Abstract
The progression rate of iron overload in hereditary hemochromatosis in individuals in the general population is unknown. We therefore examined in the general population iron overload progression rate in C282Y homozygotes. Using a cohort study of the Danish general population, The Copenhagen City Heart Study, we genotyped 9174 individuals. The 23 C282Y homozygotes identified were matched to 2 subjects each of 5 other HFE genotypes with respect to sex, age, and alcohol consumption. As a function of biologic age, transferrin saturation increased from 50% to 70% from 25 to 85 years of age and from 70% to 80% from 35 to 80 years of age in female and male C282Y homozygotes, respectively. Equivalently, ferritin levels increased from 100 to 500 μg/L and decreased from 800 to 400 μg/L in female and male C282Y homozygotes. As a function of 25 years follow-up irrespective of age, transferrin saturation and ferritin levels increased slightly in male and female C282Y homozygotes. None of the C282Y homozygotes developed clinically overt hemochromatosis. In conclusion, individuals in the general population with C282Y homozygosity at most demonstrate modest increases in transferrin saturation and ferritin levels, and clinically overt hemochromatosis is rare. Therefore, C282Y homozygotes identified during population screening, and not because of clinically overt hemochromatosis, at most need to be screened for manifestations of hemochromatosis every 10 to 20 years. (Blood. 2004;103: 2914-2919)
Introduction
Hereditary hemochromatosis is a disease caused by iron accumulation in the body due to excess iron absorption from the intestinal tract.1 This leads to increased transferrin saturation and ferritin levels, and may cause progressive organ damage such as liver cirrhosis, type 1 diabetes mellitus, hypogonadotropic hypogonadism, cardiomyopathy, arthritis, and bronze coloring of the skin. Most hereditary hemochromatosis patients are homozygous for C282Y (Cys282Tyr) of the HFE gene.2
Hereditary hemochromatosis may be one of the most common genetic disorders among people of Northern European descent.3,4 Therefore, hereditary hemochromatosis could be an ideal condition for population screening5,6 ; however, penetrance of C282Y homozygosity remains controversial.7-19 Accordingly, before such screening procedures can be recommended we need to know the iron overload progression rate among healthy C282Y homozygotes in individuals from the general population.
We genotyped 9174 individuals from the Danish general population, The Copenhagen City Heart Study.20 Measured as increases in transferrin saturation and ferritin levels, we studied iron overload progression rate in C282Y homozygotes during 25 years follow-up. In addition, we assessed all symptoms, signs, hospitalizations, and deaths of C282Y homozygotes.
Patients, materials, and methods
Subjects
The basis for this study was 9174 subjects from The Copenhagen City Heart Study (third examination, 1991-1994).20,21 These individuals were selected after age and sex stratification based on the Danish Central Population Register code to represent the Danish general population.
More than 99% were of white Danish descent. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki: the study was approved by the Steering Committee of The Copenhagen City Heart Study, Herlev University Hospital, and the Danish ethics committee for Copenhagen and Frederiksberg (No.100.2039/91). All participants gave written informed consent in accordance with the Helsinki protocol.
Design
In a cross-sectional design, each subject homozygous for C282Y was matched to 2 subjects each of wild type/wild type, H63D/wild type, H63D/H63D, C282Y/wild type, and C282Y/H63D genotypes with respect to sex, age (in 10-year groups), and alcohol consumption (in sex-specific tertiles).
To study iron overload progression rate, the matched individuals of wild type/wild type, C282Y/H63D, and C282Y/C282Y genotypes were used to follow the development of iron status from 25 to 85 years of age; we followed each individual for up to 25 years comprising 4 investigations between 1976 and 2001. Plasma samples from 1976 to 1978 and 1981 to 1983 stored at -20°C, serum samples from 1991 to 1994 stored at -80°C, as well as newly drawn serum samples from 2001 were used.
To study lifetime penetrance, we conducted a fourth examination in 2001 comprising the 20 C282Y homozygotes still alive. During this examination, blood samples were obtained and in addition patients were questioned about possible illnesses and examined physically by a hemochromatosis specialist (H.B.). Previous disease was also assessed by reviewing the Danish National Hospital Discharge Register and the Danish National Register of Causes of Deaths; these registers cover all citizens of Denmark and all Danish hospitals from 1976 through 1999.
Measurements
Hemochromatosis genotyping was as described.20 Transferrin was measured on a Nephelometer Analyzer II (Behring, Düdingen, Switzerland), while follicle-stimulating hormone levels, luteinizing hormone levels, and ferritin levels were determined on a Technicon Immuno 1 System (Bayer, Leverkusen, Germany). Ferritin level higher than 250 μg/L in men and higher than 200 μg/L in women was chosen as indication of iron overload. Aspartate aminotransferase was measured on a Hitachi 705 autoanalyzer (Hitachi, Tokyo, Japan). Albumin levels, alkaline phosphatase levels, bilirubin levels, and iron levels (except for the 1976-1978 and 1981-1983 samples) were measured on a Vitros 950 autoanalyzer (Johnson & Johnson, Rochester, NY). Because plasma samples from the 1976-1978 and 1981-1983 investigations contained EDTA (ethylenediaminetetraacetic acid), iron contents of these samples were measured by atomic absorption photometry on a Perkin-Elmer 403 photometer (Shelton, CT).
Statistical analyses
Statistical analyses were performed using SPSS.22 A P value less than .05 on a 2-sided test was significant. Correction for multiple comparisons was not performed. A priori we stratified by sex. We used Mann-Whitney U test for 2-genotype comparisons. The smoothed relation between age and transferrin saturation and ferritin levels (log transformed) in different genotype groups was investigated using local linear regression (smoother function, normal kernel, bandwidth multiplier value = 5 years); ferritin levels were log transformed to approach normal distribution.
Results
Of 9174 subjects from the Danish general population, 6135 were wild type/wild type (66.9%; 95% CI, 65.9%-67.8%), 1881 were H63D/wild type (20.5%; 95% CI, 19.7%-21.3%), 158 were H63D/H63D (1.7%; 95% CI, 1.5%-2.0%), 846 were C282Y/wild type (9.2%; 95% CI, 8.6%-9.9%), 131 were C282Y/H63D compound heterozygous (1.4%; 95% CI, 1.2%-1.7%), and 23 were homozygous for C282Y (0.25%; 95% CI, 0.16%-0.38%).20
Iron status cross-sectionally
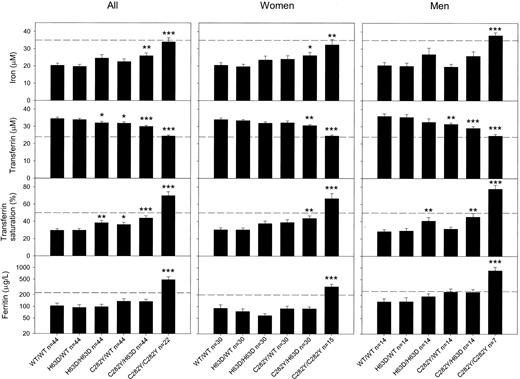
Transferrin saturation levels were increased in C282Y homozygotes (P < .001), C282Y/H63D compound heterozygotes (P < .001), C282Y/wild type (P < .05), and H63D/H63D (P < 0.01) when compared with wild type/wild type (Figure 1). These differences in C282Y homozygotes and C282Y/H63D compound heterozygotes versus wild type/wild type were similar when men and women were examined separately. Only C282Y homozygotes had average transferrin saturation levels above 50%.
Average levels with standard error of iron status parameters as a function ofHFEgenotype. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of the 5 other genotypes. Due to technical error, the sample from one of the 23 C282Y homozygotes was not measured, and consequently the 2 matched persons in each of the other genotypes were also excluded. Dashed lines are upper borders of reference intervals; for ferritin these borders differ between women and men. Levels in mutation carriers were tested against wild type/wild type (WT/WT) levels using Mann-Whitney U tests: *P = .05, **P = .01, ***P = .001. Error bars indicate the standard error of the mean.
Average levels with standard error of iron status parameters as a function ofHFEgenotype. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of the 5 other genotypes. Due to technical error, the sample from one of the 23 C282Y homozygotes was not measured, and consequently the 2 matched persons in each of the other genotypes were also excluded. Dashed lines are upper borders of reference intervals; for ferritin these borders differ between women and men. Levels in mutation carriers were tested against wild type/wild type (WT/WT) levels using Mann-Whitney U tests: *P = .05, **P = .01, ***P = .001. Error bars indicate the standard error of the mean.
Ferritin levels were increased in C282Y homozygotes compared with wild type/wild type, irrespective of whether men and women were examined separately or together (Figure 1). However, there was no difference in ferritin levels between C282Y/H63D compound heterozygotes and wild type/wild type, indicating that individuals with this genotype did not show evidence of iron overload.
Iron overload progression rate
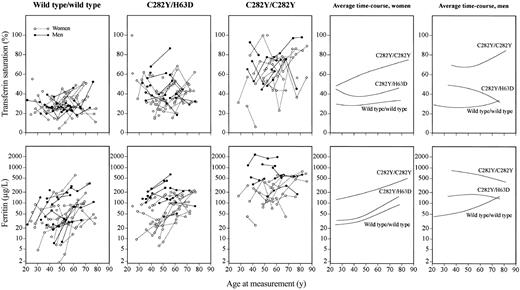
Transferrin saturation increased from 50% at 25 years of age to 70% at 85 years of age in female C282Y homozygotes and from 70% at 35 years of age to 80% at 80 years of age in male C282Y homozygotes on local linear regression analysis, whereas transferrin saturation did not increase with age in female or male wild type/wild type individuals and in female or male C282Y/H63D compound heterozygotes (Figure 2). Transferrin saturation values oscillated most in C282Y homozygotes, somewhat less in C282Y/H63D compound heterozygotes, and least in wild type/wild type individuals.
Levels of transferrin saturation and ferritin in selected genotypes over a follow-up period of up to 25 years, shown as a function of age at measurement. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of wild type/wild type and C282Y/H63D genotypes. Data for a given individual are connected with lines. Panels to the right show local linear regression curves for women and men separately.
Levels of transferrin saturation and ferritin in selected genotypes over a follow-up period of up to 25 years, shown as a function of age at measurement. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of wild type/wild type and C282Y/H63D genotypes. Data for a given individual are connected with lines. Panels to the right show local linear regression curves for women and men separately.
From 25 to 85 years of age, ferritin levels increased from 120 to 500 μg/L in female C282Y homozygotes, from 30 to 150 μg/L in female C282Y/H63D compound heterozygotes, and from 25 to 80 μg/L in female wild type/wild type individuals (Figure 2). From 35 to 80 years of age, ferritin levels declined from 800 to 400 μg/L in male C282Y homozygotes, were stable around 150 μg/L in male C282/H63D compound heterozygotes, but increased in male wild type/wild type individuals from 40 to 150 μg/L. Ferritin values oscillated more in C282Y homozygotes than in wild type/wild type individuals or in C282/H63D compound heterozygotes.
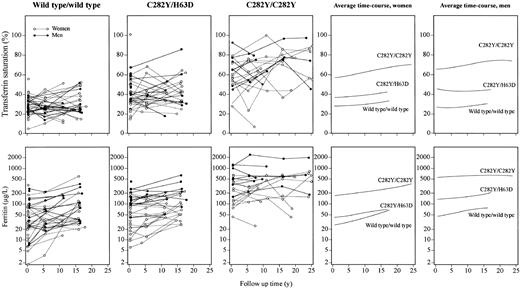
As a function of 25-, 15-, and 15-year follow-up in female and male C282Y homozygotes, C282Y/H63D compound heterozygotes, and wild type/wild type individuals, respectively, transferrin saturation increased modestly at most (Figure 3). This was also the case for ferritin levels.
Levels of transferrin saturation and ferritin in selected genotypes over a follow-up period of up to 25 years, shown as a function of follow-up time. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of wild type/wild type and C282Y/H63D genotypes. Data for a given individual are connected with lines. Panels to the right show local linear regression curves for women and men separately.
Levels of transferrin saturation and ferritin in selected genotypes over a follow-up period of up to 25 years, shown as a function of follow-up time. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of wild type/wild type and C282Y/H63D genotypes. Data for a given individual are connected with lines. Panels to the right show local linear regression curves for women and men separately.
Lifetime penetrance
Of the 23 C282Y homozygotes, 14 women and 6 men with median ages of 66 years (range, 35-85 years) and 62 years (range, 45-81 years) were alive in 2001 (Table 1). Only 4 of these had been blood donors, and only one gave more than 50 units. One patient died from lung cancer, one patient died from atherosclerotic heart disease, and one patient died from malignant melanoma.
Characteristics of C282Y homozygotes at the 2001 investigation
Subject no. . | Age at investigation, y . | Previous hemochromatosis diagnosis . | Blood donor, units . | Transferrin saturation, % . | Ferritin, μg/L . | Liver disease* . | Diabetes mellitus† . | Hypogonadism‡ . | Cardiomyopathy . | Arthritis . |
|---|---|---|---|---|---|---|---|---|---|---|
| Women | ||||||||||
| 1 | 35 | No | 0 | 58 | 330 | No | No | No | No | Arthralgia |
| 2 | 42 | No | 0 | 6 | 24 | No | No | No | No | No |
| 3 | 48 | No | 0 | 45 | 351 | No | No | No | No | No |
| 4 | 51 | No | 0 | 66 | 116 | No | No | No | No | No |
| 5§ | 52 | No | ND | 100 | 117 | No | No | No | No | No |
| 6 | 56 | No | 12 | 77 | 73 | No | No | No | No | No |
| 7 | 57 | No | 2 | 63 | 280 | No | No | No | No | Arthralgia |
| 8§ | 66 | No | ND | 73 | 646 | No | No | No | No | No |
| 9 | 65 | No | >50 | 55 | 623 | No | No | No | No | No |
| 10 | 67 | No | 0 | 44 | 43 | No | No | No | No | No |
| 11 | 73 | No | 10 | 77 | 570 | No | No | No | No | No |
| 12 | 76 | No | 0 | 86 | 156 | No | No | No | No | No |
| 13 | 77 | No | 0 | 56 | 595 | No | Yes† | No | No | No |
| 14 | 81 | No | 0 | 84 | 813 | No | No | No | No | No |
| 15 | 85 | No | 0 | 57 | 710 | No | No | No | No | No |
| 16 | 85 | No | 0 | 89 | 424 | No | No | No | No | No |
| Men | ||||||||||
| 17∥ | 45 | Yes∥ | 0 | 80 | 452 | No | No | No | No | No |
| 18§ | 58 | No | ND | 63 | 1162 | No | No | No | No | No |
| 19 | 49 | No | 0 | 50 | 511 | No | No | No | No | No |
| 20 | 60 | No | 0 | 74 | 2062 | No | No | No | No | No |
| 21 | 63 | No | 0 | 75 | 557 | No | No | No | No | No |
| 22 | 77 | No | 0 | 45 | 188 | No | No | No | No | No |
| 23 | 81 | No | 0 | 97 | 668 | No | No | No | No | No |
Subject no. . | Age at investigation, y . | Previous hemochromatosis diagnosis . | Blood donor, units . | Transferrin saturation, % . | Ferritin, μg/L . | Liver disease* . | Diabetes mellitus† . | Hypogonadism‡ . | Cardiomyopathy . | Arthritis . |
|---|---|---|---|---|---|---|---|---|---|---|
| Women | ||||||||||
| 1 | 35 | No | 0 | 58 | 330 | No | No | No | No | Arthralgia |
| 2 | 42 | No | 0 | 6 | 24 | No | No | No | No | No |
| 3 | 48 | No | 0 | 45 | 351 | No | No | No | No | No |
| 4 | 51 | No | 0 | 66 | 116 | No | No | No | No | No |
| 5§ | 52 | No | ND | 100 | 117 | No | No | No | No | No |
| 6 | 56 | No | 12 | 77 | 73 | No | No | No | No | No |
| 7 | 57 | No | 2 | 63 | 280 | No | No | No | No | Arthralgia |
| 8§ | 66 | No | ND | 73 | 646 | No | No | No | No | No |
| 9 | 65 | No | >50 | 55 | 623 | No | No | No | No | No |
| 10 | 67 | No | 0 | 44 | 43 | No | No | No | No | No |
| 11 | 73 | No | 10 | 77 | 570 | No | No | No | No | No |
| 12 | 76 | No | 0 | 86 | 156 | No | No | No | No | No |
| 13 | 77 | No | 0 | 56 | 595 | No | Yes† | No | No | No |
| 14 | 81 | No | 0 | 84 | 813 | No | No | No | No | No |
| 15 | 85 | No | 0 | 57 | 710 | No | No | No | No | No |
| 16 | 85 | No | 0 | 89 | 424 | No | No | No | No | No |
| Men | ||||||||||
| 17∥ | 45 | Yes∥ | 0 | 80 | 452 | No | No | No | No | No |
| 18§ | 58 | No | ND | 63 | 1162 | No | No | No | No | No |
| 19 | 49 | No | 0 | 50 | 511 | No | No | No | No | No |
| 20 | 60 | No | 0 | 74 | 2062 | No | No | No | No | No |
| 21 | 63 | No | 0 | 75 | 557 | No | No | No | No | No |
| 22 | 77 | No | 0 | 45 | 188 | No | No | No | No | No |
| 23 | 81 | No | 0 | 97 | 668 | No | No | No | No | No |
ND indicates no data.
Based on symptoms; aspartate aminotransferase level > 50 IU/L; alkaline phosphatase level > 275 IU/L; coagulation factors II + VII + X < 70%; bilirubin level > 17 μM.
Based on symptoms; nonfasting glucose level > 8.3 mM; HbA1c (β-N-valyl-1-deoxyfructose hemoglobin) > 0.063. Subject no. 13 was on insulin.
Based on symptoms; follicle-stimulating hormone levels < 24 IU/L for postmenopausal women, < 1.3 IU/L for others; luteinzing hormone levels < 13.5 IU/L for postmenopausal women, < 1.3 IU/L for others.
Subject nos. 5, 8, and 18 died before 2001; their data are from the 1991-1994 examination.
Subject no. 17 was diagnosed with subclinical hemochromatosis in late 1999; all his data refer to this time point.
Clinically overt hemochromatosis was not diagnosed in any of the 23 C282Y homozygotes before the study (Table 1); one was accidentally diagnosed with increased iron levels, which lead to measurement of transferrin saturation of 80% and ferritin level of 452 μg/L and the subsequent finding of C282Y homozygosity without clinically overt hemochromatosis (no.17). Among C282Y homozygotes, 13 of 16 women and 5 of 7 men developed transferrin saturation higher than 50%. Iron overload defined as ferritin level higher than 200 μg/L was found in 10 of 16 women, and iron overload defined as ferritin level higher than 250 μg/L was found in 6 of 7 men.
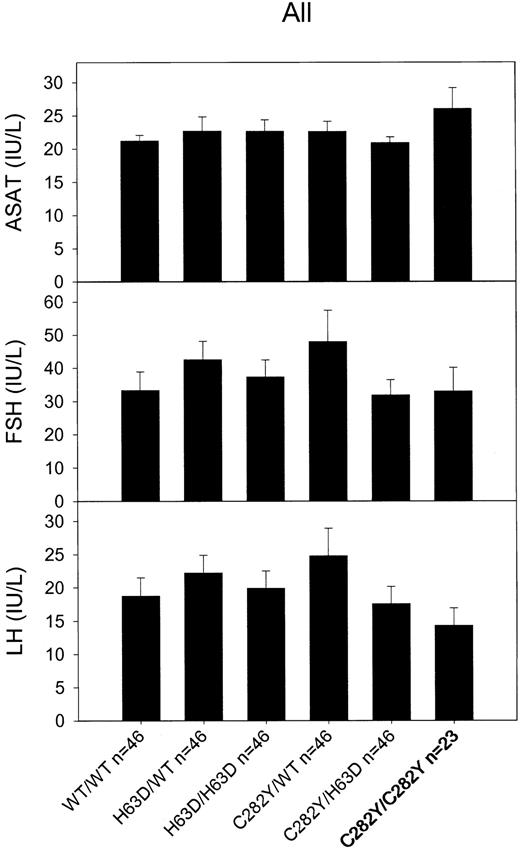
Liver disease or enlargement was not observed in any of the C282Y homozygotes (Table 1). Diabetes mellitus type 1 was only found in one C282Y homozygote, a 77-year-old woman diagnosed at 72 years of age (Table 1 no.13). Lack of secondary hypogonadism was demonstrated by normal age-adjusted values of follicle-stimulating hormone and luteinizing hormone in all C282Y homozygotes (Table 1). Levels of aspartate aminotransferase, follicle-stimulating hormone, and luteinizing hormone did not differ between C282Y homozygotes and wild type/wild type (Figure 4). None of the C282Y homozygotes received treatment for heart failure, indicating lack of hemochromatosis-associated cardiomyopathy (Table 1). Two homozygotes had acute myocardial infarction at 55 years of age (no. 21) and 68 years of age (no. 13). Two C282Y homozygotes had universal arthralgias (Table 1 nos.1 and 7), but none had clinical signs of arthritis at physical examination. None of the homozygotes had skin darkening.
Average levels with standard error of aspartate aminotransferase (ASAT), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) as a function ofHFEgenotype. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of the 5 other genotypes. Levels in mutation carriers were tested against wild type/wild type (WT/WT) levels using Mann-Whitney U tests, none were significant. Error bars indicate standard error of the mean.
Average levels with standard error of aspartate aminotransferase (ASAT), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) as a function ofHFEgenotype. Twenty-three C282Y homozygotes were each matched for sex, age, and alcohol consumption with 2 persons each of the 5 other genotypes. Levels in mutation carriers were tested against wild type/wild type (WT/WT) levels using Mann-Whitney U tests, none were significant. Error bars indicate standard error of the mean.
Discussion
Iron overload progression rate
Transferrin saturation increased from 50% to 70% from 25 to 85 years of age and from 70% to 80% from 35 to 80 years of age in female and male C282Y homozygotes, respectively, a phenomenon not observed for other genotypes. Within the same age ranges, ferritin levels increased from 100 to 500 μg/L and decreased from 800 to 400 μg/L in female and male C282Y homozygotes. Our observation of at most only modest increases in transferrin saturation and ferritin levels with increasing age in C282Y homozygotes are in accordance with results of previous cross-sectional studies.8-15
Pathophysiologically, our data are compatible with the idea that most C282Y homozygotes in the population at large will develop biochemical iron overload early in adult life. Thereafter, the rate of increase in ferritin levels and probably in accumulation of iron is not larger in C282Y homozygotes than in individuals without C282Y homozygosity. Thus, most C282Y homozygotes will only reach a level of modest iron overload.
Each C282Y homozygote had an oscillating course in ferritin levels. Some even had stable or decreasing levels, indicating lack of progressive iron loading. This is in accordance with the study of Olynyk et al,16 where 5 of 12 C282Y homozygotes had static or decreasing ferritin levels during a 4-year observation period. We have no explanation for why one patient dropped from greater than 1000 μg/L to less than half the initial value. He was not treated with phlebotomy or loosing blood in another manner.
Lifetime penetrance
Our data indicate that, although the majority of C282Y homozygotes biochemically had iron overload, none developed clinically overt hemochromatosis. The only patient known to be a C282Y homozygote before this study was accidentally diagnosed and did not suffer from hemochromatosis. Only one patient had unequivocal biochemical signs of hemochromatosis, since he had serum ferritin level higher than 2000 μg/L and aspartate aminotransferase at the upper limit of the normal range, suggesting that he might have hepatic fibrosis.23 In addition, one woman had been a blood donor more than 50 times, and therefore these phlebotomies could have protected her from developing clinically overt hemochromatosis. The C282Y homozygote with type 1 diabetes mellitus had a very late disease onset and only slightly increased ferritin levels, and therefore her diabetes mellitus might not be related to hemochromatosis. Finally, the 2 C282Y homozygotes who suffered from nonspecific arthralgias had ferritin levels of only 280 and 330 μg/L, indicating that hemochromatosis most likely was not responsible for these complaints. In accordance with this, Beutler et al7 did not observe differences between C282Y homozygotes and controls with respect to joint complaints.
Therefore, among the patients still alive in 2001, at most one C282Y homozygote had biochemical signs compatible with subclinical hemochromatosis, indicating a penetrance of 0% to 5% at 64 years of age, which is supported by 3 previous studies.7-9 Consequently, other previous studies suggesting that at least half of C282Y homozygotes ultimately will develop clinical signs of hemochromatosis16-19 clearly is not supported by our data. In the present study, all living C282Y homozygotes were informed about their genotype status, the possibility of a slightly increased risk of developing hemochromatosis, and that phlebotomy could be considered now or later on. Of the 20 C282Y homozygotes still alive in 2001, all were referred to a hospital for further control and possible treatment for hemochromatosis (in 2001), which therefore will not affect the results of the present study. Before the study, only 1 of 23 homozygotes was treated with phlebotomy, but this person did not suffer from hemochromatosis.
Potential limitations
First, the low penetrance of C282Y homozygosity in this study most likely was not due to selection bias, since all individuals were randomly selected from the general population of Copenhagen.21 Second, since clinical manifestations in hereditary hemochromatosis will not appear before 40 years of age,24 we could have studied individuals who were too young; however, median ages at investigation were 66 and 62 years in female and male C282Y homozygotes, making this possibility unlikely. Third, since women develop manifestations of hemochromatosis later in life than men and usually in a milder form,24 the minority of men in our study could have influenced the result in favor of lesser iron overload. However, this was probably not the case, since our results agree with 2 large population-based studies and a study of blood donors evaluating the clinical penetrance of C282Y homozygosity.7-9 A similar low penetrance was also suggested by a large necropsy study of patients with liver cirrhosis.25 Fourth, as we found fewer male (7 homozygotes among 4096 participants = 0.17%; 95% CI, 0.07%-0.35%) than female C282Y homozygotes (16 among 5078 participants = 0.32%; 95% CI, 0.18%-0.51%), the former could have died at an earlier age or not been able to participate in The Copenhagen City Heart Study; however, the average age of male C282Y homozygotes was similar to that of men with other genotypes. Furthermore, the C282Y genotype frequencies in our study did not differ from that predicted from the Hardy-Weinberg equilibrium (women and men, P = .35; women, P > .9; men, P = .10). Fifth, it seems unlikely that we underdiagnosed the 282Y allele, because C282Y genotype frequencies did not differ from the Hardy-Weinberg equilibrium and because the 95% confidence interval for the allele frequency for 282Y in our study of 5.3% to 5.9% overlapped that reported for 219 Danish newborns of 5.5% to 10.9%.26
Regarding progression with age, the main objective of the present study, the relatively small number of C282Y homozygotes, represents a limitation. Of the 23 patients only 7 were men, the most critical subgroup in the study, and only 3 of these men were younger than 50 years of age at the last analysis. This is a rather small group to reach firm conclusions regarding age-related changes in patients ranging from 35 to 80 years of age. Therefore, to increase the statistical power, iron load progression rate was examined according to the length of follow-up as well as a function of biologic age. Concerning transferrin saturation, the conclusion was similar from the 2 different plots, namely that transferrin saturation increased slightly with age in both female and male C282Y homozygotes. This was also the case for ferritin levels in female C282Y homozygotes; both plots demonstrated moderate increases with age. Progression in ferritin levels in male C282Y homozygotes differed in the 2 plots. As a function of increasing biologic age, ferritin levels seemed to decrease; however, this most likely was caused by the 2 relatively young males with very high ferritin levels (Figure 2). Thus, as a function of follow-up time, ferritin levels increased slightly even in male C282Y homozygotes (Figure 3).
Regarding phenotype, the work up was not completely satisfactory. A rough clinical evaluation is not enough to determine conclusively that clinical disease is negligible. Three of the 23 homozygotes had serum ferritin levels greater than 1000 up to 2433 μg/L and many had transferrin saturation greater than 75%. In such patients we cannot totally exclude liver fibrosis or cirrhosis. Therefore, initially we considered whether we should perform liver biopsies on these participants; however, for ethical reasons we abstained from performing liver biopsy because these persons appeared healthy. All C282Y homozygous individuals were referred for further control for hemochromatosis.
In conclusion, the average individual in the general population with C282Y homozygosity at most demonstrates modest increases in transferrin saturation and ferritin during 25 years follow-up and rarely develops clinically overt hemochromatosis. Therefore, C282Y homozygotes identified during population screening, and not because of clinically overt hemochromatosis, at most need to be screened for manifestations of hemochromatosis every 10 to 20 years.
The future challenge will be to identify the few C282Y homozygotes who will develop serious progressive iron accumulation leading to organ damage. Other genetic and environmental factors that regulate iron absorption and thus may modulate the phenotype of C282Y homozygotes need to be identified.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-10-3564.
Supported by the Danish Heart Foundation and Chief Physician Johan Boserup's and Lise Boserup's Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Vibeke Wohlgehagen, Hanne Damm, and Nina Kjersgaard for excellent technical assistance and Niels Fogh-Andersen for introduction to atomic absorption photometry.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal