Abstract
A major limitation to clinical stem cell–mediated gene therapy protocols is the low levels of engraftment by transduced progenitors. We report that CXCR4 overexpression on human CD34+ progenitors using a lentiviral gene transfer technique helped navigate these cells to the murine bone marrow and spleen in response to stromal-derived factor 1 (SDF-1) signaling. Cells overexpressing CXCR4 exhibited significant increases in SDF-1–mediated chemotaxis and actin polymerization compared with control cells. A major advantage of CXCR4 overexpression was demonstrated by the ability of transduced CD34+ cells to respond to lower, physiologic levels of SDF-1 when compared to control cells, leading to improved SDF-1–induced migration and proliferation/survival, and finally resulting in significantly higher levels of in vivo repopulation of nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice including primitive CD34+/CD38-/low cells. Importantly, no cellular transformation was observed following transduction with the CXCR4 vector. Unexpectedly, we documented lack of receptor internalization in response to high levels of SDF-1, which can also contribute to increased migration and proliferation by the transduced CD34+ cells. Our results suggest CXCR4 overexpression for improved definitive human stem cell motility, retention, and multilineage repopulation, which could be beneficial for in vivo navigation and expansion of hematopoietic progenitors. (Blood. 2004;103:2942-2949)
Introduction
Gene transfer into human hematopoietic stem cells (HSCs) may be a promising tool in the correction of a wide variety of hematopoietic and genetic disorders. HSC transplantation can be used to durably deliver these genetically modified cells to the bone marrow (BM), which in turn will release mature cells with the corrected gene into the circulation throughout life. Clinical and experimental HSC transplantation procedures mimic the physiologic process of HSC migration from the circulation into the BM occurring during late embryonic development and steady-state hematopoiesis in adults throughout life.1-3 One of the disadvantages of BM transplantation is the long-lasting reduced levels of immature progenitors, including long-term culture-initiating cells (LTCICs; 1 log reduction), in the BM of patients who have received transplants compared with healthy individuals.4-6 Furthermore, emerging evidence exists for impaired homing7 and low engraftment8 of retrovirally transduced human CD34+ cells. Enhanced efficacy of HSC engraftment could improve the outcome of clinical transplantations as well as gene therapy protocols and might be achieved by modulating the ability of stem cells to home to and repopulate the recipient BM.
Interactions between the chemokine stromal-derived factor 1 (SDF-1), also referred to as CXCL12, and its receptor CXCR4 play an essential role in stem cell seeding of the BM during murine embryonic development.9,10 Moreover, we have previously demonstrated in a functional preclinical model, using nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice as recipients, that both the homing and high-level multilineage repopulation of human CD34+-enriched cells are dependent on SDF-1/CXCR4 interactions.11-14 In support of these data, it has been shown that either high levels of CXCR4 expression on human CD34+ cells or high SDF-1–induced directional motility in vitro correlates with faster recovery in both allogeneic and autologous clinical transplantations with positive selection of adult CD34+ cells15,16 or whole cord blood (CB) cells.17
CXCR4 expression is dynamic, regulated by environmental factors such as cytokines, chemokines, stromal cells, adhesion molecules, and proteolytic enzymes.18 In human stem and progenitor cells, CXCR4 can be up-regulated by short-term (∼ 40 h) in vitro cytokine culture.12,19 This subsequently enhances their in vitro migration toward an SDF-1 gradient12,20 as well as their in vivo homing and repopulation capacities in NOD/SCID mice receiving transplants and via serial transplants in β2-microglobulin null (β2mnull) NOD/SCID mice,11,12 linking stem cell self-renewal and development with motility. A recent report demonstrated that longer culture periods with a cytokine cocktail result in a decrease in cell surface CXCR4 expression on human CB CD34+ cells,21 and reduced repopulation was documented with human progenitors cultured in vitro for longer periods.22 Recently, we revealed that CB CD34+/CXCR4--sorted cells harbor low levels of intracellular CXCR4, which, following short-term in vitro cytokine stimulation, can rapidly be functionally expressed on the cell surface to mediate SDF-1–dependent homing and repopulation of NOD/SCID mice receiving transplants.14
In addition to their central role in mediating directional migration of human and murine stem cells,23 SDF-1/CXCR4 interactions are also involved in other stem cell functions. Low concentrations of SDF-1 in synergy with other early acting cytokines enhance proliferation/survival of both human CD34+ cells and murine stem cells.24-28 High levels of SDF-1 induce quiescence of proliferating human LTCICs and primitive human fetal liver CD34+ cells capable of serial repopulation of NOD/SCID mice.29,30 Of importance, SDF-1/CXCR4 interactions are also involved in retention of stem and progenitor cells in the BM.9,31,32 This hypothesis has also been confirmed by other studies that demonstrated the involvement of SDF-1/CXCR4 interactions in the anchorage of human HSCs injected directly into the murine BM cavity.33,34 Interference of these interactions induces release/mobilization of both human and murine progenitors from the BM into the circulation.35-42
Therefore, overexpression of CXCR4 on human CD34+ progenitors (especially after long periods of culture in vitro) may facilitate their motility as well as retention in the BM and increase their proliferation and repopulation potential. Indeed, transgenic mice overexpressing human CD4 and CXCR4 on their CD4+ T cells have increased levels of these cells in their BM and only very low levels in the circulation.43
Lentiviral vectors have been used to introduce transgenes into SCID repopulating cells (SRCs),44-47 due to their unique ability to transduce nondividing cells.48 Therefore, we used a lentiviral gene transfer technique to overexpress CXCR4 on enriched human CD34+ stem and progenitor cells for the purpose of examining its effect on human CD34+ cell development, migratory patterns, and repopulation potential.
Materials and methods
Viral vector construction and production
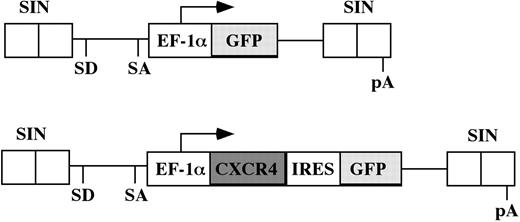
The human CXCR4 gene expression vector was constructed by isolating a 1.2-kb CXCR4 cDNA from human CB cells and linking it to the enhanced green fluorescent protein (GFP) gene via an internal ribosome entry site (IRES). The fragment containing the CXCR4-IRES-GFP was ligated with the EF-1α promoter to generate a self-inactivating (SIN) vector where the fragment containing the EF-1α-CXCR4-IRES-GFP bicistronic cassette was inserted into a pHR′-SIN vector backbone kindly provided by Dr Didier Trono (Geneva, Switzerland). The control vector lacks the CXCR4 gene and expresses only GFP (Figure 1).
Schematic representation of the lentiviral vector constructs. Only the relevant portions of the integrated provirus are depicted. The EF1-α promoter is used to drive expression of either GFP cDNA in the control vector (top panel) or the CXCR4-IRES-GFP bicistronic cassette of the experimental vector (bottom panel). SD indicates splice donor; SA, splice acceptor; pA, polyadenylation signal.
Schematic representation of the lentiviral vector constructs. Only the relevant portions of the integrated provirus are depicted. The EF1-α promoter is used to drive expression of either GFP cDNA in the control vector (top panel) or the CXCR4-IRES-GFP bicistronic cassette of the experimental vector (bottom panel). SD indicates splice donor; SA, splice acceptor; pA, polyadenylation signal.
Replication-defective, SIN HIV-derived lentiviral vector was generated by transient transfection of the 293T packaging cell line by means of FuGENE 6 transfection reagent (Roche Diagnostics, Mannheim, Germany), using a 3-plasmid system: transfer vector pHR′-EF1α-GFP-SIN (control vector) or pHR′-EF1α-CXCR4-IRES-GFP-SIN (experimental vector), the envelope coding plasmid pMD.G, and the packaging construct pCMVR8.91.49,50 Twenty-four hours after transfection, the viral supernatant was replaced with serum-free medium supplemented with 2% bovine serum albumin (BSA; Sigma, St Louis, MO), 10 μg/mL insulin (Biological Industries, Beit Haemek, Israel), 200 μg/mL transferrin (Sigma), 0.1 mM 2-mercaptoethanol, 2 mM l-glutamine (Biological Industries), 100 μg/mL streptomycin (Biological Industries), and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (pH 7.3; Biological Industries). Twenty-four hours later, viral supernatant was collected, filtered (0.45-μm Minisart filter; Sartorius, Goettingen, Germany), and used for transduction of target cells.
Preparation of human CD34+ cells
Human CB cells from full-term deliveries, or granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood (MPB) cells from healthy adult donors for clinical transplantation were obtained after informed consent was given and were used in accordance with procedures approved by the human ethics committee of the Weizmann Institute. The samples were separated on Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). CD34+ cells were enriched using the magnetic activated cell sorting (MACS) cell isolation kit and the auto-MACS magnetic cell sorter (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions, obtaining purity of about 95%. Purified cells were used freshly or frozen in 10% dimethyl sulfoxide (DMSO) for later usage.
Transduction of cells
CD34+ cells (up to 4 × 105/well) were prestimulated with stem cell factor (SCF; 50 ng/mL) in 400 μL serum-free medium for 24 hours in a 12-well plate. Viral supernatant (1.6 mL/35-mm well) supplemented with SCF (50 ng/mL), FLT-3L (50 ng/mL), both from R&D Systems (Minneapolis, MN), and interleukin 6 (IL-6; 50 ng/mL; Interpharm Laboratories, Ares-Serono Group, Ness Ziona, Israel) was added to the cells with a viral load as much as 2 × 107 TU/mL (first infection). Transduction was repeated 24 hours later (second infection). Following transduction, cells were either assayed in vitro or transplanted into sublethally irradiated NOD/SCID mice. Infection efficiency was determined according to cell surface expression of CXCR4 using specific antihuman CXCR4-phycoerythrin (PE; 12G5; BD PharMingen, San Diego, CA) and GFP (FL1 channel) by flow cytometric analysis (FACSCalibur; Becton Dickinson, San Jose, CA) 72 hours after transduction. Mock cells were cultured in the same conditions as transduced cells, without exposure to lentiviral vectors.
Mice
NOD/LtSz-Prkdcscid (NOD/SCID) mice were bred and maintained under defined flora conditions in individually ventilated (high-efficiency particle-arresting filtered air) sterile microisolater cages (Techniplast; Varese, Italy) at the Weizmann Institute. All the experiments were approved by the animal care committee of the Weizmann Institute. Eight- to 10-week-old mice were sublethally irradiated (375 cGy, from a 60 Co source) and given transplants with human cells as indicated (2 × 105 cells/mouse) 24 hours after irradiation.
Human cell engraftment
Human cell engraftment was assayed 5 weeks after transplantation by flow cytometry (FACSCalibur, Becton Dickinson) using specific antihuman CD45-allophyocyanin (APC), anti-CD19–PE, anti-CD33–PE, anti-CD34–APC, and anti-CD38–PE monoclonal antibodies (mAbs; all obtained from BD PharMingen). Noninjected mice were used as negative controls.
Colony-forming unit assay
Semisolid cultures were performed as previously described.51 The cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2 and scored 14 days later by phase contrast microscopy for GFP+ as well as myeloid or erythroid colonies by morphologic criteria.
Actin polymerization assay
Transduced cells were stimulated with SDF-1α (300 ng/mL; Peprotech, Rocky Hill, NJ) in serum-free RPMI at 37°C for indicated times. Reaction was stopped by adding 3 volumes of 3.7% paraformaldehyde at room temperature for 10 minutes, followed by washing with phosphate-buffered saline (PBS) and permeabilization on ice for 2 minutes with 0.1% Triton-HEPES (20 mM HEPES, 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 0.1% Triton). Cells were then stained with fluorescein isothiocyanate (FITC)–phalloidin (2 mg/mL; Sigma) for 30 minutes at room temperature, washed, and analyzed by flow cytometry.
Migration assay
RPMI (600 μL) supplemented with 10% fetal calf serum (FCS) containing either 10 ng/mL or 125 ng/mL SDF-1α was added to the lower chamber of a Costar 24-wells transwell (pore size, 5 μm; Corning, NY). Then, 1 × 105 transduced CD34+ cells in 100 μL medium were loaded to the upper chamber and were allowed to migrate for 4 hours at 37°C. Migrating cells were collected from the lower chamber and counted for 30 seconds using a FACSCalibur. Control spontaneous migration was performed without SDF-1α in the lower chamber.
Proliferation assay
CB CD34+ cells were cultured for 7 days in serum-free medium supplemented with SCF 50 ng/mL, FLT-3L 50 ng/mL, and IL-6 50 ng/mL in the presence or absence of SDF-1 50 ng/mL. Cells were counted daily and viability was evaluated by trypan blue exclusion.
Homing assay
Cells were recovered from the murine BM and spleen either 2 or 16 hours after transplantation and analyzed for the presence of human cells using human-specific anti-CD34 and anti-CD38 mAbs acquiring at least 106 cells/sample.
Intracellular CXCR4 staining
Cell surface CXCR4 was blocked with nonconjugated antihuman CXCR4 mAb (clone 12G5, 10 μg/mL, 1 hour, 4°C). Cells were fixed with paraformaldehyde (4%, 20 minutes at room temperature; BDH, Poole, United Kingdom) and then permeabilized with Triton X-100 (0.5%-1%, 10 minutes at room temperature; Sigma, St Louis, MO). Antihuman CXCR4-PE mAb was used to label the cells for flow cytometry for 30 minutes at 4°C. The cells were washed with PBS without Mg++/Ca++ after each step.
Statistics
Results of experimental points are reported as mean ± SE. Significance levels were determined by Student t test for differences in means.
Results
CXCR4-transduced human CD34+ cells have increased surface CXCR4 expression
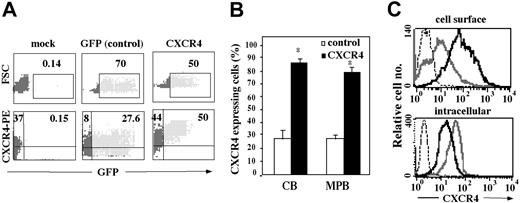
CXCR4 was overexpressed on human CB and MPB CD34+-enriched cells using an HIV-derived lentiviral gene transfer system. Transduced cells were analyzed for expression of the transgene by simultaneous determination of GFP fluorescence and CXCR4 surface expression. Both CB and MPB CD34+ cells showed high transduction efficiencies as scored by flow cytometry, reaching 70% GFP+ cells in GFP vector (control) transduced cells and 50% in CXCR4-transduced cells (Figure 2A upper panel). Furthermore, CXCR4-infected CD34+ cells were 87% ± 2.7% (CB) and 80% ± 4% (MPB) positive for cell surface CXCR4 expression, whereas only 28% ± 3.1% of both CB and MPB CD34+ cells infected with the GFP vector expressed endogenous CXCR4, resembling levels of nontransduced cells (mock cells; Figure 2A lower panel [representative] and Figure 2B). Interestingly, CXCR4-transduced cells showed a higher mean fluorescence intensity (MFI) of 89.4 compared with 10.2 of GFP vector–transduced cells (Figure 2C). However, their intracellular CXCR4 expression was lower than their control (GFP) counterparts (Figure 2C). Notably, cells transduced with the CXCR4 vector had fewer GFP+ cells, consistent with earlier reports showing reduced expression levels of genes that are placed downstream from an IRES.52 GFP vector–transduced cells demonstrated the same responses as mock cells in all parameters examined and were therefore used as the relevant control in further experiments.
Cell surface CXCR4 expression on lentiviral-transduced CD34+ ,cells. Following mock or lentiviral infection, CB and MPB CD34+ cells were analyzed by flow cytometry for either GFP expression alone or GFP together with CXCR4 expression using an anti-hCXCR4–PE antibody. Quadrants were set according to isotype-matched negative controls and mock-infected cells. (A) Data show a representative FACS analysis from CB CD34+ cells. Numbers indicate percent of total CD34+ cells. (B) Results indicate percentage of CB and MPB CD34+ cells expressing CXCR4 and represent mean ± SE of 7 independent experiments. *P < .01 compared to control GFP-infected cells. (C) Immunofluorescence detection of cell surface (top panel) and intracellular (bottom panel) CXCR4 expression of GFP-transduced (gray line), CXCR4-transduced (black line), or isotype control (dotted line) cells.
Cell surface CXCR4 expression on lentiviral-transduced CD34+ ,cells. Following mock or lentiviral infection, CB and MPB CD34+ cells were analyzed by flow cytometry for either GFP expression alone or GFP together with CXCR4 expression using an anti-hCXCR4–PE antibody. Quadrants were set according to isotype-matched negative controls and mock-infected cells. (A) Data show a representative FACS analysis from CB CD34+ cells. Numbers indicate percent of total CD34+ cells. (B) Results indicate percentage of CB and MPB CD34+ cells expressing CXCR4 and represent mean ± SE of 7 independent experiments. *P < .01 compared to control GFP-infected cells. (C) Immunofluorescence detection of cell surface (top panel) and intracellular (bottom panel) CXCR4 expression of GFP-transduced (gray line), CXCR4-transduced (black line), or isotype control (dotted line) cells.
CXCR4-overexpressing human CD34+ cells maintain their in vitro differentiation potential and primitive CD34+/CD38-/low cell population
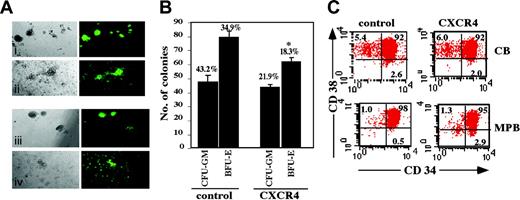
To further assess the transduction efficiency of colony-forming cell (CFC) progenitors, CB CD34+ cells were plated in methylcellulose with cytokines 72 hours following transduction. Transgene expression did not affect the ability of the cells to differentiate toward the myeloid and erythroid lineages. Transduced CD34+ cells showed multilineage differentiation into GFP+ CFC colonies including erythroid burst-forming units (BFU-Es) and granulocyte-macrophage colony-forming units (CFU-GMs; Figure 3A). The number of GFP+ CFC colonies from control cells was 2-fold higher than for CXCR4-transduced cells, in accordance with the lower percentage of GFP+ cells following transduction with this vector (Figure 3B). Interestingly, whereas both control as well as CXCR4-transduced cells produced the same number of CFU-GM colonies, there was a 25% (P = .004) reduction in BFU-E colonies from CXCR4-transduced cells (Figure 3B), confirming previous findings of Gibellini et al showing that SDF-1/CXCR4 interactions suppress erythroid lineage differentiation.53 Furthermore, MPB CD34+ CXCR4-transduced cells demonstrated a higher percentage (2.9%) of CD34+/CD38-/low population compared with 0.5% in control cells. This effect was not observed in CB CD34+ cells (Figure 3C). CXCR4 transduction may therefore better preserve or even increase the MPB CD34+/CD38-/low primitive population in vitro.
Clonogenic progenitor and CD34+/CD38-/low content of transduced CD34+ cells. Transduced CB CD34+ cells were seeded in semisolid methylcellulose culture. (A) GFP+ CFC colonies were analyzed by phase contrast microscopy at × 10 original magnification on day 14. Top panel indicates BFU-E (i) and CFU-GM (ii) colonies from control cells. Bottom panel indicates BFU-Es (iii) and CFU-GMs (iv) from CXCR4-transduced cells. A representative experiment is shown. (B) Data indicate total number of CFU-GM and BFU-E colonies. Numbers above bars indicate percentage of GFP+ colonies out of total colonies. Bars represent mean ± SE of 3 independent experiments. *P = .004 compared to BFU-E colonies of control cells. (C) CB and MPB CD34+ cells were labeled with human anti-CD34 and anti-CD38 mAbs. Numbers indicate percent positive cells from entire population. A representative experiment of 3 is shown.
Clonogenic progenitor and CD34+/CD38-/low content of transduced CD34+ cells. Transduced CB CD34+ cells were seeded in semisolid methylcellulose culture. (A) GFP+ CFC colonies were analyzed by phase contrast microscopy at × 10 original magnification on day 14. Top panel indicates BFU-E (i) and CFU-GM (ii) colonies from control cells. Bottom panel indicates BFU-Es (iii) and CFU-GMs (iv) from CXCR4-transduced cells. A representative experiment is shown. (B) Data indicate total number of CFU-GM and BFU-E colonies. Numbers above bars indicate percentage of GFP+ colonies out of total colonies. Bars represent mean ± SE of 3 independent experiments. *P = .004 compared to BFU-E colonies of control cells. (C) CB and MPB CD34+ cells were labeled with human anti-CD34 and anti-CD38 mAbs. Numbers indicate percent positive cells from entire population. A representative experiment of 3 is shown.
CXCR4 expressed on transduced CD34+ cells is functional
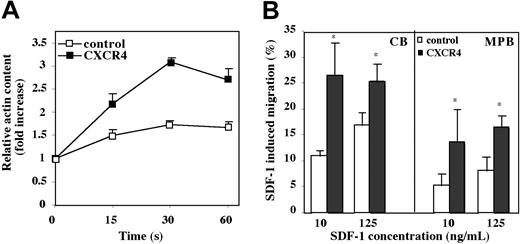
As a means for determining functionality of the inserted receptor, we examined the effect of CXCR4 overexpression on SDF-1–induced activation of the motility machinery. Motility of cells requires cytoskeletal rearrangement and particularly actin polymerization. We found that CB CD34+ cells exhibited a peak of actin polymerization after 30 seconds of stimulation with SDF-1 (Figure 4A). At this time point, CXCR4-overexpressing cells demonstrated a 3 ± 0.11-fold (P < .001) increase in actin polymerization versus a 1.5 ± 0.07-fold (P = .002) increase in control cells when compared to unstimulated cells (Figure 4A). In light of these results, we investigated the migration potential of CXCR4-transduced CB and MPB CD34+ cells to a gradient of SDF-1 (125 ng/mL) in a transwell migration assay. Cells overexpressing CXCR4 demonstrated a significantly increased response to SDF-1–mediated chemotaxis of 1.5 ± 0.04-fold (P < .04) for CB and 2 ± 0.3-fold (P = .03) for MPB when compared to control cells (Figure 4B). All together, these data suggest that overexpression of CXCR4 on human CD34+ progenitor cells results in enhanced SDF-1–induced signaling, leading to an increase in cell motility.
Functionality of CXCR4 expressed on transduced CD34+ cells. (A) CXCR4-transduced CB CD34+ cells were stimulated with SDF-1 (300 ng/mL) for the indicated times and intracellular F-actin content was measured by FACS. Data indicate fold increase in F-actin content following stimulation with SDF-1 compared to unstimulated cells. Data represent mean ± SE of 3 independent experiments. (B) CXCR4-transduced CB and MPB CD34+ cells were tested in a transwell migration assay for their migration toward different SDF-1 concentrations as indicated. Data indicate percent migrating cells to SDF-1. Bars represent mean ± SE of 5 independent experiments. *P < .04 (CB), *P = .03 (MPB) compared to control GFP-transduced cells at 125 ng/mL SDF-1. *P < .05 (CB), *P < .05 (MPB) compared to control GFP-transduced cells at 10 ng/mL SDF-1.
Functionality of CXCR4 expressed on transduced CD34+ cells. (A) CXCR4-transduced CB CD34+ cells were stimulated with SDF-1 (300 ng/mL) for the indicated times and intracellular F-actin content was measured by FACS. Data indicate fold increase in F-actin content following stimulation with SDF-1 compared to unstimulated cells. Data represent mean ± SE of 3 independent experiments. (B) CXCR4-transduced CB and MPB CD34+ cells were tested in a transwell migration assay for their migration toward different SDF-1 concentrations as indicated. Data indicate percent migrating cells to SDF-1. Bars represent mean ± SE of 5 independent experiments. *P < .04 (CB), *P = .03 (MPB) compared to control GFP-transduced cells at 125 ng/mL SDF-1. *P < .05 (CB), *P < .05 (MPB) compared to control GFP-transduced cells at 10 ng/mL SDF-1.
CXCR4-transduced CD34+ cells are more responsive to low SDF-1 concentrations
We hypothesized that overexpression of CXCR4 on CD34+ cells may render them more responsive to low SDF-1 concentrations. To test this, we performed in vitro migration of transduced cells to different SDF-1 concentrations. Indeed, we found that already at low concentrations of SDF-1 (10 ng/mL), the migration of CB CXCR4-transduced cells had reached peak levels of 25%, similar to migration of these cells at a high SDF-1 concentration (125 ng/mL; Figure 4B). At the lower SDF-1 concentration (10 ng/mL), CXCR4-transduced cells showed up to 2.5-fold (P = .05) increase in migration compared with their control counterparts, whereas at higher SDF-1 concentrations (125 ng/mL), this increase was less significant (1.5-fold; Figure 4B). Similarly, even though the percent of migrating cells was much lower than for CB CD34+ cells, CXCR4-transduced MPB CD34+ cells showed a 2.6-fold (P = .05) increase in migration to 10 ng/mL SDF-1 compared with a 2-fold increase to 125 ng/mL SDF-1 (Figure 4B).
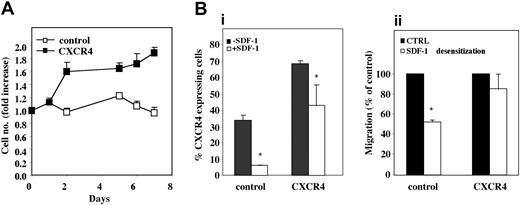
Low concentrations of the chemokine SDF-1 in synergy with cytokines have been shown to enhance proliferation of human CD34+ cells as well as both human and murine progenitor cell survival.24-27 We therefore monitored the effect of SDF-1 at low concentrations on proliferation of CXCR4-overexpressing cells. Transduced cells were incubated with SDF-1 (50 ng/mL) in combination with a cytokine cocktail (SCF, FLT-3L, and IL-6) known to support stem cells in vitro, and cell number was assessed at 24-hour intervals for 1 week. CXCR4-transduced CB CD34+ cells had almost doubled their seeded amount (P < .05) already 48 hours after seeding and this effect could be seen for up to 7 days (P = .001) in culture. Control cells, however, only increased their cell number on day 5 by up to only 1.2 ± 0.05-fold, and by day 7 their number had decreased to below the original amount seeded (Figure 5A). Notably, this enhanced proliferative effect of CXCR4-overexpressing cells was not detected at higher (100 ng/mL) SDF-1 concentrations (data not shown). Longer time points in culture led to cell differentiation, suggestive of nontransformed cell phenotype following lentiviral transduction. Taken together, our results indicate that overexpression of CXCR4 on CD34+ cells enhances their response to low SDF-1 concentrations, increasing both their motility and proliferation/survival, with a lesser response to high concentrations.
Response of CXCR4-overexpressing CB CD34+ cells to different SDF-1 concentrations. (A) Lentiviral-transduced CB CD34+ cells were incubated for 7 days in serum-free conditions with SDF-1 (50 ng/mL) in combination with SCF (50 ng/mL), FLT-3L (50 ng/mL), and IL-6 (50 ng/mL). Results are shown as fold increase in number of viable cells compared to cells incubated in the absence of SDF-1. Results represent mean ± SE of 3 independent experiments performed in duplicate. (B) Lentiviral-transduced CB CD34+ cells were incubated overnight with 1 μg/mL SDF-1 and examined for (i) CXCR4 expression by immunostaining and (ii) SDF-1 (125 ng/mL) induced in vitro migration using a transwell migration system. Bars represent mean ± SE of 2 independent experiments performed in duplicate. *P <.05 compared to untreated cells (▪).
Response of CXCR4-overexpressing CB CD34+ cells to different SDF-1 concentrations. (A) Lentiviral-transduced CB CD34+ cells were incubated for 7 days in serum-free conditions with SDF-1 (50 ng/mL) in combination with SCF (50 ng/mL), FLT-3L (50 ng/mL), and IL-6 (50 ng/mL). Results are shown as fold increase in number of viable cells compared to cells incubated in the absence of SDF-1. Results represent mean ± SE of 3 independent experiments performed in duplicate. (B) Lentiviral-transduced CB CD34+ cells were incubated overnight with 1 μg/mL SDF-1 and examined for (i) CXCR4 expression by immunostaining and (ii) SDF-1 (125 ng/mL) induced in vitro migration using a transwell migration system. Bars represent mean ± SE of 2 independent experiments performed in duplicate. *P <.05 compared to untreated cells (▪).
CXCR4-overexpressing cells are less responsive to SDF-1–induced desensitization
It has been documented that SDF-1 at high concentrations (1 μg/mL and above) induces desensitization and internalization, via endocytosis, of the cell surface CXCR4 molecule, which eventually can be recycled to the cell surface.54 We therefore tested the effect of high SDF-1 concentrations on cells overexpressing CXCR4. To this end, CXCR4-transduced CB CD34+ cells were incubated overnight with 1 μg/mL SDF-1 and analyzed for CXCR4 cell surface expression. Unexpectedly, there was only a 40% (P < .05) decrease in cell surface receptor expression in CXCR4-overexpressing cells, whereas in control cells this decrease reached up to 90% (P < .05; Figure 5Bi). Desensitized cells were also assayed in vitro for directional migration toward an SDF-1 (125 ng/mL) gradient. Control cells showed a significant (P < .05) decrease in SDF-1–mediated migration, whereas the migration of CXCR4-transduced cells was hardly affected (Figure 5Bii). This suggests that the internalization of CXCR4 is compensated for by constant overexpression of the receptor, which continuously remains functional.
CXCR4 overexpression improves SRC engraftment of NOD/SCID mice
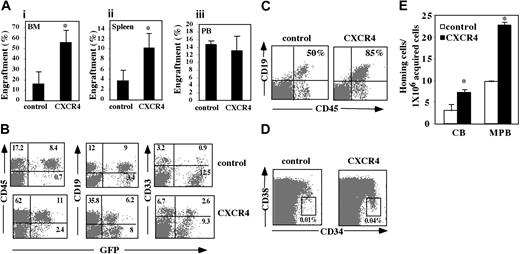
To assess the effect of CXCR4 overexpression on the SDF-1/CXCR4-dependent engraftment of human CB CD34+ cells, transduced progenitors were transplanted in NOD/SCID mice. CXCR4-transduced cells demonstrated increased engraftment of human cells in the murine BM of up to 4 ± 0.7-fold (P = .001) for CB CD34+ cells when compared to control cells (Figure 6Ai). Similarly, CXCR4-transduced cells showed a 2.7 ± 0.8-fold (P = .05) increase in repopulation of the spleen (Figure 6Aii). Interestingly, no significant differences were observed between the numbers of circulating human cells in mice engrafted with control versus CXCR4-transduced cells (Figure 6Aiii). Furthermore, in mice engrafted with CXCR4-overexpressing cells 11.9% ± 1.68% of the human cells in the circulation were transduced compared with 24% ± 3.12% transduced human cells in the BM (P = .01). Together these observations suggest a major role for CXCR4 in retention of human progenitor cells within the BM microenvironment.
In vivo multilineage reconstitution and GFP expression of CXCR4-overexpressing SRCs. CXCR4-transduced CB CD34+ cells were injected into sublethally irradiated NOD/SCID mice. Five weeks after transplantation mice were examined for the presence of repopulating human cells when compared to control GFP-transduced cells. (A) Murine BM (i), spleen (ii), and peripheral blood (PB; iii) were analyzed for human cell engraftment by FACS analysis of % of human CD45+ cells. Bars represent mean ± SE of 9 independent experiments performed in duplicate or triplicate. *P < .005 compared to control GFP-transduced cells. (B) Lymphoid and myeloid differentiation of human SRCs in a representative NOD/SCID transplant recipient is shown by CD19 and CD33 antibody staining, respectively. Numbers indicate percent of positive cells of total live population. (C) BM cells were stained for the human-specific pan leukocyte marker CD45 and the B-cell lineage differentiation marker CD19 and analyzed by FACS. Numbers indicate percentage of CD19 cells calculated from total CD45 population. Data show a representative experiment. (D) BM cells were stained with human-specific anti-CD34 and anti-CD38 mAbs and analyzed by flow cytometry. Numbers indicate percentage of CD34+/38-/low cells. Data show a representative experiment. (E) Homing of cells to the spleen was determined 16 hours (MPB) or 2 hours (CB) after transplantation by staining with human-specific anti-CD34 and anti-CD38 mAbs. Bars represent mean ± SE of 3 independent experiments performed in duplicate. *P < .05 compared to control cells.
In vivo multilineage reconstitution and GFP expression of CXCR4-overexpressing SRCs. CXCR4-transduced CB CD34+ cells were injected into sublethally irradiated NOD/SCID mice. Five weeks after transplantation mice were examined for the presence of repopulating human cells when compared to control GFP-transduced cells. (A) Murine BM (i), spleen (ii), and peripheral blood (PB; iii) were analyzed for human cell engraftment by FACS analysis of % of human CD45+ cells. Bars represent mean ± SE of 9 independent experiments performed in duplicate or triplicate. *P < .005 compared to control GFP-transduced cells. (B) Lymphoid and myeloid differentiation of human SRCs in a representative NOD/SCID transplant recipient is shown by CD19 and CD33 antibody staining, respectively. Numbers indicate percent of positive cells of total live population. (C) BM cells were stained for the human-specific pan leukocyte marker CD45 and the B-cell lineage differentiation marker CD19 and analyzed by FACS. Numbers indicate percentage of CD19 cells calculated from total CD45 population. Data show a representative experiment. (D) BM cells were stained with human-specific anti-CD34 and anti-CD38 mAbs and analyzed by flow cytometry. Numbers indicate percentage of CD34+/38-/low cells. Data show a representative experiment. (E) Homing of cells to the spleen was determined 16 hours (MPB) or 2 hours (CB) after transplantation by staining with human-specific anti-CD34 and anti-CD38 mAbs. Bars represent mean ± SE of 3 independent experiments performed in duplicate. *P < .05 compared to control cells.
A representative FACS staining with CD19 and CD33 mAbs demonstrates that multilineage hematopoiesis of transduced cells into lymphoid and myeloid populations, respectively, was maintained in the murine BM (Figure 6B) with a trend to more B-cell lymphopoiesis in mice receiving CXCR4-transduced cells (Figure 6C), most probably because SDF-1 is also a pre–B-cell growth factor.55 Furthermore, an average of 36% ± 19% (range, 7.5%-77%) of the CD45+ cells were found to express GFP (Figure 6B). Transgene expression was also detected in both myeloid and lymphoid populations (Figure 6B). Mice injected with CXCR4-overexpressing cells showed a 4-fold increase in the primitive CD34+/CD38-/low cell population compared with mice injected with control cells (Figure 6D), suggesting that the higher engraftment levels of CXCR4-overexpressing cells are due to increased repopulation of the more primitive cell population.
We have previously demonstrated that homing of immature human CD34+38-/lowCXCR4+ cells to the murine BM and spleen is dependent on CXCR4/SDF-1 interactions.11 We therefore examined whether overexpression of CXCR4 on human CD34+ cells could improve their homing to the BM and spleen of sublethally irradiated NOD/SCID mice. We observed that 2 hours (CB) or 16 hours (MPB) after transplantation CXCR4-transduced cells showed a more than 2-fold increase in homing to the spleen compared with their control counterparts (Figure 6E). We did not detect these differences in their homing capacity to the BM (data not shown). These results suggest that CXCR4-transduced cells may first home short-term to the spleen before repopulating (5 weeks after transplantation) the BM as previously suggested.11,56
Discussion
The cell surface expression of the CXCR4 chemokine receptor is a major determinant in the migration and repopulation capacity of hematopoietic stem and progenitor cells.11,12 However, the exact role of CXCR4 in these processes still needs to be clarified. In the present study we developed a novel system for overexpressing a functional CXCR4 receptor on human HSCs and examined its effect on several cellular functions in response to SDF-1.
Using a lentiviral gene transfer system previously reported to be efficient in the transduction of CD34+ SRCs,44-47,57 we successfully overexpressed cell surface CXCR4 on CB and MPB CD34+ cells. Furthermore, we show that overexpression of CXCR4 on human CB CD34+ cells significantly improves their long-term in vivo repopulation of NOD/SCID BM and spleen 5 weeks after transplantation. This finding is in accordance with Sawada et al who showed an increase of CXCR4-overexpressing CD4+ T cells in the BM of transgenic mice.43 We further observed that CXCR4-overexpressing engrafted cells maintained their differentiation potential into the myeloid and lymphoid lineages. This is supported by our in vitro data demonstrating that transduced cells retain their multilineage colony-forming potential in methylcellulose cultures. However, we observed a 25% reduction in erythroid colonies from CXCR4-transduced cells, confirming previous data showing suppression of erythroid lineage differentiation by SDF-1/CXCR4 interactions.53 SDF-1/CXCR4 interactions are also involved in early B-lymphocyte development9 and SDF-1 is a pre–B-cell growth-stimulating factor.55 In agreement with these data, we observed an increased tendency toward B-cell lineage differentiation in mice engrafted with CXCR4-transduced cells. Therefore, besides its role in repopulation, we suggest that CXCR4 overexpression may determine the fate of lineage commitment in transduced cells.
The enhanced engraftment levels of CXCR4-transduced cells may be explained by an increase in HSC response to SDF-1 involving either motility, proliferation, survival, or retention in the BM microenvironment. Indeed, at a ligand concentration optimal for SDF-1–mediated transwell migration in vitro (125 ng/mL), both CB and MPB CD34+ cells overexpressing CXCR4 showed an increase in migration when compared to control cells. Notably, this increase in migration was more significant for MPB CD34+ cells. Very low CXCR4 expression has been observed on MPB CD34+ cells and their in vitro migration toward a gradient of SDF-1 is reduced compared with CB and BM CD34+ cells.12,58 Therefore, MPB CD34+ cells may migrate better following CXCR4 overexpression, as opposed to CB CD34+ cells, which have a higher basal level migratory capacity. Interestingly, we found that CB and MPB CD34+ cells overexpressing CXCR4 already reached peak migration levels at a low SDF-1 concentration (10 ng/mL). In support of the increased migration following CXCR4 expression, we further show that CB CD34+ cells overexpressing CXCR4 have increased SDF-1–induced actin polymerization compared with control cells. Enhanced in vitro cell motility and actin polymerization suggests that transduced cells respond better to the SDF-1 chemoattracting gradient from the murine BM and spleen and may therefore demonstrate enhanced in vivo homing ability. Both CB and MPB CD34+ cells overexpressing CXCR4 demonstrated at least a 2-fold increase in homing to the spleen; however, this effect was not observed in the BM. Papayannopoulou has suggested that intravenously administered cells are initially distributed in other organs such as the spleen and may at a later stage home specifically to the BM.56 Furthermore, we have previously shown that more CB CD34+ cells reach the spleen shortly following infusion (homing),11 suggesting that CXCR4-overexpressing cells may preferentially home to the spleen before residing long-term in the BM. We further suggest that other mechanisms such as increased retention or proliferation in the BM may also facilitate the increased repopulation. Indeed, we observed an increase in in vitro proliferation of up to 2-fold in human CB CXCR4-transduced CD34+ cells compared with control cells following coculture with a cytokine cocktail in combination with a low SDF-1 concentration (50 ng/mL), and an almost 6-fold increase in in vitro primitive CD34+/CD38- cell proliferation of CXCR4-overexpressing MPB cells compared with control cells. SDF-1 at low concentrations has been shown to be a survival factor for progenitor cells and enhances proliferation of human CD34+ cells and murine progenitors.24-28,59 Therefore, it may be possible that on arrival in the BM niche, transduced cells show increased proliferation in response to SDF-1, providing another explanation for the higher engraftment levels of CXCR4-overexpressing cells. Moreover, our results suggest that overexpression of CXCR4 on transduced cells renders them more responsive to low SDF-1 concentrations both in the context of directional migration toward an SDF-1 gradient as well as in the context of cell proliferation. Interestingly, although low concentrations of SDF-1 induce increased cell proliferation, Cashman et al demonstrated that in vivo administration of 2 consecutive injections of a high dose of SDF-1 (10 μg) increases the number of repopulating human fetal liver stem cells in NOD/SCID mice receiving serial transplants by enhancing the levels of quiescent cells.30 SDF-1 therefore exhibits pleiotropic concentration dependent effects on HSC proliferation and cell cycle status.
Cellular responses to chemoattractants can be reduced by receptor-mediated desensitization. Similarly, SDF-1 induces receptor internalization.54 Unexpectedly, we report that in cells overexpressing CXCR4, there was only a 40% decrease in cell surface receptor expression compared with a 90% decrease in control cells following in vitro exposure to high levels of SDF-1 (1 μg/mL). Higher cell surface receptor expression following SDF-1 desensitization may be explained by its increased turnover, as a result of constitutive presentation on the cell surface. This is supported by the lower intracellular CXCR4 expression we observed on CXCR4-overexpressing cells.
CXCR4 has been suggested to play a role in the retention of HSCs in the murine BM.9,31-34 Similarly, Ma et al have proposed that CXCR4 is required for the retention of B-lineage and granulocytic precursors in the BM.31 Therefore, maintenance of high CXCR4 expression on transplanted cells together with continuous expression of SDF-1 in the murine BM following total body irradiation13 and during steady-state homeostasis may facilitate better anchorage and proliferation of HSCs in the BM microenvironment. Despite higher engraftment levels within the BM of mice injected with CXCR4-transduced cells, these mice showed similar numbers of circulating cells compared with controls, supporting a role for the constitutively overexpressed receptor in BM retention. Furthermore, the higher percentage of transduced human cells in the BM compared with the circulation observed in mice engrafted with CXCR4-overexpressing cells suggests that more of the transduced cells are maintained in the BM. This strengthens the notion that CXCR4 is also involved in human stem cell retention within the BM. We therefore suggest that CXCR4 overexpression may promote retention and development of SRCs in their specialized BM niches.
In conclusion, we demonstrate that CXCR4 overexpression on CD34+ stem and progenitor cells promotes their increased in vivo engraftment of NOD/SCID mice. Further expansion of a CXCR4-overexpressing system may serve as an effective tool to study regulation and functionality of this unique receptor as well as to improve the compromised homing and engraftment following gene therapy protocols.7,8 A significant clinical breakthrough in gene therapy was made in patients with human SCID-X1 resulting in full correction of disease phenotype,60,61 proving that gene therapy can work in practice. However, the risk of malignant transformation is prevalent in some patients.60 Other cell types such as mesenchymal stem cells possess the ability to migrate to various organs; however, their levels of engraftment are particularly low. For example, human bone marrow transplantation in children with severe osteogenesis imperfecta resulted in only a transient improvement in bone formation.62 The development of a system facilitating constitutive or transient CXCR4 expression on the cell surface together with induction of SDF-1 expression in the target organ could be beneficial for directional migration in vivo, as well as long-term repopulation and development of various cell types in the organ of interest in patients, as part of organ repair. Recently it was shown that SDF-1 overexpressed in cardiac fibroblasts infused in the rat heart could functionally attract CD117+ stem cells to injured myocardium.63 We therefore suggest overexpression of CXCR4 as a universal system for regulating stem cell function and development, which could improve the outcome of many clinical protocols.
Supported in part by grants from the Ares-Serono Group, the Israel Science Foundation (ISF), MINERVA, EUROCORD III, and the Gabriella Rich Center for Transplantation Biology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-07-2607.
We would like to extend our gratitude to Mrs Loya Abel for her expert assistance in experiments performed. We also thank Merav Darash-Yahana for kindly providing the double-infection protocol.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal