Abstract
SERPINB6 (PI6) is a member of the intracellular serine protease inhibitors (serpins). Previous studies showed that SERPINB6 is localized mainly in the cytoplasm of endothelial cells, some epithelial cells, monocytes, and neutrophils. In these cells SERPINB6 is thought to prevent cellular damage by scavenging leaking lysosomal proteases. We show here, using novel, well-defined monoclonal antibodies, that SERPINB6 is abundantly expressed by mast cells in all organs and by the human mast cell line HMC-1. Gel filtration experiments revealed that the latter cells contain a high-molecular-weight form of SERPINB6, which consists of sodium dodecyl sulfate (SDS)-stable complexes of this inhibitor with monomeric β-tryptase. Expression of SERPINB6 by mast cells was compared with those of tryptase and CD117 (c-kit) in biopsies from patients with different forms of mast cell disease. In all cases the lesional mast cells expressed SERPINB6, and, in diffuse cutaneous mastocytosis and mastocytoma, SERPINB6 was expressed by a substantially higher number of mast cells when compared with tryptase. In conclusion, SERPINB6 is abundantly expressed by normal mast cells and by mast cells in mastocytoma lesions. We suggest that in mast cells, SERPINB6 serves to regulate the activity of endogenous β-tryptase in the cytoplasm. (Blood. 2004;103:2710-2717)
Introduction
SERPINB6 (PI6) is a member of the superfamily of serine protease inhibitors (serpins). Serpins are structurally related proteins regulating the activity of serine proteases involved in diverse processes such as coagulation, fibrinolysis, inflammation, apoptosis, and tumorigenesis.1-3 SERPINB6 belongs to the subfamily of intracellular serpins, which are unique in that they lack a cleavable N-terminal signal sequence and, therefore, reside mainly intracellularly.4 Hence, it is thought they exert their functions primarily, if not exclusively, intracellularly. However, at least 2 intracellular serpins, SERPINB2 (PAI-2) and SERPINB3 (SCCA), are also found extracellularly. SERPINB2 is partially secreted through a yet unidentified secretory pathway,5 whereas SERPINB3 is thought to be passively released into the circulation.6
Intracellular serpins share a higher degree of homology (more than 50% amino acid identity) than other serpin family members.4 Homology does not encompass the so-called reactive site loop (RSL) region. The P1 residue within the RSL largely determines the specificity of the serpin by mimicking a protease substrate sequence. On binding of the target protease to the serpin, the RSL is cleaved at its P1 residue and the protease is covalently linked to the serpin and becomes inactivated.7 Serpins most homologous to SERPINB6 are SERPINB8 and SERPINB9 (up to 68% amino acid identity).8 The P1 residue of SERPINB6 and SERPINB8 is arginine, whereas SERPINB9 has glutamic acid as its P1 residue, suggesting that the target specificity and function of SERPINB6, SERPINB8, and SERPINB9 are distinct.
Arginine as the P1 residue of SERPINB6 predicts that it inhibits trypsinlike proteases. Indeed, SERPINB6 has been identified as a placental thrombin inhibitor9 —it inhibits several trypsinlike proteases in vitro, such as thrombin, trypsin, urokinase, plasmin, and factor Xa.10,11 However, the physiologic relevance of this inhibition is not yet clear because these concern extracellular proteases. A previous study showed SERPINB6 expression in monocytes, granulocytes, and myelomonocytic cell lines,12 in which SERPINB6 is complexed to cathepsin G. The authors proposed that SERPINB6 protects these cells from leaky lysosomes. SERPINB6 is expressed not only by hematopoietic cells but also by endothelial cells and some subpopulations of epithelial cells.13 However, the target proteases and physiologic function(s) in these cell types remain unknown. To study the (patho)physiologic role of SERPINB6 in more detail, we generated a panel of novel, SERPINB6-specific monoclonal antibodies and used these antibodies to evaluate SERPINB6 expression in normal tissues. We showed strong SERPINB6 expression by mast cells in different organs (skin, gut, lung, muscle, uterus, lymphoid organs) and in mastocytosis lesions. In addition, we analyzed the expression of SERPINB6 by the human mast cell line HMC-1 and identified a high-molecular-weight (HMW) form of this inhibitor as a complex with monomeric mast cell β-tryptase.
Patients, materials, and methods
Tissue distribution and patient material
Slides with sections of formalin-fixed (10% [vol/vol] formalin for 18 hours), paraffin-embedded, normal human tissue and biopsy samples from patients with cutaneous or systemic mastocytosis were selected from the tissue bank of the Department of Pathology, VU University Medical Center.
Tissue samples from 13 patients with cutaneous mastocytosis (5 patients with urticaria pigmentosa, 4 with diffuse cutaneous mastocytosis, and 4 with mastocytoma) and from 2 patients with systemic mastocytosis (1 colon and 1 bone marrow sample) were processed as described for normal human tissue. Diseases in these patients complied with the recent World Health Organization (WHO) classification14 and are further described in Table 1.
Expression of CD117, tryptase, and SERPINB6 in relation to subtype of mastocytosis
. | Patient characteristics . | . | . | Positive tumor cells, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Site of biopsy . | Sex . | Age . | CD117 . | Tryptase . | SERPINB6 . | ||||
| Cutaneous mastocytosis, n = 13 | ||||||||||
| Urticaria pigmentosa | Trunk | M | 21 y | 75-100 | 75-100 | 75-100 | ||||
| Urticaria pigmentosa | Leg | F | 30 y | 75-100 | 75-100 | 50-75 | ||||
| Urticaria pigmentosa | Arm | F | 26 y | 75-100 | 75-100 | 75-100 | ||||
| Urticaria pigmentosa | Trunk | M | 45 y | 75-100 | 75-100 | 25-50 | ||||
| Urticaria pigmentosa | Leg | F | 27 y | 75-100 | 75-100 | 5-25 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 9 mo | 75-100 | 75-100 | 75-100 | ||||
| Diffuse cutaneous mastocytosis | Buttock | M | 7 mo | 75-100 | 50-75 | 75-100 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 9 mo | 75-100 | 25-50 | 50-75 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 6 mo | 75-100 | 25-50 | 75-100 | ||||
| Mastocytoma | Foot | M | 11 mo | 75-100 | 75-100 | 75-100 | ||||
| Mastocytoma | Leg | M | 6 mo | 75-100 | 5-25 | 75-100 | ||||
| Mastocytoma | Trunk | M | 9 mo | 75-100 | 25-50 | 75-100 | ||||
| Mastocytoma | Trunk | F | 6 mo | 75-100 | 50-75 | 75-100 | ||||
| Systemic mastocytosis, n = 2 | ||||||||||
| Systemic mastocytosis | Colon | M | 40 y | 75-100 | 50-75 | 50-75 | ||||
| Systemic mastocytosis | Bone marrow | F | 53 y | 75-100 | 50-75 | 50-75 | ||||
. | Patient characteristics . | . | . | Positive tumor cells, % . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis . | Site of biopsy . | Sex . | Age . | CD117 . | Tryptase . | SERPINB6 . | ||||
| Cutaneous mastocytosis, n = 13 | ||||||||||
| Urticaria pigmentosa | Trunk | M | 21 y | 75-100 | 75-100 | 75-100 | ||||
| Urticaria pigmentosa | Leg | F | 30 y | 75-100 | 75-100 | 50-75 | ||||
| Urticaria pigmentosa | Arm | F | 26 y | 75-100 | 75-100 | 75-100 | ||||
| Urticaria pigmentosa | Trunk | M | 45 y | 75-100 | 75-100 | 25-50 | ||||
| Urticaria pigmentosa | Leg | F | 27 y | 75-100 | 75-100 | 5-25 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 9 mo | 75-100 | 75-100 | 75-100 | ||||
| Diffuse cutaneous mastocytosis | Buttock | M | 7 mo | 75-100 | 50-75 | 75-100 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 9 mo | 75-100 | 25-50 | 50-75 | ||||
| Diffuse cutaneous mastocytosis | Trunk | F | 6 mo | 75-100 | 25-50 | 75-100 | ||||
| Mastocytoma | Foot | M | 11 mo | 75-100 | 75-100 | 75-100 | ||||
| Mastocytoma | Leg | M | 6 mo | 75-100 | 5-25 | 75-100 | ||||
| Mastocytoma | Trunk | M | 9 mo | 75-100 | 25-50 | 75-100 | ||||
| Mastocytoma | Trunk | F | 6 mo | 75-100 | 50-75 | 75-100 | ||||
| Systemic mastocytosis, n = 2 | ||||||||||
| Systemic mastocytosis | Colon | M | 40 y | 75-100 | 50-75 | 50-75 | ||||
| Systemic mastocytosis | Bone marrow | F | 53 y | 75-100 | 50-75 | 50-75 | ||||
Cell culture
Jurkat cells were cultured in RPMI 1640 medium, supplemented with 5% (vol/vol) heat-inactivated fetal bovine serum (FBS; Life Technologies, Rockville, MD), 50 U/mL penicillin, and 50 μg/mL streptomycin. The human mast cell line-1 (HMC-1; a kind gift from Dr J. Butterfield, Mayo Clinic, Rochester, MN) was cultured as originally described.15 Cell lysates were prepared as described previously16 and were stored at -80°C until use. Protein concentration was measured using the Bradford analysis (Bio-Rad Laboratories, Munchen, Germany) according to the instructions of the manufacturer.
Plasmids, cloning, and retroviral transduction
The constructs SERPINB6-pcDNA3, SERPINB8-pcDNA3, and SERPINB9-pcDNA3.1, comprising the cDNA sequence encoding the full-length proteins, were prepared as previously described.17 From these plasmids, full-length SERPINB6, SERPINB8, and SERPINB9 cDNAs were subcloned into the retroviral vector pMSCV-puro (Clontech, Palo Alto, CA). Production of retroviruses and stable cell lines was performed essentially as described previously.18,19 Jurkat cells were transduced for 30 minutes with viral supernatant in the presence of polybrene (8 μg/mL) while centrifuged at 500g at room temperature. Subsequently, cells were incubated for another 1.5 hours at 37°C. The infection procedure was repeated 4 times. Cells were then washed in phosphate-buffered saline (PBS), pH 7.4, and were incubated overnight in RPMI medium at 37°C. Infected cells were selected with puromycin (2.5 mg/mL), amplified, and tested for the expression of the protein of interest.
Expression and purification of recombinant human serpins
Yeast plasmids pHIL-D2-SERPINB6, pPIC3-SERPINB8, and pHIL-D2-SERPINB9, containing histidine-tagged human recombinant serpins, were kind gifts from Dr W. Kisiel (Department of Pathology, New Mexico School of Medicine, Albuquerque).20 Recombinant serpins were expressed as described previously for SERPINB8.20 Next, yeast cells were harvested by centrifugation and lysed in YPER (Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Recombinant proteins were purified by affinity chromatography using a 0.1 M NiSO4 HIStrap column (Pharmacia Biotech, Uppsala, Sweden). Purified recombinant proteins rSERPINB6, rSERPINB8, and rSERPINB9 were stored at -80°C until use. Ten micrograms recombinant protein was radiolabeled with sodium iodide I 125 (125I) using an Iodo-Gen protocol (Merck, Darmstadt, Germany).
Polyclonal anti-SERPINB6 antibodies
Rabbits were immunized with 50 μg yeast-expressed, histidine-tagged rSERPINB6 emulsified in complete Freund adjuvant, followed by 3 monthly boosts with rSERPINB6 emulsified in incomplete adjuvant. Serum antibody responses were screened using a radioimmunoassay (RIA) with antirabbit immunoglobulin-Sepharose beads and 125I-labeled rSERPINB6, as previously described for anti-C3 antibodies.21 Four days after the final injection, citrated blood was collected and processed into plasma. Immunoglobulin G (IgG) was purified from plasma with protein-G affinity chromatography (Pharmacia Biotech).
Production and purification of anti-SERPINB6 monoclonal antibodies
BALB/c mice were immunized by intravenous and intraperitoneal injections with 10 μg yeast rSERPINB6 emulsified in complete Freund adjuvant, followed by a boost every 3 weeks with rSERPINB6 emulsified in incomplete adjuvant (5-7 times). Serum antibody titers were screened by RIA with 125I-labeled rSERPINB6, as described previously for anti-C3 antibodies.21 Spleen and lymph node cells were used for fusion with SP2/0-Ag-14 mouse myeloma cells according to standard procedures.21 Hybridoma culture supernatants were screened for the presence of specific anti-SERPINB6 antibodies by RIA.21 As controls, preimmune and postimmune serum samples of the immunized mouse were included in the assay. Cross-reactivity of the antibodies tested was assessed using 125I-SERPINB8 and 125I-SERPINB9. Nonspecific binding of the radiolabeled tracer was excluded in experiments with irrelevant monoclonal antibodies. After subcloning, the anti-SERPINB6 antibodies were purified by protein-A affinity chromatography (Pharmacia Biotech). Monoclonal antibodies (mAbs) were biotinylated using long-chain biotinyl-N-hydroxysuccinimide ester sulfonic acid (Pierce Chemical) according to the manufacturer's instructions.
SERPINB6 ELISA
Microtiter plates (Maxisorb Immunoplates; Nunc, Copenhagen, Denmark) were coated with 0.1 μg/well purified anti-SERPINB6 polyclonal antibody (pAb) in 0.1 M sodium carbonate buffer, pH 9.6, for 16 hours at room temperature. The plates were washed with PBS/0.02% (wt/vol) Tween-20 and washed again after each of the following incubation steps. Residual binding sites were blocked by 30-minute incubation with high-performance enzyme-linked immunosorbent assay (ELISA) buffer (HPE; Sanquin Research at CLB, Amsterdam, the Netherlands) containing 1% (vol/vol) normal mouse serum (HPE-NMS). All samples to be tested and standards (purified yeast rSERPINB6) were diluted in HPE-NMS and were incubated for 1 hour. Next, plates were incubated for 1 hour with biotinylated mAb PI6-12 (1 μg/mL), followed by incubation for 30 minutes with streptavidin-polymerized horseradish peroxidase (strep-polyHRP; Sanquin Research). Bound peroxidase was visualized by incubation with a solution of 100 μg/mL 3,3′,5,5′-tetramethylbenzidine (TMB; Merck) and 0.003% (vol/vol) H2O2 in 0.11 M sodium acetate buffer, pH 5.5. The reaction was stopped by the addition of an equal volume of 2 M H2SO4, and absorbance at 450 nm was measured on a Titer-Tek Multiscan plate reader (Labsystems, Helsinki, Finland). For competition ELISA, purified anti-SERPINB6 mAbs were used as coating antibodies and biotinylated anti-SERPINB6 mAbs as detecting antibodies. Purified rSERPINB6 was used as standard. As control, the same mAb was included as the coating and detecting antibody, which abolished the signal. As positive control, the pAb anti-SERPINB6 was included as the coating and detecting antibody. An mAb was considered to bind similar or overlapping epitopes when the absorbance at 450 nm was reduced by more than 50%.
Immunoprecipitation
pAb anti-SERPINB6 was coupled to cyanogen bromide activated (CNBr)-Sepharose according to the manufacturer's instructions. HMC-1 lysate in the presence or absence of 2 M NaCl was added to pAb anti-SERPINB6 coupled to Sepharose beads. After head-over-head rotation for 16 hours at 4°C, the Sepharose was washed 4 times with PBS/0.1% (vol/vol) Tween-20. Pellets were dissolved in nonreducing sodium dodecyl sulfate (SDS) sample buffer and were analyzed by electrophoresis on a 10% (wt/vol) SDS-polyacrylamide gel, under either nonreducing or reducing conditions, according to standard procedures. After electrophoresis, Western blotting was performed as described previously.16 SERPINB6 and tryptase were detected using mAb PI6-25 (IgG1; 2 μg/mL) and mAb AA1 (IgG1; 1:500; DAKO, Glostrup, Denmark), respectively. Bound antibodies were visualized with a chemiluminescence development reagent (ECL system; Amersham Biosciences Europe, Roosendaal, the Netherlands) according to the manufacturer's instructions.
Molecular size of SERPINB6
Gel filtration chromatography on an ACA-54 column (Ciphergen BioSephra, Cergy-Saint-Christophe, France), volume 50 mL, was used to determine the molecular size of SERPINB6. A flow rate of 0.5 mL/min was used routinely. Running buffer was PBS in the presence or absence of 2 M NaCl. Before run, the column was equilibrated with 2-column volumes of the appropriate buffer. In each experiment, 1 mL (40 × 106 cells) HMC-1 lysate, 1:1 diluted with running buffer, was applied to the column. Fractions of 0.5 mL were collected, and the concentration of SERPINB6 was determined using SERPINB6 ELISA. In some experiments, SERPINB6 was immunoprecipitated from selected fractions of the column, as in the previous paragraph. The column was calibrated with phenol red (5 kDa), albumin (66 kDa), IgG (160 kDa), and dextran blue (void volume) in the presence and absence of 2 M NaCl.
Immunohistochemistry
Immunohistochemical staining of formalin-fixed, paraffin-embedded tissues was performed essentially as previously described for SERPINB8 and SERPINB9.16,17,19 For detection of SERPINB6, mast cell tryptase, or CD117, mAb PI6-18 (IgG1, 5μg/mL), mAb AA1 (IgG1, 1:200), or pAb anti-CD117 (c-kit, 1:50; DAKO) were used, respectively. Tissue sections were subjected to antigen retrieval by boiling in 10 mM sodium citrate buffer, pH 6, for 10 minutes in a microwave oven. Bound antibodies were visualized with 3-amino-9-ethylcarbazole (AEC; Zymed, San Francisco, CA). Negative control slides were stained with mouse IgG of the appropriate subclass.
Double staining was performed on skin and tonsil after antigen retrieval sections were preincubated with normal goat serum. Then SERPINB6 was visualized in green by subsequent incubations with biotinylated mAb PI6-18, fluorescein isothiocyanate (FITC)-labeled streptavidin, rabbit-anti-tetramethyl rhodamine isothiocyanate (FITC), and Alexa 488-labeled goat-antirabbit. Tryptase was visualized in red using TRITC-labeled goat-antimouse IgG1. CD117 was visualized in red using pAb CD117, biotinylated swine-antirabbit, strept ABComplexes (sABC), and fluorescein tyramide (FT). Slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Images were recorded using a Zeiss LSM-510 confocal laser scanning microscope (Weesp, the Netherlands) equipped with argon and helium/neon lasers. Excitation was measured at 488 nm for FITC and 568 nm for rhodamine, respectively.
Results
Generation of SERPINB6-specific mAbs
From a fusion experiment of a mouse immunized with rSERPINB6, 6 anti-SERPINB6 mAbs were selected, all of the IgG1 subclass. High-resolution electrophoresis showed a unique migration pattern for each anti-SERPINB6 mAb (not shown), indicating that the antibodies originated from different hybridoma clones. All mAbs were tested for cross-reactivity against the most homologous serpins, SERPINB8 and SERPINB9, using soluble radiolabeled recombinant serpins in RIA and solid-phase recombinant serpins in ELISA. All selected mAbs showed a specific binding to rSERPINB6 and did not cross-react with the 2 other serpins (not shown). Competition ELISA experiments revealed 3 groups of anti-SERPINB6 mAbs that bound similar or overlapping epitopes (data not shown)—group 1 (mAbs PI6-10 and PI6-25), group 2 (mAbs PI6-12 and PI6-18), and group 3 (mAbs PI6-17 and PI6-23). All antibodies were tested for their ability to bind soluble rSERPINB6 in a sandwich ELISA, and based on these results a SERPINB6 ELISA was developed. Plates were coated with pAb anti-SERPINB6, and bound SERPINB6 was detected with mAb PI6-12. This sandwich ELISA for SERPINB6 not only detected the soluble yeast rSERPINB6, it also detected SERPINB6 expressed by Jurkat cells (Figure 1A-B). No cross-reaction was observed when rSERPINB8 and rSERPINB9, expressed by yeast or by Jurkat cells, were tested (Figure 1A-B). In addition, the mAbs were tested for their ability to detect SERPINB6 in Western blot analysis and immunohistochemistry. Of all antibodies tested, mAb PI6-25 displayed optimal reactivity with SERPINB6 during Western blotting and did not cross-react with SERPINB8 or SERPINB9, expressed by transformed Jurkat cells (Figure 1C) or with recombinant proteins (results not shown). Expression of SERPINB8 or SERPINB9 in the Jurkat cells was assessed with specific antibodies against these proteins, as previously published16,17 (results not shown).
Specificity of SERPINB6 mAbs in ELISA, Western blot analysis, and immunohistochemistry. (A) Sandwich ELISA with pAb anti-SERPINB6 as catching antibodies (1 μg/mL) and biotinylated mAb PI6-12 as detecting antibody. Serial dilutions of rSERPINB6, SERPINB8, or SERPINB9 were tested. Absorbance values at 450 nm (A450/540) are plotted against the concentration of recombinant serpin (pg/mL). (B) Sandwich ELISA with pAb anti-SERPINB6 as catching antibodies (1 μg/mL) and biotinylated mAb PI6-12 as detecting antibody. Serial dilutions of lysates of Jurkat cells stably transfected with cDNA encoding SERPINB6, SERPINB8, or SERPINB9 were tested. Absorbance values at 450 nm (A450/540) are plotted against the sample dilution in the system. (C) Specificity of mAb PI6-25 in Western blot analysis. Ten microliters cell lysate (4 × 105 cells) of serpin-transfected and wild-type Jurkat cells and HeLa cells was separated on 10% SDS-PAGE and subjected to Western blotting. Blots were incubated overnight with mAb PI6-25. Arrow on the right indicates 42 kDa. (D) Specificity of mAb PI6-18 in immunocytochemistry. Cytospins of Jurkat cells, either wild-type or stably transfected with cDNAs encoding SERPINB6, SERPINB8, or SERPINB9, were analyzed by immunocytochemistry using mAb PI6-18, as described in “Patients, materials, and methods.” Nuclei were counterstained with hematoxylin. Original magnification, × 630.
Specificity of SERPINB6 mAbs in ELISA, Western blot analysis, and immunohistochemistry. (A) Sandwich ELISA with pAb anti-SERPINB6 as catching antibodies (1 μg/mL) and biotinylated mAb PI6-12 as detecting antibody. Serial dilutions of rSERPINB6, SERPINB8, or SERPINB9 were tested. Absorbance values at 450 nm (A450/540) are plotted against the concentration of recombinant serpin (pg/mL). (B) Sandwich ELISA with pAb anti-SERPINB6 as catching antibodies (1 μg/mL) and biotinylated mAb PI6-12 as detecting antibody. Serial dilutions of lysates of Jurkat cells stably transfected with cDNA encoding SERPINB6, SERPINB8, or SERPINB9 were tested. Absorbance values at 450 nm (A450/540) are plotted against the sample dilution in the system. (C) Specificity of mAb PI6-25 in Western blot analysis. Ten microliters cell lysate (4 × 105 cells) of serpin-transfected and wild-type Jurkat cells and HeLa cells was separated on 10% SDS-PAGE and subjected to Western blotting. Blots were incubated overnight with mAb PI6-25. Arrow on the right indicates 42 kDa. (D) Specificity of mAb PI6-18 in immunocytochemistry. Cytospins of Jurkat cells, either wild-type or stably transfected with cDNAs encoding SERPINB6, SERPINB8, or SERPINB9, were analyzed by immunocytochemistry using mAb PI6-18, as described in “Patients, materials, and methods.” Nuclei were counterstained with hematoxylin. Original magnification, × 630.
Several antibodies (mAbs PI6-17, PI6-18, PI6-23, and PI6-25) reacted with formalin-fixed, paraffin-embedded tissues, of which mAb PI6-18 was selected for further use in immunohistochemistry. This mAb did not cross-react with other serpins when tested on cytospins of transformed Jurkat cells (Figure 1D).
SERPINB6 is expressed by human mast cells
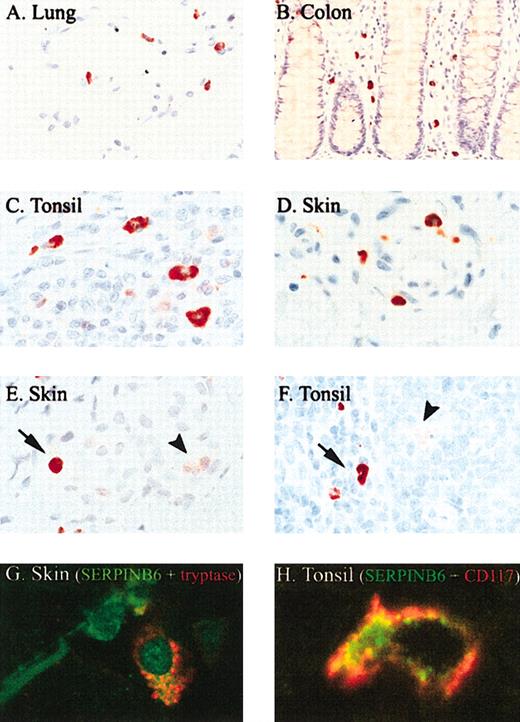
Immunohistochemical survey of SERPINB6 expression in normal human tissues showed expression in certain endothelial cells, in ciliated cells of the fallopian tube, in cells within the exocrine and endocrine pancreas, in the upper layer of stratified squamous epithelium such as in epidermis and esophagus, in some adnexal structures, and in the kidney (data not shown). These results confirm and extend findings from a previous study describing SERPINB6 expression.22 However, the most remarkable observation was intense staining of mast cells throughout the body. Mast cells in the lung (Figure 2A), throughout the gastrointestinal tract (colon; Figure 2B), in lymphoid organs such as tonsil (Figure 2C, F, H), skin (Figure 2E, G), subcutaneous tissue, and other organs (uterus, breast, muscle) all showed strong SERPINB6 staining. The identity of mast cells was confirmed morphologically and through double staining of SERPINB6 with a mast cell-specific marker such as tryptase (Figure 2G) or CD117 (Figure 2H) showing coexpression in mast cells in the skin (Figure 2G) and tonsil (Figure H). Mast cells with oval nuclei and those with a more elongated morphology stained positively for SERPINB6. The main location of SERPINB6 in the mast cells was cytoplasmic. In contrast to the homologous serpin PI8,16 no nuclear localization of SERPINB6 was observed. Interestingly, sometimes a granular staining of SERPINB6 in mast cells was also observed, as shown in Figure 2G. In addition to its appearance in mast cells, weak SERPINB6 expression was observed in several types of tissue macrophages (starry sky macrophages, Kupffer cells, sinus macrophages in the lymph node, alveolar macrophages in the lung), as reported for several monocyte/macrophage cell lines in vitro.12 However, the staining intensity of SERPINB6 in these cells was faint compared with that observed in mast cells, as shown in Figure 2F of a starry sky macrophage (arrowhead) in the vicinity of a mast cell (arrow). Plasma cells, eosinophils, and lymphocytes were all negative for SERPINB6. In normal, nonpathologic tissues, neutrophils did not stain for SERPINB6, but in inflammatory infiltrates, such as in acute dermatitis, neutrophils were occasionally faintly positive (Figure 2E, arrowhead), suggesting that SERPINB6 is up-regulated after neutrophil activation.
SERPINB6 is expressed by mast cells in different tissues. Tissue sections were stained for SERPINB6 expression (mAb PI6-18; A-F) and mast cell tryptase (mAb AA1; G-H), as described in “Patients, materials, and methods.” Slides were stained with AEC and counterstained with hematoxylin. Arrow indicates mast cell showing strong SERPINB6 staining (E-F); arrowhead, neutrophil (E) or starry sky macrophage (F) with weak SERPINB6 staining. Original magnifications, × 400 (B), × 630 (A, F), and × 1000 (C-E). (G) Fluorescent double staining of SERPINB6 (green) with tryptase (red) in skin. (H) Fluorescent double staining of SERPINB6 (green) with CD117 (red) in tonsil. In the latter a clear membranous staining of CD117 is seen. Original magnifications, × 1000 (G-H).
SERPINB6 is expressed by mast cells in different tissues. Tissue sections were stained for SERPINB6 expression (mAb PI6-18; A-F) and mast cell tryptase (mAb AA1; G-H), as described in “Patients, materials, and methods.” Slides were stained with AEC and counterstained with hematoxylin. Arrow indicates mast cell showing strong SERPINB6 staining (E-F); arrowhead, neutrophil (E) or starry sky macrophage (F) with weak SERPINB6 staining. Original magnifications, × 400 (B), × 630 (A, F), and × 1000 (C-E). (G) Fluorescent double staining of SERPINB6 (green) with tryptase (red) in skin. (H) Fluorescent double staining of SERPINB6 (green) with CD117 (red) in tonsil. In the latter a clear membranous staining of CD117 is seen. Original magnifications, × 1000 (G-H).
SERPINB6 is complexed to mast cell β-tryptase
Because tissue distribution identified mast cells as the major producers of SERPINB6, we studied SERPINB6 expression in mast cells in more detail using HMC-1, which indeed expressed SERPINB6 antigen as determined by sandwich ELISA (approximately 4.5 ng/106 cells) and Western blot analysis. Western blot analysis of HMC-1 extracts with mAb PI6-25 showed a dominant protein band migrating at approximately 42 kDa, similar to that of rSERPINB6 (Figure 3A, arrow). This migration pattern agreed well with the predicted molecular weight of SERPINB6. In addition, a second major protein band migrating approximately at 78 kDa was observed (Figure 3A, arrowhead). This band was present under reducing and nonreducing conditions (not shown). Complexes between serpins and serine proteases in general are stable under SDS conditions.1 Hence, the 78-kDa band may represent a complex of SERPINB6 with a serine protease.
Characterization of SERPINB6 expressed by HMC-1 cells. (A) Ten microliters HMC-1 cell lysate (4 × 106 cells) was separated on 10% SDS-PAGE and subjected to Western blotting with mAb PI6-25 (1.5 μg/mL) to detect PI6 (lane 1). As positive control, rSERPINB6 was included (lane 2). The position of the molecular mass marker bands is indicated on the left. (B) One microliter HMC-1 lysate (40 × 106 cells) was applied to an ACA-54 column and was eluted with either PBS (black line) or PBS/2 M NaCl (gray dotted line) at 0.5 mL/min. SERPINB6 was measured in the column fractions by SERPINB6 ELISA. Positions of the markers albumin and IgG are indicated for both buffers. (C) HMC-1 lysate and the peak fractions of SERPINB6 of the ACA54 column were immunoprecipitated with pAb anti-SERPINB6 coupled to Sepharose beads and were analyzed on Western blot using mAb PI6-25 or mAb AA1 (1:200) to detect SERPINB6 (lanes 1-2, 5-6) and β-tryptase (lanes 3-4, 7-8), respectively. (arrow, lane 7) 32 kDa β-tryptase. (arrowhead, lanes 6, 8) 78 kDa SERPINB6-β-tryptase complex. The position of the molecular mass marker band is indicated on the left.
Characterization of SERPINB6 expressed by HMC-1 cells. (A) Ten microliters HMC-1 cell lysate (4 × 106 cells) was separated on 10% SDS-PAGE and subjected to Western blotting with mAb PI6-25 (1.5 μg/mL) to detect PI6 (lane 1). As positive control, rSERPINB6 was included (lane 2). The position of the molecular mass marker bands is indicated on the left. (B) One microliter HMC-1 lysate (40 × 106 cells) was applied to an ACA-54 column and was eluted with either PBS (black line) or PBS/2 M NaCl (gray dotted line) at 0.5 mL/min. SERPINB6 was measured in the column fractions by SERPINB6 ELISA. Positions of the markers albumin and IgG are indicated for both buffers. (C) HMC-1 lysate and the peak fractions of SERPINB6 of the ACA54 column were immunoprecipitated with pAb anti-SERPINB6 coupled to Sepharose beads and were analyzed on Western blot using mAb PI6-25 or mAb AA1 (1:200) to detect SERPINB6 (lanes 1-2, 5-6) and β-tryptase (lanes 3-4, 7-8), respectively. (arrow, lane 7) 32 kDa β-tryptase. (arrowhead, lanes 6, 8) 78 kDa SERPINB6-β-tryptase complex. The position of the molecular mass marker band is indicated on the left.
To further analyze this HMW band of SERPINB6, HMC-1 lysate was fractionated with ACA-54 gel filtration chromatography in the absence of salt. This yielded 2 SERPINB6 peaks, as determined by SERPINB6 ELISA (Figure 3B)—a large peak consisting of fractions 56 to 64 (peak 1) with an apparent molecular size of approximately 42 kDa, and a smaller peak consisting of fractions 44 to 47 (peak 2) with an estimated size of approximately 78 kDa. Peak 1 likely represented uncomplexed SERPINB6, whereas peak 2 presumably represented complexes of SERPINB6 with unknown protease(s). This was confirmed using Western blot analysis (Figure 3C). Peak 1 migrated at approximately 42 kDa, that is, at the position of uncomplexed SERPINB6 (lane 1). The SERPINB6 nature of this band indeed was confirmed by immunoprecipitation with pAb anti-SERPINB6 (lane 2). In peak 2, we observed 2 additional HMW protein bands migrating at 59 and 78 kDa, respectively (lane 5). Each band was precipitated with pAb anti-SERPINB6 (lane 6).
Given that SERPINB6 has an arginine at the P1 position, which predicts specificity for trypsinlike proteases,11,23 we hypothesized that in mast cells, tryptase would be an obvious candidate target protease for SERPINB6. To study this we analyzed whether β-tryptase was coprecipitated with SERPINB6 from the fractions of peaks 1 and 2. Mast cell β-tryptase was present in peak 1 and in peak 2 and migrated at 32 kDa on Western blot (lanes 3, 7). In peak 2, a faint HMW tryptase-positive band was detected (lane 7, arrowhead) that had the same apparent MW as the 78-kDa protein band detected with the anti-SERPINB6 antibody (lanes 5-6). Lane 8 shows that only this HMW band, and not the 32-kDa, β-tryptase band, coprecipitated with SERPINB6, indicating that the former consisted of an SDS-stable SERPINB6-β-tryptase complex. The size of the 78-kDa complex suggests that SERPINB6 is complexed to the monomeric form of β-tryptase. In HMC-1 cells, β-tryptase is stored in the secretory granules in its active tetrameric conformation.24 These tetramers are composed of monomers, which are noncovalently linked. Under SDS conditions these multimers dissociate in monomers on SDS-polyacrylamide gel electrophoresis (SDS-PAGE), which explains the migration of β-tryptase around 32 kDa on Western blot in peaks 1 and 2 (lanes 3, 7). Because mast cell β-tryptase tetramers are stabilized in the presence of high ionic strength,25 we also tested the SERPINB6 elution profile during ACA-54 gel filtration chromatography in the presence of 2 M NaCl. This resulted in a reduced HMW SERPINB6 peak (Figure 3B, gray dotted line), leading us to conclude that dissociation of the tetramer hardly occurs and, therefore, that monomeric β-tryptase is unavailable for binding to SERPINB6. Control experiments verified the specific precipitation of SERPINB6 from HMC-1 lysate with SERPINB6 antibodies because the bands with apparent MWs of 42 kDa and 78 kDa were not detected when SERPINB6 antibodies were substituted with irrelevant antibodies (results not shown).
Tumor cells in cutaneous and systemic mastocytosis express SERPINB6
SERPINB6 expression by mast cells in mastocytosis was studied in 15 patients with cutaneous or systemic mastocytosis (Table 1). In all patients, SERPINB6-positive lesional mast cells were detected, and in most (13 of 15) patients, most (more than 50%) mast cells were positive for SERPINB6. Figure 4 shows an example of SERPINB6 and tryptase expression in a cutaneous mastocytoma (patient 10; Figure 4C-D). SERPINB6 expression in the lesions was compared with that of CD117 and mast cell tryptase. Interestingly, in diffuse cutaneous mastocytosis and localized mastocytoma, all mast cells except those in patient 8 expressed high levels of SERPINB6, whereas in 6 of 8 patients, the percentages of tryptase-positive mast cells were substantially lower (Table 1; Figure 4A-B). Furthermore, the staining intensity of SERPINB6 in these patients was equal to, or even higher than, nontumor mast cells in the deep subcutaneous tissue, whereas in tryptase the staining intensity of tumor cells was often weaker (patient 12; Figure 4B). On the other hand, none of the patients with urticaria pigmentosa showed loss of tryptase staining, in contrast to SERPINB6, which showed a diminished staining in 3 of 5 patients tested. In the 2 biopsy samples of systemic mastocytosis, SERPINB6 and tryptase showed equal staining (eg, patient 14; Figure 4E-F).
Immunohistochemical detection of SERPINB6, tryptase, and CD117 in mastocytosis. Tissue sections of 2 cutaneous mastocytomas in patient 12 (A-B, G) and patient 10 (C-D, H) and of systemic mastocytosis in patient 14 (E-F, I) were stained with mAb PI6-18 (A, C, E), antitryptase mAb AA1 (B, D, F), or anti-CD117 (G-I). Slides were stained with AEC (A-F) or DAB (G-I) and were counterstained with hematoxylin. Original magnification, × 400 (A-I).
Immunohistochemical detection of SERPINB6, tryptase, and CD117 in mastocytosis. Tissue sections of 2 cutaneous mastocytomas in patient 12 (A-B, G) and patient 10 (C-D, H) and of systemic mastocytosis in patient 14 (E-F, I) were stained with mAb PI6-18 (A, C, E), antitryptase mAb AA1 (B, D, F), or anti-CD117 (G-I). Slides were stained with AEC (A-F) or DAB (G-I) and were counterstained with hematoxylin. Original magnification, × 400 (A-I).
Discussion
In this paper we show expression of the intracellular serpin SERPINB6 in human mast cells, in mast cell disorders (cutaneous and systemic mastocytosis), and in the human mast cell line HMC-1. One of the major mast cell serine proteases, human β-tryptase, was coprecipitated from HMC-1 with SERPINB6, suggesting that SERPINB6 may regulate the activity of this protease. Together, these data suggest a role for SERPINB6 in mast cell biology.
As a first step to elucidate the function of SERPINB6, a panel of 6 novel, high-affinity mAbs directed against SERPINB6 was generated. Because intracellular serpins share a high degree of homology (more than 50%), antibodies directed against 1 of these proteins may cross-react with its family members. Indeed such cross-reactivity was clear from experiments with the anti-SERPINB6 pAb, which also bound to SERPINB9 and, to a lesser extent, to SERPINB8 (not shown). Moreover, we previously identified an mAb raised against SERPINB9, mAb anti-PI9-1, which cross-reacts with SERPINB6 and SERPINB8.17 Hence, it was necessary to analyze binding of the SERPINB6 mAbs under denaturing and nondenaturing conditions to the serpins SERPINB8, SERPINB9, and SERPINB2, which share a high degree of homology with SERPINB6—that is, approximately 65% for SERPINB8 and SERPINB9 and 50% for SERPINB2, respectively.8 Anti-SERPINB6 mAbs recognized SERPINB6 under native (ELISA and immunoprecipitation) and denatured (Western blotting, immunohistochemistry) conditions and did not cross-react with the other serpins. With our antibodies, a sensitive and specific SERPINB6 ELISA was developed that allowed quantification of SERPINB6 levels in cell lysates.
Immunohistochemical analysis of SERPINB6 expression in normal human tissues confirmed findings from a previous study.22 In addition, we observed SERPINB6 expression in some other tissues, such as the ciliated cells of the fallopian tube, cells within the exocrine and endocrine pancreas, and the upper layer of stratified squamous epithelium (epidermis, esophagus, cervix). It has been suggested that SERPINB6 is involved in keratinocyte differentiation13 and in protection of monocytes and neutrophils from proteolytic damage by lysosomal proteases.12 The function of SERPINB6 in other tissues remains unknown.
The most remarkable observation in our study was that human mast cells throughout the body strongly stained for SERPINB6. The intensity of SERPINB6 staining was much stronger in mast cells than in macrophages. Neutrophils were only occasionally positive for SERPINB6 and only when involved in an inflammatory response. Cells in mastocytosis lesions were also positive for SERPINB6 and tryptase, again confirming SERPINB6 expression by mast cells. Notably, in diffuse cutaneous mastocytosis and mastocytoma, tryptase expression was less than in normal mast cells, though for SERPINB6 in these lesions no marker loss was observed. Thus, SERPINB6 is expressed by normal and “neoplastic” mast cells and might be used as a new marker to identify mastocytosis by immunohistochemistry. As expected, SERPINB6 staining was mostly cytoplasmic, although sometimes (course) granular staining was observed. However, this granular pattern was different from the pattern observed with tryptase, and it was unclear whether this resulted from a fixation artifact.
Human mast cells are multifunctional immune cells involved in the pathogenesis of various (patho)physiologic processes such as cardiovascular disorders, asthma, and rheumatoid arthritis.26-29 The precise role of mast cells in these conditions is not completely known, though secretion of a variety of active mediators likely is important. Among the mediators released by mast cells are the serine proteases chymase and tryptase, which have chymotrypsinlike and trypsinlike specificities, respectively.30 The actual amount of each protease in mast cells may differ in various tissues. For example, mast cells in subcutaneous connective tissue contain tryptase and chymase, whereas those in the lungs and mucosal tissues express relatively little chymase compared with tryptase.31 Deduced from its sequence, SERPINB6 is predicted to interact mainly with trypsinlike proteases, but through other residues in its reactive site loop it also may interact with chymotrypsinlike proteases.31 The abundant expression of SERPINB6 in mast cells throughout the body corresponded better with the expression pattern of tryptase than with that of chymase because the latter is more limited. Hence, we focused on a potential interaction of SERPINB6 with tryptase and used HMC-1 cells as a model.
Mast cells express several forms of tryptase in vivo.30,33,34 In contrast, HMC-1 cells only express β-, δ-, and γ-tryptase, of which β-tryptase is the major form.33,35,36 The pro-form β-tryptase is spontaneously secreted, whereas mature active tetrameric β-tryptase is retained in their secretory granules.24 Crystallography studies have shown that this enzymatically active β-tryptase is a ringlike tetramer structure of approximately 130 kDa, with the active sites of the monomers facing the central pore.37,38 Localization of these active sites in the central pore limits accessibility of these sites to macromolecular substrates and inhibitors. The only naturally occurring tryptase inhibitor identified thus far is the Kazal-type leech-derived tryptase inhibitor (LDTI), which is smaller than other inhibitors.39 Hence, SERPINB6 is predicted to be too large to fit in the central pore of tetrameric tryptase and to inhibit the activity of this protein complex. Yet, using gel filtration chromatography and immunoprecipitation, we observed that β-tryptase was coprecipitated with SERPINB6 from the HMC-1 cell lysate (Figure 3C). The size of the precipitated protein complex (75 kDa), as determined by gel filtration chromatography, suggested that it did not represent SERPINB6 bound to the tetrameric form of β-tryptase but that SERPINB6 bound to the monomeric form (Figure 3B-C).
Under physiologic conditions, β-tryptase is stored in secretory granules as a tetrameric structure. The monomers within this tetramer are noncovalently bound and are stabilized by association with negatively charged glycosaminoglycan chains, such as heparin or high ionic strength conditions in vitro.25 On activation and degranulation of mast cells, β-tryptase is released as a tetramer bound to heparin and, therefore, retains its activity. In the absence of heparin or at low salt conditions, β-tryptase tetramers dissociate rapidly and irreversibly into monomers. Furthermore, tetramerization requires an acidic pH (lower than 6.5).40 Fajardo and Pejler41 have shown that incubation of tetrameric β-tryptase at neutral pH and at 37°C results in dissociation of the tetramer into monomers and, in contrast to previous reports, that these monomers are active and functionally distinct from tetrameric β-tryptase. In contrast to the hidden active sites in the tetrameric form of β-tryptase, the active site of monomeric β-tryptase is accessible for macromolecular proteins and inhibitors. For example, fibrinogen is a substrate for monomeric β-tryptase but not for tetrameric β-tryptase.41 Our observations imply that the active site of monomeric β-tryptase is also accessible to SERPINB6, and that the activity of this protease may be regulated by SERPINB6. In this view, SERPINB6 might neutralize the activity of monomeric β-tryptase, which may be released from the granules to the interior of the cell because of faults in the routing of the protease to the granules or faults in the degranulation process. This has been previously suggested for other intracellular serpins as well.42
In conclusion, we demonstrated marked expression of the intracellular serpin SERPINB6 by normal and pathologic mast cells. We provide evidence that this inhibitor can form complexes with β-tryptase monomers and that it functions as an endogenous inhibitor of β-tryptase. These findings point to an important role for SERPINB6 in mast cell biology.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-08-2981.
Supported by VUMC verruiming middelenkader.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal