Abstract
Two siblings with hypofibrinogenemia have lifelong trauma-related bleeding. Recently, the brother experienced recurrent thrombosis after cryoprecipitate infusions following surgery. The sister had 6 miscarriages. Plasma clots in each were resistant to compression and fibrinolysis and were soluble in 5 M urea. Examination by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) revealed only the presence of crosslinked γ–γ fibrin chain dimers without high polymers of αn. Fibrin clots contained an abnormal 35-kDa constituent recognized by an antibody to the mature fibrinogen Aα–chain residues 241-476 but not by antibodies to Aα219-348 or Aα349-406. DNA analysis revealed a heterozygous CAA → TAA mutation at the codon for amino acid 328 of the Aα gene in these siblings and 2 asymptomatic family members. The Gln328stop mutation (fibrinogen Keokuk) predicted a 46% truncation and the production of a 35-kDa Aα chain. Analysis of purified fibrinogen revealed expression of the abnormal Aα chain in 4 family members but found no normal fibrinogen in the 2 hypofibrinogenemic patients. This paradox was resolved when they and their asymptomatic mother were found to be heterozygous for a second Aα mutation, a GT → TT splice site mutation in intron 4 (IVS4 + 1 G> T). However, compound heterozygosity for both mutations was required for the expression of severe hypodysfibrinogenemia and for clinical symptoms.
Introduction
Fibrinogen is an approximately 340-kDa disulfide-bonded dimer of 3 different protein chains. The Aα,Bβ, and γ chains are encoded by 3 separate genes within a 50-kilobase (kb) region of chromosome 4q28-q31 (for a review, see Nieuwenhuizen et al1 ). The glycoprotein is synthesized in hepatocytes and is secreted to yield a normal steady state plasma concentration of 1.5 to 3.5 mg/mL with a half-life of approximately 4 days.2 As the precursor of the clot-forming protein fibrin and as a mediator of platelet aggregation, fibrinogen is a key component of the hemostatic system.1 As evidenced by the high rate of spontaneous abortions in women with hypofibrinogenemia,3 fibrinogen is also important for the successful conclusion of pregnancy.
Fibrinogen abnormalities can be classified according to whether there are low or no circulating levels of normal protein (hypofibrinogenemia or afibrinogenemia), a mutated species (dysfibrinogenemia), or a combination (hypodysfibrinogenemia). Reports1,4 on approximately 350 families with dysfibrinogenemia reveal that approximately half the cases are clinically silent, a quarter show a tendency toward bleeding, and another quarter show a predisposition for thrombosis with or without bleeding. Mutations have been found on all the fibrinogen chain genes, but those of the Aα chain have been most prevalent,5,6 particularly a mutation of the invariant 5′ splice site of intron 4 from GT → TT (IVS4 + 1 G>T), which results in afibrinogenemia in the homozygous state.7 Several nonsense mutations leading to a truncated fibrinogen Aα-chain have been reported.7-14 Clinical complications can vary widely, from asymptomatic to afibrinogenemic, depending on whether the patient is heterozygous or homozygous and on the extent of the truncation and any confounding mutations.
In this study we describe a novel fibrinogen mutation—designated fibrinogen Keokuk—that truncates the Aα-chain at Gln328 of the mature protein. Four family members were shown to be heterozygous for this mutation, but only 2 of them had hemostatic complications. These 2 were found to be compound heterozygotes for the truncation and for the IVS4 + 1 G>T mutation, resulting in hypodysfibrinogenemia.
Patients, materials, and methods
Patients
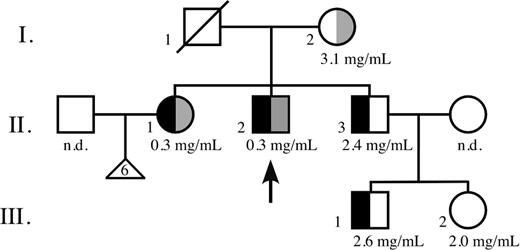
Three generations of a nonconsanguineous American family of European descent were studied: the mother (referred to as I.2), her 3 children (II.1, II.2, II.3), and 2 grandchildren (III.1, III.2) from her third child. II.1 and II.2 have no offspring, though II.1 had 6 miscarriages. The husband of II.1 and the wife of II.3 (the mother of III.1 and III.2) were not studied. The father (I.1), who died of lung cancer, was not known to have any hemostatic disorders. Only II.1 and II.2 had hypofibrinogenemia and problems with bleeding and thrombosis. Informed consent was obtained from all participants.
Sample collection and preparation
Blood for analysis of hemostasis was collected into tubes containing 0.1 volume of 0.105 M trisodium citrate anticoagulant (Becton Dickinson, Franklin Lakes, NJ). Platelet-rich plasma was obtained by centrifugation (100g for 10 minutes at room temperature [RT]). The remaining blood was centrifuged (1000g for 10 minutes at 4°C) to obtain platelet-poor plasma (PPP), aliquoted, and stored at -80°C. Blood for DNAanalysis was collected into tubes containing 0.011 volume 15% K3 EDTA (ethylenediaminetetraacetic acid). Unfractionated samples were transported at RT for up to 3 days before analysis. Genomic DNA was isolated from whole blood as previously described.15
Hemostasis functional analysis
Total fibrinogen concentration was measured using the Laurell immunoelectrophoresis method.16 Because the Laurell method has a sensitivity level of only 0.45 mg/mL, fibrinogen levels for II.2, II.3, and III.2 were also measured by fibrinopeptide A release as previously described.17
Platelet count was performed with a Thrombocounter C (Beckman Coulter, Brea, CA). Platelet aggregation was studied using a PAP-4 (Bio-Data, Horsham, PA) by adding 5 μL of 2 μM calcium ionophore U-46619 (Sigma, St Louis, MO) to 250 μL platelet-rich plasma and stirring (1000 rpm for 4 minutes at 37°C).
Enzyme-linked immunosorbent assay (ELISA) kits were used for measuring tissue plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1) levels (BioPool, Burlington, ON, Canada) and anticardiolipin antibodies (TheraTest Laboratories, Chicago, IL). Analysis for antifibrinogen and antifactor XIII (anti-FXIII) antibodies was carried out by ELISA as previously described,18 using purified fibrinogen or FXIII as antigens.
FXIII activity was assessed by examining clot solubility in 5 M urea.19,20 Activated partial thromboplastin time (aPTT), prothrombin time (PT), mixing tests, and assays for factors VIII, IX, and XI and clottable fibrinogen (using a modified Clauss method21,22 ) were performed using an MLA Electra 800 instrument, and antithrombin levels were measured using an MLA Electra 900 instrument (both from Medical Laboratory Automation, Pleasantville, NY). Thrombin time (TT) and plasminogen activity were determined with the respective STA kits, whereas lupus anticoagulant and functional protein C and protein S levels were measured by the respective STACLOT procedures, all performed on an ST-4 instrument (Diagnostica Stago, Asnieres-Sűr-Seine, France). Whole-blood clotting time was determined by observing 1.0 mL whole blood in a glass clotting tube, incubated at 37°C for 2 minutes, then tilted every 15 seconds until the tube could be inverted without significant movement of the blood in the tube.
Fibrinogen purification
Three milliliters PPP was dialyzed against 10 mM sodium phosphate, pH 6.5, 1 mM EDTA, and was subjected to anion exchange chromatography on a DEAE-Sepharose column equilibrated with the same buffer. After loading, the column was washed with 10 mM sodium phosphate, pH 6.0, 1 mM EDTA until the absorbance at 280 nm returned to baseline. Remaining plasma proteins, including fibrinogen, were eluted with 10 mM sodium phosphate, pH 6.0, 1 mM EDTA, and 200 mM NaCl, and fractions corresponding to these proteins were pooled. Fibrinogen was purified from this pool by precipitation with 35% saturated ammonium sulfate. After washing the pellet twice with 40% saturated ammonium sulfate, it was resuspended in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, and 150 mM NaCl and dialyzed against the same buffer. Normal fibrinogen was purified from 3 mL plasma using the same method or by precipitation with 22% saturated ammonium sulfate. The pellet was washed twice with 25% saturated ammonium sulfate, resuspended in HEPES buffer, and dialyzed.
Fibrin clot function and structure
TPA-induced fibrinolysis for II.1 and II.2 was determined in duplicate by the release of sodium iodide 125 (125I)–fibrinogen (196 μCi/mg [7.25 MBq/mg]; Amersham Pharmacia Biotech, Arlington Heights, IL) from a number of thrombins (kindly provided by J.W. Fenton, New York State Department of Health, Albany, NY)–induced PPP clots over several time points, as previously described.23 The percentage of lysed fibrinogen was calculated as [(supernatant cpm - background cpm)/(total cpm - background cpm)] × 100 and was plotted on a histogram compared with time incubated at 37°C. The time to achieve 50% lysis was derived from the curves fitted to those points.
Plasma clot compaction was determined in triplicate by mixing 60 μL PPP with 2.5 mM CaCl2 and 5 U/mL bovine thrombin (Instrumentation Laboratory, Lexington, MA) and immediately injecting the solution into heparinized microhematocrit capillary tubes (Curtin Matheson Scientific, Houston, TX), which were then sealed with Seal-ease tube sealing compound (Clay Adams, Parsippany, NJ). A tiny air bubble was left between the plug and the plasma to assist the clot in disengaging from the glass to ensure more uniform compaction. The plasma was allowed to clot for 2 to 3 minutes at RT and was then centrifuged (12 600g for 3 minutes at RT) in a TRIAC Centrifuge (Clay Adams). The length of the compacted clot was measured on a Leitz DM IL inverted microscope (Leica, Wetzlar, Germany) using an ocular ruler. To test normal fibrinogen clot compaction at a concentration similar to that of the proband, clots were made from 6 μL normal PPP (3 mg/mL fibrinogen) mixed with 54 μL fibrinogen-depleted plasma, obtained from the supernatant of PPP heated to 56°C for 5 minutes, and was then centrifuged at 1500g for 15 minutes.
Thrombin-catalyzed fibrin polymerization was initiated by adding 20 μL of l U/mL thrombin (Thrombostat; Parke-Davis, Morris Plains, NJ) to purified fibrinogen (0.15 mg/mL in 180 μL of 20 mM HEPES, pH 7.4, 150 mM NaCl), and absorbance at 350 nm was monitored for 50 minutes at RT.24 Triplicate measurements were made on fibrinogen purified from control and patient II.2 plasma.
Fibrin cross-linking was carried out with 100 μL or 200 μL (for hypofibrinogenemic samples) PPP brought to a total volume of 500 μL with buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10 000 IU/mL Trasylol, 12.5 U/mL bovine thrombin, and either 1 mM EDTA or 10 mM CaCl2). After incubation (60 minutes, 37°C), 0.5 mL of 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 1 mM EDTA was added, and each clot was centrifuged (20 000g for 10 minutes, 4°C). Pellets were washed twice with 1 mL of 50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 1 mM EDTA, centrifuged, and solubilized in 150 μL or 75 μL (for hypofibrinogenemic samples) of 50 mM Tris-HCl, pH 6.8, 9 M urea, 2% SDS, and 40 mM dithiothreitol for 30 minutes at 37°C before sodium dodecyl sulfate–polyacrimide gel electrophosphoresis (SDS-PAGE).25,26 Six microliters or 8 μL (hypofibrinogenemic samples) of the solubilized clots were applied for electrophoresis on 10% polyacrylamide gels. Gels were stained with Coomassie brilliant blue R, destained, dried, and scanned.
Electrophoretic and immunologic analysis of clots and purified fibrinogen preparations
Plasma was diluted with MilliQ water and was matched for similar fibrinogen loading on gels (approximately 0.02 μg). Samples were electrophoresed on a 7.5% reducing SDS-PAGE gel, electroblotted onto nitrocellulose, blocked with casein blocking solution (5% [wt/vol] low-fat milk powder in Tris-buffered saline with Tween-20), and incubated for 1 hour at RT with rabbit polyclonal antibody to human fibrinogen (immunoglobulin fraction, 1:1000 dilution in casein blocking solution; DAKO, Glostrup, Denmark). Antibody staining was visualized with an enhanced chemiluminescence (ECL) Western blotting analysis system (Amersham Pharmacia Biotech) using a secondary antibody specific to rabbit immunoglobulin G (IgG) (1:10 000 dilution) and X-Omat AR film (Kodak, Rochester, NY).
Solubilized clots (EDTA samples only) were matched for similar protein loading (3-8 μL/well), electrophoresed, and electroblotted onto nitrocellulose,27 blocked with Blotto (2% nonfat dry milk in phosphate-buffered saline [PBS]), and incubated overnight at RT with antibodies to whole human fibrinogen (rabbit immunoglobulin fraction, 1:20 000 dilution in Blotto; DAKO, Carpenteria, CA) or its Aα241-476 (rabbit antiserum HS2-6, 1:100 000 dilution in Blotto; kindly provided by J. Sobel, Columbia University, New York, NY), Aα291-348 or Aα349-406 segments (mouse monoclonal hybridoma supernatants from Dr G. Samokhin, Northwestern University, Chicago, IL). Antibody staining was visualized with an ECL Western blotting system (Amersham Pharmacia Biotech) using secondary antibodies specific to rabbit or mouse IgG (1:10 000 dilution) and Biomax ML film (Kodak).
DNA sequence analysis
Exon 5 of the fibrinogen Aα-chain gene was amplified by 37 cycles of polymerase chain reaction (PCR) using the following primers: AαVF corresponding to nucleotides 3715 to 3732 (5′CAGGAACTCAATAGACGT 3′) and AαVR primer (5′GACCAGTTTTTCTGTGTGGTACTC 3′) corresponding to the complementary nucleotides 4577 to 4554.28 The PCR product was run on a 1% agarose gel, and the band with the expected size was purified with the Gel Extraction Kit (QIAGEN, Santa Clarita, CA). Purified products were sequenced using AαVF and AαVR primers (GlaxoWellcome DNA sequence facility; University of North Carolina at Chapel Hill).
All exons and intron-exon boundaries of the fibrinogen genes were amplified from genomic DNA using standard PCR protocols. PCR products were purified in MultiScreen96 PCR plates (Millipore, Billerica, MA) and were sequenced using the BigDye Terminator version 3.1 Cycle Sequencing Kit and a 3100-Avant capillary DNA sequencer (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Primers were designed from the published FGA (GenBank M64982), FGB (GenBank M64983), and FGG (GenBank M10014) sequences.
Results
Case histories
Two adult siblings of white European descent, a 53-year-old woman (II.1) and a 49-year-old man (II.2) (arrow in Figure 1), have life-long histories of prolonged bleeding following trauma. In addition, II.1 had 6 miscarriages. Both were previously diagnosed with hypofibrinogenemia and were reported to be negative for FV Leiden. Their parents (I.1, I.2), their 44-year-old brother (II.3), and his 2 children (III.1, III.2) have no history of bleeding or thrombosis. As an adult, II.1 reported having undergone oral surgery for impacted molars. Cryoprecipitate was infused before the procedure, and “a few units” were infused every 3 days after surgery until the bleeding stopped, all without complication.
Family pedigree. Functional fibrinogen concentration, if available, is listed under family member. n.d. indicates not determined; □, male; ○, female; ▵, miscarriage; diagonal line, deceased; black, Fib Keokuk mutation; and gray, AαIVS4 + 1G>T splice site mutation. Arrow points to thrombophilic proband.
Family pedigree. Functional fibrinogen concentration, if available, is listed under family member. n.d. indicates not determined; □, male; ○, female; ▵, miscarriage; diagonal line, deceased; black, Fib Keokuk mutation; and gray, AαIVS4 + 1G>T splice site mutation. Arrow points to thrombophilic proband.
At age 43, II.2 underwent total hip arthroplasty because of aseptic necrosis of the left hip. He was prepared for surgery with cryoprecipitate to raise his circulating fibrinogen concentration to approximately 1.5 mg/mL. The procedure was uneventful, and after surgery he was given 10 to 15 units of cryoprecipitate daily to maintain the fibrinogen concentration between 1.0 and 1.5 mg/mL. Five days after surgery, he began experiencing weakness and malaise, and on day 8 cryoprecipitate administration was discontinued. On day 10, after reporting chest pain, imaging studies revealed multiple pulmonary emboli and thrombi in both femoral veins. An inferior vena cava filter was inserted, and he was treated with anticoagulants for 3 months.
Eighteen months after surgery, II.2 had leg ischemia secondary to emboli originating from a thrombus in the thoracic descending aorta. The aortic thrombus was removed, and cryoprecipitate was given after surgery for several days. Once again, pulmonary emboli developed, and intravenous catheters became occluded by thrombi. He was treated with subcutaneous low-molecular–weight heparin, and the thrombi slowly resolved. Three years after the initial hip surgery, thrombectomy was performed to remove femoral arterial clots. No cryoprecipitate was administered, and no excessive bleeding was observed. He is currently receiving long-term anticoagulation with low-molecular–weight heparin and aspirin, and has had no recent thrombotic episodes.
Hemostasis factor studies
Results of coagulation studies for the 2 hypofibrinogenemic patients (II.1, II.2) are presented in Table 1. PT and TT times were greatly prolonged, but the admixture of normal plasma normalized both. FVIII levels were elevated in II.1 (Table 1) and in family members I.2 and II.3 (data not shown). Plasminogen levels for both II.1 and II.2 were normal; t-PA and PAI-1 levels were normal in II.1 but were elevated in II.2, as might be expected in a patient experiencing thrombosis.29,30 Each had a normal platelet count, and normal platelet aggregation was obtained with the calcium ionophore U-46619. Protein C, protein S, and antithrombin levels for II.2 were normal (levels for II.1 were not determined), and test results for lupus anticoagulant, antifibrinogen, anti-FXIII, and anticardiolipin antibodies were negative for II.1 and II.2.
Hemostatic indices for patients II.1 and II.2
. | . | Patient . | . | |
|---|---|---|---|---|
| Test . | Normal range . | II.1 . | II.2 . | |
| Fibrinogen (clottable), mg/mL | 1.5-3.5 | 0.3 | 0.3 | |
| Fibrinogen (total), mg/mL | 1.5-3.5 | ND | 0.5 | |
| APTT, sec | 22-30 | 30 | 34 | |
| PT, sec | 11-13 | 22 | 21 | |
| TT, sec | 16-18 | 46 | 52 | |
| Whole blood clotting time, min | 3-8 | 15 | 10 | |
| Plasma clot lysis time, min | 27 | 101 | 104 | |
| Compressed clot, mm | 0.8-1.0* | ND | 1.6 | |
| t-PA, ng/mL | 4-6 | 6 | 12 | |
| PAI-1, ng/mL | 8-28 | 18 | 48 | |
| Platelet count/μL, × 10-3 | 150-400 | 218 | 242 | |
| FVIII, % | 55-183 | 197 | 178 | |
| FIX, % | 50-150 | 70 | 95 | |
| FXI, % | 56-164 | 92 | 100 | |
. | . | Patient . | . | |
|---|---|---|---|---|
| Test . | Normal range . | II.1 . | II.2 . | |
| Fibrinogen (clottable), mg/mL | 1.5-3.5 | 0.3 | 0.3 | |
| Fibrinogen (total), mg/mL | 1.5-3.5 | ND | 0.5 | |
| APTT, sec | 22-30 | 30 | 34 | |
| PT, sec | 11-13 | 22 | 21 | |
| TT, sec | 16-18 | 46 | 52 | |
| Whole blood clotting time, min | 3-8 | 15 | 10 | |
| Plasma clot lysis time, min | 27 | 101 | 104 | |
| Compressed clot, mm | 0.8-1.0* | ND | 1.6 | |
| t-PA, ng/mL | 4-6 | 6 | 12 | |
| PAI-1, ng/mL | 8-28 | 18 | 48 | |
| Platelet count/μL, × 10-3 | 150-400 | 218 | 242 | |
| FVIII, % | 55-183 | 197 | 178 | |
| FIX, % | 50-150 | 70 | 95 | |
| FXI, % | 56-164 | 92 | 100 | |
Normal plasma (fibrinogen = 2-3 mg/mL). Clots from normal plasma, diluted to 0.3 mg/mL with defibrinogenated plasma, were compressed to 0.007 mm. ND indicates not determined
Fibrinogen and fibrin clot studies
No fibrinogen could be detected in the PPP of patients II.1 and II.2 by immunoelectrophoresis, but, as indicated in Table 1, low concentrations of fibrinogen were found with the modified Clauss procedure and fibrinopeptide quantitation. Fibrinogen concentrations were normal in the other family members (Figure 1).
Plasma clot lysis induced by t-PA was greatly retarded, and plasma clots from II.2 were resistant to compression by centrifugation, especially when compared with normal plasma clots made with the same fibrinogen levels as for II.2 (Table 1). In addition, clots from II.2 were soluble in 5 M urea.
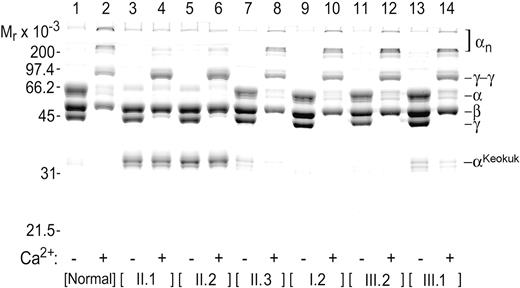
Examination of fibrin chain profiles by SDS-PAGE (Figure 2) revealed the presence of an abnormal fibrin(ogen). Thrombin-induced clots of PPP from patients II.1 and II.2 had essentially no normal α chain (compare lanes 3 and 5 with lane 1 in Figure 2). Perhaps the most remarkable feature of these clots was the presence of a new abnormal band at approximately 35 kDa (αKeokuk; Figure 2, lanes 3-8, 13, 14). The weak Coomassie blue staining at the position of normal α chains (66 kDa) (Figure 2, lanes 3, 5) probably resulted from trapped albumin, which would be expected to migrate in this position in the presence and absence of Ca2+. Further evidence for the absence of normal α chains in II.1 and II.2 was a failure to form cross-linked polymeric αn structures when clotting was carried out in the presence of Ca2+ (compare lanes 4 and 6 with lane 2 in Figure 2). However, the β and γ chain patterns (Figure 2, lanes 3, 5), including the formation of γ-γ chain dimers (Figure 2, lanes 4, 6), were normal for II.1 and II.2.
Electrophoretic profile of fibrin clots. SDS-PAGE showing fibrin chain profiles of clots generated in the absence (odd-numbered lanes) and presence (even-numbered lanes) of Ca2+ from the citrated plasma of II.1, II.2, their mother (I.2), brother (II.3), and his children (III.1, III.2). II.1 and II.2 lack normal α chain, and a much shorter constituent chain (αKeokuk) that cannot produce cross-linked αn polymers is seen in II.1, II.2, II.3, and III.1 samples.
Electrophoretic profile of fibrin clots. SDS-PAGE showing fibrin chain profiles of clots generated in the absence (odd-numbered lanes) and presence (even-numbered lanes) of Ca2+ from the citrated plasma of II.1, II.2, their mother (I.2), brother (II.3), and his children (III.1, III.2). II.1 and II.2 lack normal α chain, and a much shorter constituent chain (αKeokuk) that cannot produce cross-linked αn polymers is seen in II.1, II.2, II.3, and III.1 samples.
The proportion of truncated α chains in the simple heterozygotes was calculated from the gel band intensities as [αKeokuk % = (2 × αKeokuk)/[(2 ×αKeokuk) +αnormal)] × 100]. A correction factor of 2 was used to compensate for the reduced capacity of the approximately half-normal αKeokuk chain to bind the Coomassie blue stain, and allowance was made for the presence of normal α chain cleavage products that migrated close to the truncated α chain position, in that α(total) always includes any cleavage products. For normal samples, it is all cleavage products; for fibrinogen Keokuk samples, it would be a combination, probably proportional to the amount of normal fibrinogen. From subsequent gene analysis, we concluded that patients II.1 and II.2 carried only the truncated α chain and that family members I.2 and III.2 were healthy. From densitometric scanning of the gel in Figure 2, we estimated that approximately 10% of the circulating fibrinogens in II.3 and his son III.1 was derived from the truncated species.
Immunoblotting experiments (shown for patient II.2 in Figure 3) with an antiserum specific for the Aα241-476 segment of human fibrinogen confirmed that the approximately 35-kDa constituent was a derivative of the Aα chain of the normal protein.
Characterization of aberrant Aα chain. (A) Immunoblot of plasma, after SDS-PAGE, with antibody to Aα241-476 before and after the addition of thrombin. Lanes 1 and 2, normal control; lanes 3 and 4, patient II.2. The patient lacks normal Aα chains, but fibrinopeptide A is successfully removed from the N-terminus of the defective chain by thrombin, suggesting C-terminal truncation. (B) Immunoblot of SDS-PAGE using antiserum to whole fibrinogen. Lane 1, normal plasma; lane 2, patient II.2; lane 3 homozygote for AαOtago truncation with a predicted mass of 30 862 Da.
Characterization of aberrant Aα chain. (A) Immunoblot of plasma, after SDS-PAGE, with antibody to Aα241-476 before and after the addition of thrombin. Lanes 1 and 2, normal control; lanes 3 and 4, patient II.2. The patient lacks normal Aα chains, but fibrinopeptide A is successfully removed from the N-terminus of the defective chain by thrombin, suggesting C-terminal truncation. (B) Immunoblot of SDS-PAGE using antiserum to whole fibrinogen. Lane 1, normal plasma; lane 2, patient II.2; lane 3 homozygote for AαOtago truncation with a predicted mass of 30 862 Da.
A slightly larger form of this abnormal chain was present in citrated plasma rather than in the clot (compare the band marked AαKeokuk of lane 3 with that of αKeokuk in lane 4 of Figure 3A for patient II.2), consistent with the notion that thrombin cleavage produced the AαKeokuk to αKeokuk conversion by removing the fibrinopeptide A moiety. It was further evident from the findings in lane 4 that the abnormal αKeokuk chain could not form high-molecular–weight cross-linked homopolymers under FXIIIa-catalyzed conditions (compare lanes 2 and 4 of Figure 3A), beyond perhaps a certain extent of dimerization (that would yield a protein band close to the position of the normal Aα chain in lane 4).
Taken together, the results indicated that the C-terminal half of the Aα chain was missing in protein expressed from the Keokuk mutation (similar results were obtained for samples from patient II.1; data not shown). Additional immunoblots were negative, with monoclonal antibodies to Aα291-348 and to Aα349-406 (data not shown), suggesting that truncation occurs upstream of residue 348 but downstream of the Otago truncation at 271. Indeed, as presented in Figure 3B for purified fibrinogen preparations, the AαKeokuk chain is larger (approximately 35 kDa, lane 2) than the previously described AαOtago (approximately 30 kDa, lane 3).11
The nature of the defect implied by the prolonged thrombin time (Table 1) was explored further by examining the kinetics of thrombincatalyzed polymerization. Polymerization curves on purified fibrinogen (Figure 4) showed significant defects in all stages of fibrin polymerization, with a lag phase of 430 seconds, a Vmax of 0.78 × 10-4 U/s, and a final turbidity of 0.023 U compared with control values of 220 seconds, 2.22 × 10-4 U/s, and 0.111 U, respectively.
Fibrin polymerization curves. The clotting defect in patient II.2 is characterized by a slower than normal rate of fibrin assembly and by an approximately 5-fold reduction of clot turbidity (ordinate).
Fibrin polymerization curves. The clotting defect in patient II.2 is characterized by a slower than normal rate of fibrin assembly and by an approximately 5-fold reduction of clot turbidity (ordinate).
DNA analysis
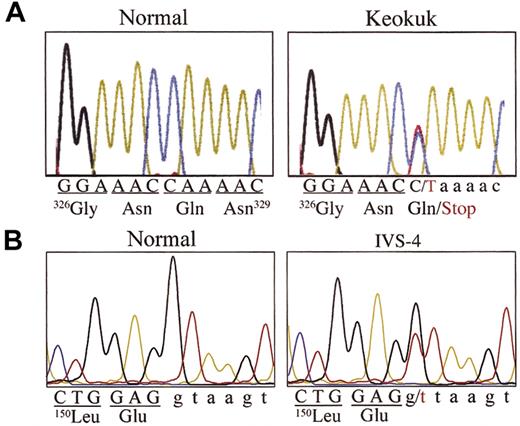
Because of the presumed site of the Keokuk putative mutation deduced from immunoblotting (Figure 3A), DNA sequence analysis focused first on examining exon 5 of the fibrinogen Aα-chain gene. A C>T point mutation was found that changed the triplet CAA coding for AαGln328 to a TAA stop codon (Figure 5A). Family members II.1, II.2, II.3, and III.1 were heterozygous for this mutation (named fibrinogen Keokuk, after the family's town of origin). However, to explain the fact that only 2 of the 4 family members had extreme hypofibrinogenemia and experienced coagulation problems, further genetic analysis was deemed necessary. These tests revealed another mutation at the 5′ end of intron 4 of the Aα gene. The mutation converts the invariant GT splice site to TT (Figure 5B), and 3 family members (I.2, II.1, II.2) were found to be heterozygous for this mutation. Interestingly, the heterozygous carriers with fibrinogen Keokuk (II.3, III.1) or the IVS4 + 1 G>T (I.2) mutation alone have normal concentrations of fibrinogen and seem to be free of disease, whereas the compound heterozygous combination of these mutations found in II.1 and II.2 causes extreme hypodysfibrinogenemia and coagulopathy. Complete sequencing of all exons and intronexon boundaries of the 3 fibrinogen subunit genes from II.2 found no other mutations.
DNA sequence analysis. (A) DNA sequencing of exon 5 of the fibrinogen Aα gene showed that II.1 and II.2, their brother (II.3), and his son (III.1) had a heterozygous C>T mutation that changed the CAA codon for residue Gln328 to a TAA stop codon and was the cause for the Keokuk truncation of the Aα constituent chain of the protein. (B) II.1 and II.2 and their mother (I.2) had a heterozygous mutation (IVS4 + 1 G>T) in intron 4 at the invariant 5′ splice site of this gene. Representative examples of each mutation are shown.
DNA sequence analysis. (A) DNA sequencing of exon 5 of the fibrinogen Aα gene showed that II.1 and II.2, their brother (II.3), and his son (III.1) had a heterozygous C>T mutation that changed the CAA codon for residue Gln328 to a TAA stop codon and was the cause for the Keokuk truncation of the Aα constituent chain of the protein. (B) II.1 and II.2 and their mother (I.2) had a heterozygous mutation (IVS4 + 1 G>T) in intron 4 at the invariant 5′ splice site of this gene. Representative examples of each mutation are shown.
Discussion
DNA sequencing established that the 2 symptomatic siblings (II.1, II.2) with hypofibrinogenemia were compound heterozygotes for 2 different Aα gene mutations: the IVS4 + 1G>T splice site mutation and a novel stop mutation at codon AαGln328 of the mature protein (the Keokuk mutation). Heterozygosity for either the splice site or the Keokuk mutation, of themselves, caused neither clinical symptoms nor hypofibrinogenemia. Fibrinogen concentrations were 3.1 mg/mL for I.2 and 2.4 and 2.6 mg/mL for II.3 and III.1, respectively. Homozygosity for the splice mutation is recognized as a frequent cause of afibrinogenemia,14 indicating that no viable Aα chains should be produced from the aberrantly spliced allele. Thus, the compound heterozygotes should express only a truncated Aα chain of 35 977 Da. This was confirmed in SDS-PAGE of their purified fibrinogen (Figure 2), which showed a single Aα band migrating above the truncated AαOtago chains measuring 30 862 Da.
Nonsense mutations have been reported along the entire length of the fibrinogen Aα-chain gene, and homozygosity can result in dysfibrinogenemia,9,13 hypodysfibrinogenemia,8,11 or often afibrinogenemia7,12,14,31 depending on the extent of the truncation. As with fibrinogen Keokuk, heterozygous family members usually had normal fibrinogen concentrations, although with diminished allelic expression,8,11 and in some cases a significant polymerization defect.10,32 For instance, heterozygotes for fibrinogen Perth32 and fibrinogen Otago11 (with Aα truncations of 15% and 56%, respectively) had normal fibrinogen concentrations but decreased expression of the truncated chains; the ratio of αPerth to Aα was 0.2:1.0 and there were no αOtago chains in the plasma fibrinogen of fibrinogen Otago heterozygotes. The 46% truncation in fibrinogen Keokuk led to an allelic expression ratio (αKeokuk/αA) of only approximately 0.1:1.0, as seen in II.3 and III.1. This suggests that the AαC domain is involved in assembly of the fibrinogen molecule in the hepatocyte—the shorter the α chain, the less the truncated species is found in circulating fibrinogen.
The AαC domain of fibrin is intimately involved in all stages of polymerization. Although cleavage of fibrinopeptide A is the primary driver of polymerization, cleavage of fibrinopeptide B not only exposes the GlyHisArg sequence, it facilitates the release of the αC domain from intramolecular association with the central E domain. These tethered αC appendages associate intermolecularly and are thought to promote lateral aggregation of the protofibrils.33 Polymerization curves for the clotting of purified fibrinogen Keokuk support this scenario with a doubling of the lag phase, indicating a delay in the onset of protofibril formation and a 3-fold decrease in Vmax that indicates impaired protofibril assembly into fibers. The most significant aberration, however, was that the final turbidity (measured by absorbance at 350 nm) was only approximately one fifth of the normal control, implying that the Keokuk clot was composed of thinner fibers.
The deletion of Aα residues 328-610 results in the removal of key side chains directly involved in the FXIIIa-mediated covalent crosslinking of the Aα chain, notably Gln328 and Gln366 and Lys508.34 Although analysis of plasma clots by SDS-PAGE (Figure 1, lanes 4, 6) indicated normal γ-γ dimer formation, covalent αn polymers were notably absent. Interestingly, plasma clots of fibrinogen Keokuk were readily soluble in 5 M urea, indicating that although the covalent fusion of γ chains along the linear array of fibrin molecules might be necessary for stabilizing the clot network,35 it is the FXIIIa-catalyzed “spot-welding” of α chains36 that renders a clot insoluble in urea.
Although bleeding episodes can be readily explained by a combination of low fibrinogen, severely impaired polymerization kinetics, and defective α chain cross-linking, the principal abnormalities were thrombosis in II.2 and recurrent miscarriages in II.1. Thin fibers and decreased pore size led to clots that were physically more resistant to degradation, and this might have contributed to the thrombosis history of II.2. Other mutations within the AαC domain have been associated with rigid clots showing diminished final turbidities, thin fibers, and history of thrombosis.8,9,37-39 It is well established that fibrinogen contains 2 cryptic plasminogen and t-PA binding sites (Aα148-160 and γ312-324) that become available in fibrin. However, more recent expression studies using purified full-length AαC (residues 221-610) and its N- and C-terminal fragments established that the AαC domain contains additional independent cryptic t-PA and plasminogen binding sites located in the region from 392 to 610.40 The loss of these sites in fibrinogen Keokuk through truncation at residue 328 might have contributed to the thrombotic phenotype observed in II.2. The prothrombotic signals commonly generated by major surgery presumably acted on the fibrinolysis-resistant fibrinogen Keokuk to form small clots, which might have accumulated over time and combined with the large quantity of added fibrinogen to eventually result in the thrombotic complications that finally became clinically significant after more than a week of cryoprecipitate infusions. Patients with similar hypodysfibrinogenemias, including II.1 of the Keokuk family, have been reported to be tolerant of moderate fibrinogen replacement therapy,11 but the levels infused into II.2 might have been excessive, especially in light of major surgery successfully completed later without any cryoprecipitate infusions.
The miscarriages experienced by II.1 all occurred in the first trimester, at approximately 6 to 8 weeks. Similarly, the fibrinogen Otago patient had 4 miscarriages in the 6th to 8th weeks of pregnancy. These miscarriages occurred when trophoblasts infiltrated the myometrium. Experimentally, afibrinogenemic mice also miscarry at the time of trophoblast infiltration (days 9-10).41
This family study provides a unique insight into how 2 mutations, without untoward consequences in carriers of either mutation, may produce severe manifestations in compound heterozygotes originally diagnosed with simple hypofibrinogenemia. Regardless of the precise molecular explanations for thrombogenicity, the near-fatal postsurgical thrombosis after the administration of cryoprecipitate to II.2 illustrates the risk associated with aggressive replacement therapy for increasing the circulating concentration of fibrinogen without adequately characterizing the nature of the hypodysfibrinogenemia.42
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-07-2316.
Supported by National Institutes of Health grants HL-16346 (L.L.) and HL31048 (S.T.L.), the Rehabilitation Institute of Chicago Atherosclerosis Research Program (D.G.), and American Heart Association fellowship 120498U (K.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the families for their participation in this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal