCs.14 Although the Thy-1.1Neg population exhibits rapid and robust thymic engraftment, it also contributes to B-cell and myeloid lineages. To further define the prothymocyte potential within this population, we tested other markers for their ability to segregate specific hematopoietic potentials. Interestingly, the adhesion molecule L-selectin (CD62L), which is expressed by both primitive thymocytes23 and early bone marrow hematopoietic progenitors,24-26 is also expressed by a majority of cells in both the Thy-1.1Neg progenitor population and the Thy-1.1Lo HSC-containing fraction (Figure 1A-B). We found that 36% ± 5% of Thy-1.1Neg progenitors are positive for L-selectin expression (L-selectinPos), whereas 8% ± 1% of Thy-1.1Neg progenitors do not express this marker (L-selectinNeg; Figure 1A). Likewise, 43% ± 5% of LinNeg Sca-1Pos c-kitHi Thy-1.1Lo progenitors are positive for L-selectin expression and 32% ± 3% are negative (Figure 1B). We also assessed mouse thymocytes lacking CD4 and CD8 (double-negative [DN] thymocytes) for expression of c-kit and L-selectin. This analysis revealed that essentially all DN thymocytes expressing c-kit at high levels also express L-selectin (Figure 1C). Because thymic ETPs represent a subset of c-kitHi thymocytes,13 we conclude that thymic ETPs are also L-selectinPos. Totaled, the c-kitHi L-selectinPos fraction represents 6.6% ± 0.7% of DN thymocytes, whereas c-kitHi L-selectinNeg compartment comprises less than 0.5%.
Expression of L-selectin among primitive bone marrow and thymic progenitors. Representative expression of L-selectin and c-kit on LinNeg Sca-1Pos mouse bone marrow (A-B) and CD4- CD8- thymus cells (C). For bone marrow statistics, percentages were averaged from 9 separate sorting experiments representing marrow pooled from 68 total donor mice. Thymic statistics represent 9 separate mouse thymuses. In all cases, error indicates SEM.
Expression of L-selectin among primitive bone marrow and thymic progenitors. Representative expression of L-selectin and c-kit on LinNeg Sca-1Pos mouse bone marrow (A-B) and CD4- CD8- thymus cells (C). For bone marrow statistics, percentages were averaged from 9 separate sorting experiments representing marrow pooled from 68 total donor mice. Thymic statistics represent 9 separate mouse thymuses. In all cases, error indicates SEM.
Expression of L-selectin separates hematopoietic populations with distinct thymic engraftment properties
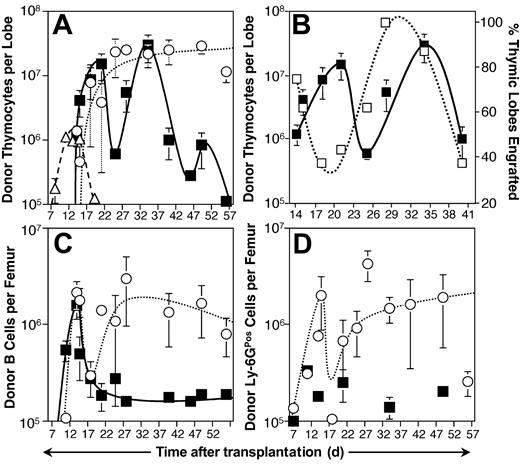
To determine if T-lineage progenitors are enriched in either of the subsets of Thy-1.1Neg cells defined by L-selectin as shown in Figure 1A, we conducted a series of transplant experiments into sublethally irradiated mice using CD45 allelic variants to enable specific tracking of donor-derived engraftment.27 Lineage reconstitution mediated by the progenitor populations was assessed in the 8 weeks following intravenous transplantation. Thymic lobes and the marrow of one femur were probed for donor-derived cells, using a threshold of 105 donor-derived cells to define positive engraftment. Analysis of thymic reconstitution revealed that both L-selectin fractions were capable of repopulating the thymus (Figure 2A). Of the 2 subsets, L-selectinPos progenitors mediated slightly faster thymic engraftment kinetics, generating an average of more than 107 thymocytes per engrafted thymic lobe within 21 days after transplantation. However, 103 L-selectinPos progenitors was not a saturating cell dose at early time points, and on day 21, only 7 (44%) of 16 thymic lobes assayed were positively engrafted (Figure 2B). Parallel transplants with the L-selectinNeg population produced a somewhat higher engraftment frequency, with 75% of thymic lobes engrafting by day 21; nevertheless, thymic engraftment from L-selectinNeg progenitors was delayed 4 days relative to L-selectinPos progenitor engraftment, in that L-selectinNeg progenitors did not produce an average of 107 donor-derived thymocytes per engrafted lobe until day 25 (Figure 2A). These results suggest that L-selectinNeg progenitors are more primitive relative to L-selectinPos progenitors.
Kinetic analysis of engraftment following intravenous transplantation of L-selectinPos and L-selectinNeg bone marrow populations. (A) Progenitors were collected from B6-Thy-1.1-Ly-5.1 mice and transplanted intravenously into sublethally irradiated (6.5 Gy) B6 host mice. Each animal received either 103 L-selectinPos progenitors (▪), 103 L-selectinNeg progenitors (○), or 2.5 × 107 normal thymocytes (▵). Thymic lobes were collected on the days indicated and assayed by immunofluorescent staining and flow cytometry for donor-derived (Ly-5.1Pos Thy-1.1Pos) thymocytes. Thymic lobes were considered engrafted if they contained equal to or more than 105 donor-derived thymocytes. Each symbol represents the mean donor-derived thymocytes per engrafted thymic lobe observed at that time point from 6 to 18 total lobes assayed over 3 independent sorting and transplantation experiments. Error bars represent SEM. (B) Host mice receiving L-selectinPos progenitors for the experiment described in panel A were analyzed for the frequency of engrafted thymic lobes relative to the kinetics of thymocyte expansion. The figure shows the percent positive thymic lobes at each time point (right axis, □) and the mean donor-derived thymocytes per engrafted lobe (left axis, ▪). Error bars represent SEM. (C-D) Bone marrow was collected from the cohort of 62 mice given transplants with L-selectinPos progenitors (▪) and 48 mice receiving L-selectinNeg progenitors (○) for the experiment described in panel A and analyzed by immunofluorescent staining and flow cytometry to identify donor-derived cells expressing either CD19 (B cells) or Ly-6G (granulocytes and macrophages). Each symbol represents the mean donor-derived B cells (C) or Ly-6G cells (D) observed per engrafted femur at the indicated times. Error bars represent SEM. Ly-6GPos engraftment following L-selectinPos progenitor transplantation was too sporadic for a trend to be extrapolated.
Kinetic analysis of engraftment following intravenous transplantation of L-selectinPos and L-selectinNeg bone marrow populations. (A) Progenitors were collected from B6-Thy-1.1-Ly-5.1 mice and transplanted intravenously into sublethally irradiated (6.5 Gy) B6 host mice. Each animal received either 103 L-selectinPos progenitors (▪), 103 L-selectinNeg progenitors (○), or 2.5 × 107 normal thymocytes (▵). Thymic lobes were collected on the days indicated and assayed by immunofluorescent staining and flow cytometry for donor-derived (Ly-5.1Pos Thy-1.1Pos) thymocytes. Thymic lobes were considered engrafted if they contained equal to or more than 105 donor-derived thymocytes. Each symbol represents the mean donor-derived thymocytes per engrafted thymic lobe observed at that time point from 6 to 18 total lobes assayed over 3 independent sorting and transplantation experiments. Error bars represent SEM. (B) Host mice receiving L-selectinPos progenitors for the experiment described in panel A were analyzed for the frequency of engrafted thymic lobes relative to the kinetics of thymocyte expansion. The figure shows the percent positive thymic lobes at each time point (right axis, □) and the mean donor-derived thymocytes per engrafted lobe (left axis, ▪). Error bars represent SEM. (C-D) Bone marrow was collected from the cohort of 62 mice given transplants with L-selectinPos progenitors (▪) and 48 mice receiving L-selectinNeg progenitors (○) for the experiment described in panel A and analyzed by immunofluorescent staining and flow cytometry to identify donor-derived cells expressing either CD19 (B cells) or Ly-6G (granulocytes and macrophages). Each symbol represents the mean donor-derived B cells (C) or Ly-6G cells (D) observed per engrafted femur at the indicated times. Error bars represent SEM. Ly-6GPos engraftment following L-selectinPos progenitor transplantation was too sporadic for a trend to be extrapolated.
Transplantation of 2.5 × 107 normal thymocytes mediated engraftment of the thymus 6 days faster than could either L-selectin subset, due to the high dose of cells transplanted and the presence of rapidly expanding progenitors in the thymus.13,28 However, both L-selectin subsets were capable of producing 106 donor thymocytes within approximately the same time frame as could a thymocyte graft (14 days; Figure 2A). Similarly, experiments with grafts enriched for HSCs did not produce thymic engraftment until day 21 and required until day 28 to produce 107 donor-derived thymocytes per thymic lobe.14 These data indicate that both L-selectin populations within the Thy-1.1Neg compartment mediate thymic engraftment with kinetics similar to thymocytes.
After the initial burst of thymic colonization from both L-selectin subsets, distinctly different kinetics emerged. Following day 21, thymic reconstitution mediated by the L-selectinPos subset dropped precipitously to less than 106 progeny per lobe on day 25. This span of engraftment reflected the 11-day engraftment cycle observed in normal thymocyte transplants. However, unlike engraftment from thymocytes, thymic colonization following L-selectinPos progenitor transplantation resurged for a second 11-day cycle, peaking above 107 donor-derived thymocytes per engrafted lobe at day 34 before dissipating between days 40 and 56 after transplantation (Figure 2A).
The frequency of engrafted thymic lobes over time reflected the sinusoidal pattern of thymocyte expansion (Figure 2B). However, engraftment frequency (dashed line) was distinctly in advance of the observed expansion kinetics (solid line). The greatest number of engrafted thymic lobes preceded the greatest thymocyte expansion by a full week. This pattern repeated itself over the second wave of engraftment, with 100% engraftment on day 28, and peak donor thymocyte levels (2.9 × 107 ± 1.3 × 107) following 6 days later. By this time, engraftment frequency had decreased to 88%.
L-selectinNeg progenitors mediated thymocyte expansion that exceeded 107 donor-derived cells per engrafted lobe within 25 days after transplantation. Unlike expansion following transplantation of the L-selectinPos subset, however, this level of engraftment persisted for the 8-week duration of the study (Figure 2A). Likewise, the frequency of engrafted thymic lobes increased steadily through day 28, when an engraftment frequency of 100% was achieved and then maintained for the remaining 4 weeks of the study. Similar to the L-selectinNeg population, HSCs also generate persistent thymic progeny in irradiated hosts but require an additional week to do so.14 The marked difference in engraftment kinetics between the L-selectinPos and L-selectinNeg populations confirms that L-selectin expression separates functionally distinct T-lineage progenitors within the Thy-1.1Neg compartment.
Expression of L-selectin coincides with increased bias for the T lineage among Thy-1.1Neg progenitors
Analysis of B-lymphoid and myeloid engraftment in the bone marrow showed distinct differences in lineage potentials of the subsets of cells defined by L-selectin expression. Both subsets contributed to the B lineage during the first 2 weeks after transplantation (Figure 2C), and the numbers of B cells per femur subsequently decreased in both cases. However, whereas the L-selectinNeg cells maintained high numbers of B-lineage cells from week 3 onward, the L-selectinPos cells failed to maintain production of B cells. Further, L-selectinPos cells produced few myeloid progeny in comparison to the L-selectinNeg subset (Figure 2D). These data demonstrate that L-selectin expression correlates with decreased myeloid and B-lineage potential among Thy-1.1Neg progenitors, a lineage potential that is very similar to the thymic ETP population.13 Combined with the rapid, cyclic thymic engraftment kinetics, as well as phenotypic similarities between the 2 populations, these data indicate that the L-selectinPos population includes bone marrow progenitors highly analogous to the thymic ETP population.
The L-selectinNeg population is highly enriched for B-cell precursors
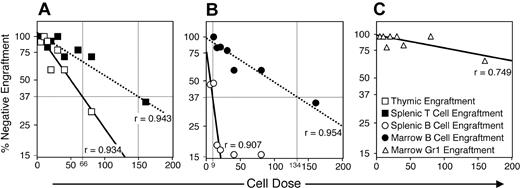
The diverse engraftment mediated by the L-selectinNeg population suggested the presence of multipotent progenitors. To evaluate the frequency of progenitors for T, B, and myeloid lineages within this population, we conducted a limiting dilution analysis29 (Figure 3).
Limiting dilution analysis of L-selectinNeg progenitor lineage potential after intravenous transplantation. L-selectinNeg progenitors were collected and transplanted intravenously into radiation-conditioned hosts as described for Figure 2 with doses transplanted per mouse ranging from 5 to 160 cells. From 3 to 13 mice were given transplants at each dose (70 mice total) over 4 replicate experiments. Engraftment was assessed between 27 and 29 days after transplantation by analyzing host tissues for donor-derived cells in specific lineages. Horizontal bars mark the 37% negative engraftment point predicting active progenitor frequencies, which are shown by vertical bars and dose numbers along the x-axis. Correlation coefficients (r) are given for each curve extrapolated from the data. Thymic T cells, □; splenic T cells, ▪; splenic B cells, (○); marrow B cells, (•); marrow myeloid cells, (▵).
Limiting dilution analysis of L-selectinNeg progenitor lineage potential after intravenous transplantation. L-selectinNeg progenitors were collected and transplanted intravenously into radiation-conditioned hosts as described for Figure 2 with doses transplanted per mouse ranging from 5 to 160 cells. From 3 to 13 mice were given transplants at each dose (70 mice total) over 4 replicate experiments. Engraftment was assessed between 27 and 29 days after transplantation by analyzing host tissues for donor-derived cells in specific lineages. Horizontal bars mark the 37% negative engraftment point predicting active progenitor frequencies, which are shown by vertical bars and dose numbers along the x-axis. Correlation coefficients (r) are given for each curve extrapolated from the data. Thymic T cells, □; splenic T cells, ▪; splenic B cells, (○); marrow B cells, (•); marrow myeloid cells, (▵).
This experiment indicated that 1 in 66 L-selectinNeg progenitors was capable of thymic colonization, whereas 1 in 149 was capable of generating sufficient thymic progeny to result in detectable CD4+ or CD8+ progeny in the spleen within 4 weeks of intravenous transplantation (Figure 3A). B-lineage potential was highly enriched within the L-selectinNeg population, because 1 in 9 L-selectinNeg progenitors produced detectable CD19+ progeny in the spleen, and 1 in 134 mediated B-cell colonization of the marrow that persisted through week 4 (Figure 3B). Surprisingly, at low L-selectinNeg cell doses, myeloid engraftment was sparse (Figure 3C). Collectively, these limiting dilution data indicate that L-selectinNeg progenitors are strongly biased toward lymphoid commitment, favoring B relative to T-lineage potential. We did not attempt limiting dilution analysis of the L-selectinPos subset due to the variation in the frequency of positive thymic lobes with time (Figure 2B).
L-selectin fractions show distinct cloning efficiencies and in vitro expansion characteristics
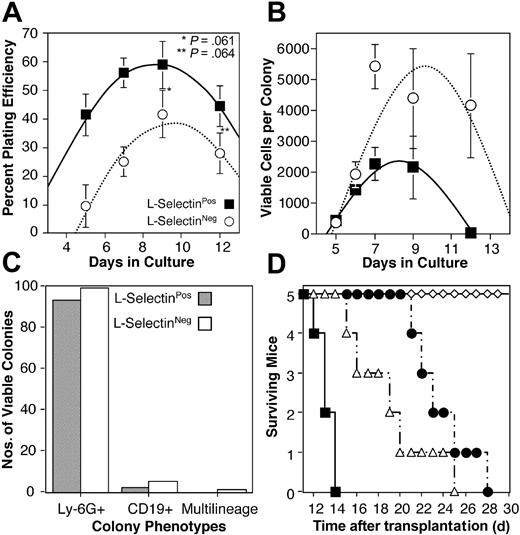
L-selectinPos and L-selectinNeg fractions of the Thy-1.1Neg population were also tested in methylcellulose cultures supplemented with stem cell factor, IL-7, Flt-3L, IL-3, IL-6, G-CSF, and EPO. Cloning efficiency kinetics were tabulated over a series of times between 5 and 12 days in culture. For both populations, mean cloning efficiencies ranged between 10% and 60%; however, at each time point the L-selectinPos fraction produced significantly more colonies per cells plated (P < .1; Figure 4A). The peak cloning efficiency observed for both subsets was at 9 days in culture, with averages of 59% and 42% for L-selectinPos and L-selectinNeg fractions, respectively.
In vitro and rescue potentials of L-selectin subsets. (A) L-selectinNeg (○) and L-selectinPos (▪) fractions were collected and cultured in methylcellulose as described in “Materials and methods.” Colonies visible with the aid of a dissecting microscope were counted at the indicated days of culture and plotted as a percent of total cells plated. Each symbol represent the average of 4 to 8 methylcellulose plates counted over 3 replicate experiments. Error bars represent SEM. (B) Colonies formed by L-selectinNeg (○) and L-selectinPos (▪) fractions were collected, stained with PI, and analyzed by flow cytometry. Each symbol represents the average number of viable cells (PINeg) per colony analyzed at the times indicated. Eight to 24 colonies were analyzed for each data point. Data were pooled from 3 replicate experiments and error bars represent SEM. (C) Viable colonies collected for analysis in panel B were evaluated for Ly-6G and CD19 expression. Bars represent the number of colonies containing equal to or more than 30 PINeg cells that expressed either or both (multilineage) of these markers. A total of 144 colonies from L-selectinPos progenitors and 128 from L-selectinNeg progenitors were analyzed for panels B and C. (D) Lethally irradiated (13 Gy) mice were given transplants intravenously with 103 Thy-1.1Neg c-kitHi L-selectinNeg (▵) or Thy-1.1Lo c-kitHi L-selectinPos (•) progenitors, or 103 Thy-1.1Lo c-kitHi hematopoietic stem/progenitor cells (⋄), or did not receive transplants (▪).
In vitro and rescue potentials of L-selectin subsets. (A) L-selectinNeg (○) and L-selectinPos (▪) fractions were collected and cultured in methylcellulose as described in “Materials and methods.” Colonies visible with the aid of a dissecting microscope were counted at the indicated days of culture and plotted as a percent of total cells plated. Each symbol represent the average of 4 to 8 methylcellulose plates counted over 3 replicate experiments. Error bars represent SEM. (B) Colonies formed by L-selectinNeg (○) and L-selectinPos (▪) fractions were collected, stained with PI, and analyzed by flow cytometry. Each symbol represents the average number of viable cells (PINeg) per colony analyzed at the times indicated. Eight to 24 colonies were analyzed for each data point. Data were pooled from 3 replicate experiments and error bars represent SEM. (C) Viable colonies collected for analysis in panel B were evaluated for Ly-6G and CD19 expression. Bars represent the number of colonies containing equal to or more than 30 PINeg cells that expressed either or both (multilineage) of these markers. A total of 144 colonies from L-selectinPos progenitors and 128 from L-selectinNeg progenitors were analyzed for panels B and C. (D) Lethally irradiated (13 Gy) mice were given transplants intravenously with 103 Thy-1.1Neg c-kitHi L-selectinNeg (▵) or Thy-1.1Lo c-kitHi L-selectinPos (•) progenitors, or 103 Thy-1.1Lo c-kitHi hematopoietic stem/progenitor cells (⋄), or did not receive transplants (▪).
Colonies were plucked at each of the times indicated in Figure 4B and examined for viable cells by PI staining and flow cytometry. Little difference in progenitor expansion potentials was seen between the 2 subsets at early time points; however, after 6 days in culture, the L-selectinNeg fraction produced on average from 2- to 100-fold more viable cells per colony for the remainder of the assay (Figure 4B), despite producing significantly fewer total colonies (Figure 4A). Cytokines were added only at the inception of each culture and were not replenished during the course of these assays, which may explain the reduced viability after day 9. Analysis of CD19 or Ly-6G expression revealed that both L-selectin subsets generated B cells and granulocytes at relatively equal frequencies, but only the L-selectinNeg fraction produced multilineage colonies with combined CD19+ and Ly-6G+ cells.
Both L-selectin subsets predominantly generated Ly-6GPos cells in culture and relatively few CD19+ cells (Figure 4C), although the frequency of CD19+ colonies produced by the L-selectinNeg population approximates the frequency of B-lineage progenitors detected in the in vivo limiting dilution analysis (Figure 3B). These results agree with previous data indicating that the unseparated Thy-1.1Neg c-kitHi population primarily generates myeloid progeny in culture,30 and further supports research suggesting that in vitro lineage potential does not necessarily reflect in vivo transplantation performance due to the higher sensitivity of in vitro assays for detecting progenitors with low proliferation potentials.31,32
L-selectinNeg progenitors cannot rescue lethally irradiated mice
Like L-selectinNeg progenitors, HSCs are also LinNeg, Sca-1Pos, and c-kitHi. In mice expressing the Thy-1.1 allele of CD90, HSCs are confined to the Thy-1.1Lo portion of this compartment.22 Interestingly, nearly one third of this fraction is also L-selectinNeg (Figure 1B). Previous studies have suggested that HSCs do not express L-selectin.25,33 The high frequency of multilineage engraftment following intravenous transplantation of the Thy-1.1Neg L-selectinNeg fraction in the experiments described, as well as the similarities in phenotype between this population and the stem cell compartment, suggested the possibility that some HSCs were also present in the L-selectinNeg fraction. To rule out this possibility, we transplanted into lethally irradiated mice 103 cells each of LinNeg Sca-1Pos c-kitHi Thy-1.1Lo HSC, LinNeg Sca-1Pos c-kitHi Thy-1.1Lo L-selectinPos progenitors, or LinNeg Sca-1Pos c-kitHi Thy-1.1Neg L-selectinNeg progenitors (Figure 4D). All mice were maintained in parallel conditions, and their health was monitored daily. By day 10, all mice that had not received transplants had developed white foot pads suggestive of anemia, and by day 14, all members of this cohort had died. Transplants of Thy-1.1Neg L-selectinNeg progenitors extended the lives of recipient mice for several days beyond the controls; however, all mice had lost color in their foot pads and 4 mice had expired by day 20. The fifth mouse died on day 26.
Similarly, mice receiving Thy-1.1Lo L-selectinPos progenitors only persisted until day 28. In contrast, mice receiving Thy-1.1Lo c-kitHi HSCs never developed white foot pads and all survived through day 35 when the experiment was terminated. The length of time that Thy-1.1Neg L-selectinNeg and Thy-1.1Lo L-selectinPos progenitors sustained mice that had received transplants suggests that these compartments contain limited platelet and erythrocyte potential. These data confirm that HSCs in B6 mice do not express L-selectin.
Discussion
Emerging evidence suggests that during commitment to the T- or B-lymphoid lineages, a variety of intermediate stages of development are subject to extrinsic controls that can switch the fate of progenitor cells.34 The strongest evidence for extrinsically controlled lineage plasticity comes from experiments in which T- or B-lineage antigen-specific receptor rearrangements initiate but fail to complete as the cell is redirected to an alternative developmental pathway.13,35 In light of these observations, it is difficult to establish lineage relationships in experimental settings because cells isolated from discrete stages of early lymphoid development are likely to respond to experimental conditions and commit to differentiation pathways distinct from those that occur in a natural setting. The role of environmental influences in early lymphopoiesis has clouded recent attempts to resolve the early stages of T- and B-lineage commitment. We have attempted to address this issue by establishing an experimental model to follow T-lineage progenitor activity in prospectively isolated cell populations while minimizing artificial environmental influences such as cell culture in hematopoietic cytokines. In developing our assay, we reasoned that T-lineage progenitors should rapidly and efficiently home to the thymus following intravenous transplantation and proliferate sufficiently to generate a sizable population of thymocytes within a short period of time. We also evaluated reconstitution of bone marrow and spleen to detect myeloid and B-lymphocyte progeny of injected cells and to follow the emergence of mature T lymphocytes after transplantation. Using this model system, we hope to identify the primary differentiation pathway that leads from the bone marrow to the majority of mature peripheral T lymphocytes.
One difficulty encountered in the dissection of the hematopoietic hierarchy is the phenotypic similarities between progenitor populations and HSCs. We have approached this problem by relying on our previous observation that the Thy-1.1 molecule is expressed by virtually all HSCs in B6-Thy-1.1 mice.22 Negative selection for Thy-1.1 expression within the Sca-1Pos LinNeg bone marrow compartment eliminates HSC activity and enriches for lymphoid progenitors,36 in a manner analogous to selection for Flk-2/Flt3 expression among Sca-1Pos LinNeg cells.37,38 Within the Thy-1.1Neg population, 3 subsets of cells can be resolved based on levels of c-kit expression14 ; all 3 of these subsets have been shown to include T- and B-lymphoid progenitors. The most primitive of these, based on biologic activity and gene expression analysis, is characterized by activation of the recombinase gene loci and is found within the c-kitHi fraction of Sca-1Pos LinNeg bone marrow.21 These cells, termed early lymphocyte progenitors (ELPs), reconstitute the thymus after intravenous transplantation and also generate B lymphocytes as well as NK cells. ELPs do not express IL7-Rα, which along with the c-kitLow phenotype is the characteristic marker of CLPs.2 Although capable of producing both T and B lineages in clonal assay models, CLPs are deficient in thymic homing potential and thus are relatively inefficient progenitors of the T lineage in intravenous transplantation experiments. Recent experiments have identified a second type of CLP (CLP-2), which is characterized by a lack of c-kit expression and a B220+ CD19- surface antigen phenotype. These cells show a remarkable ability to accumulate in the thymus after intravenous transplantation, although their ability to proliferate within the thymus is limited3 ; short-term cell culture indicates that these cells are progeny of CLPs. Based on c-kit levels and B220 expression, the cell populations characterized in the studies reported here do not overlap with either type of CLP. Our previous work has indicated persistent thymic reconstitution in the intravenous transplantation model only after transplantation of c-kitHi cells.14 Analysis of early thymic subsets has identified the thymic ETP, which apparently arises independently of the CLP and therefore of CLP-2 as well.13 Attempts to integrate all of these subsets into a unified model of lymphoid development1 are frustrated by the probability that the developmental fate of cells at this stage of development is not absolutely tied to surface antigen expression and that environmental influences introduced by culture manipulations and transplantation are likely to influence lineage commitment. Furthermore, it is likely that multiple pathways can lead to the development of T cells in the thymus.
In this study, we have focused on the c-kitHi subset of Sca-1Pos LinNeg bone marrow, a population that includes the ELP.21 We have shown that L-selectin expression separates this population into 2 subsets that are functionally distinct. Although both of these harbor considerable T-lymphocyte potential, L-selectinPos progenitors are strongly biased to the lymphoid lineages following intravenous transplantation and produce a single wave of B-lineage engraftment along with recurrent waves of thymic engraftment that occur in the absence of appreciable recurrence of B-lineage or myeloid engraftment (Figure 2). As a result of these observations, we consider the L-selectinPos subset to be strongly biased toward T-lineage development and suggest that this subset of cells includes the bone marrow counterpart of the thymic ETP population.13 L-selectinPos progenitors share several characteristics with thymic ETP, including the LinNeg Sca-1Pos c-kitHi phenotype and the expression of L-selectin (Figure 1C). Both subsets express IL-7Rα receptor at frequencies much lower than is reported for CLPs, and the thymic ETPs have further been shown to be unresponsive to IL-7 in vitro. Additionally, previous studies have shown that cells at the earliest stages of thymic development are negative for Thy-1.1 antigen expression,39 and that DN thymocytes are predominantly L-selectinPos.23
L-selectinPos progenitors and thymic ETPs also share striking functional similarities, in that both populations predominantly generate T-lineage progeny after transplantation, with fewer B-cell and scarce myeloid progeny (Figure 2A,C-D). Allman and colleagues13 reported that thymic ETPs colonize the thymus poorly after intravenous transplantation, although 400 thymic ETPs transferred intrathymically generated 105 thymocytes within 2 weeks. In contrast, 103 L-selectinPos progenitors transplanted intravenously reliably generate more than 105 thymic progeny within 14 days (Figure 2A). The difference in thymic reconstitution following intravenous transplantation suggests that this ability is rapidly lost after thymic colonization by bone marrow progenitors. The observation that L-selectinPos progenitors repopulate the thymus following intravenous transplantation with kinetics similar to those of thymic ETPs following intrathymic transplantation shows the high degree of T-lineage competency inherent within the L-selectinPos population.
The extent to which L-selectinPos progenitors colonize the thymus immediately after transplantation is unknown. The kinetic analysis shown in Figure 2A indicates that the thymus contains progenitors that can reconstitute thymocytes slightly faster than can L-selectinPos progenitors following intravenous transplantation. Additionally, the distinct waves of thymic colonization that follow L-selectinPos progenitor transplantation indicate that at least a portion of the transplanted cells colonize the bone marrow and function to reseed the thymus.28,40 Foss and colleagues observed a similar duration between waves of thymic seeding in irradiated hosts.19 This correlation may indicate that L-selectinPos progenitors are sufficiently lymphoid-biased to respond to the native signals that initiate normal thymic seeding. It is likely that both L-selectinNeg and L-selectinPos progenitors initially seed the marrow, where they expand and respond to the overwhelming demand for leukocytes created by the radiation conditioning of the host. Following this initial engraftment phase, the L-selectinNeg population continues to seed several leukocyte lineages in a consistent, stem cell–like manner. However, the L-selectinPos population is already biased to the T lineage; therefore, after the initial demands of crisis hematopoiesis are satisfied, L-selectinPos progenitors continue to expand as primitive pro-T cells in the marrow until thymic niches become available for engraftment. Further experiments will be required to test this hypothesis.
In addition to the steady thymic engraftment kinetics generated by L-selectinNeg progenitors, limiting dilution analysis indicates that this population is predominated by B-cell potential. One in 9 L-selectinNeg progenitors proved capable of generating more than 105 splenic B cells following intravenous transplantation in these assays (Figure 3B). This compares to 1 in 66 L-selectinNeg progenitors that was capable of repopulating a thymic lobe with more than 105 thymocytes (Figure 3A). These data suggest that the L-selectinNeg compartment is highly enriched for relatively primitive lymphoid progenitors, some of which may be committed to the B-cell lineage. Single-cell studies will be necessary to establish this conclusively.
Our experiments do not address the role of L-selectin expression in early T-lineage development. Indeed, gene-targeting studies have shown that L-selectin is not required for this process41,42 ; however, L-selectin is necessary for naive T cells to home to and traverse high endothelial venules and enter lymph nodes.42 Further, L-selectin allows leukocytes to escape circulation and enter sites of inflammation within tissues.41,43,44 Accordingly, it is plausible that L-selectin may play a role in the homing of bone marrow progenitors to the thymus. This concept is supported by the regulation of L-selectin by the transcription factor Ikaros, which is essential for lymphocyte development on many levels.45 It has recently been observed that inhibiting Ikaros activity blocks L-selectin expression in hematopoietic cell lines,46 resulting in a reduction of spontaneous migration. Extrapolating this observation suggests that L-selectin expression may aid the migration of primitive prothymocytes from the bone marrow to the thymus. This is supported by the fact that c-kitHi DN thymocytes (and therefore the thymic ETPs) also express L-selectin (Figure 1C). Any functional role for L-selectin in thymic homing must be redundant, however, because L-selectin itself is not necessary for T-cell development. This agrees with data presented by Allman and colleagues, showing that Ikaros-deficient mice still accumulate thymic ETPs but lack CLPs.13
In addition to describing a bone marrow counterpart to the thymic ETP population, these studies confirm that L-selectin is not expressed by hematopoietic stem and progenitor cells capable of rescuing mice from lethal irradiation (Figure 4D).25,33 Along with expression of Flk-2/Flt337,38 and other markers,47 this property will be useful in refining stem cell isolation within the LinNeg Sca-1Pos c-kitHi population. Furthermore, these data suggest that isolating the L-selectinPos fraction of this primitive bone marrow compartment is an effective way to enrich lymphoid progenitors for specific study in the absence of HSC contamination.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-09-3030.
Supported by grants from the National Institutes of Health (T32 DK07115 and RO1 DK57899), and the Brian Rooney Fund of the Lymphoma Foundation. S.T. is a research scholar of the American Cancer Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal