Hepatitis B virus (HBV) reactivation is a well-recognized and potentially fatal complication of cytotoxic chemotherapy in cancer patients who are known to be HBV surface-antigen seropositive.1-4 Studies have not, however, investigated the occurrence of HBV reactivation in individuals who are surface-antigen negative.
Here, patients with biopsy-proven systemic AIDS-related lymphoma (ARL) who received combination cytotoxic chemotherapy with curative intent were identified from a cohort of 5832 HIV-1–positive individuals followed up in the highly active antiretroviral therapy (HAART) era, commenced in 1996 (when HAART became freely available to our patients). HBV core (total) antibodies and the presence of HBV surface antigen were detected on stored sequential samples from these patients using the Axsym system (Abbott Laboratories, Chicago, IL). In those individuals who tested positive for HBV core antibodies and were surface-antigen negative, detection and quantification of HBV DNA were performed using real-time polymerase chain reactions (RealArt HBV RG PCR; Artus, Hamburg, Germany). Patient samples were obtained before, during, and following cytotoxic chemotherapy to be examined for evidence of chemotherapy-induced HBV reactivation. All patients were from the Chelsea and Westminster Hospital, one of the largest HIV cohorts in Europe, and the study received appropriate ethical approval.
Of 61 ARL patients, 30 were HBV core-antibody positive and 31 were core-antibody negative. Interestingly, none of the 31 core-antibody–positive group of patients tested positive for HBV surface antigen in prechemotherapy samples. There were no statistically significant differences in patient characteristics between these 2 groups for age, median CD4 cell count, HIV viral load, stage III/IV disease, B symptoms, Eastern Cooperative Oncology Group (ECOG) performance status, prior AIDS defining illness, and HAART prior to ARL diagnosis.
Sequential samples from 30 HBV core antibody patients were tested for evidence of HBV reactivation during chemotherapy, and in 5 of these individuals HBV viral loads increased during chemotherapy. There were no significant differences in the aforementioned baseline characteristics of these 5 male patients, compared with the other 56 individuals in the cohort, including subgroup analyses of HBV core-antibody positive and negative comparisons. Interestingly, only 1 of the 5 patients had evidence of lymphomatous liver involvement, compared with 20 in the remainder.
The changes in HBV viral load before, during, and following chemotherapy for these 5 individuals and the corresponding changes in serum alanine aminotransferase (ALT) are shown in Table 1. The median rise in HBV viral load was 2500 copies/mL and there were no significant differences between ALT changes in patients with reactivation versus others. Patients 1 and 4 became surface-antigen positive during chemotherapy. The remaining 3 patients with HBV reactivation did not demonstrate evidence of HBV surface antigenemia during chemotherapy.
Changes in HBV viral load and ALT before, during, and after chemotherapy in 5 individuals with evidence of reactivation
. | Before chemotherapy . | Maximum during chemotherapy . | After chemotherapy . |
|---|---|---|---|
| HBV viral load (age, y) | |||
| Patient no. 1 (41) | 0 | 2.3 × 109 | 1.4 × 109 |
| Patient no. 2 (36) | 0 | 2500 | 0 |
| Patient no. 3 (38) | 0 | 7500 | 0 |
| Patient no. 4 (41) | 0 | 1150 | 45 350 |
| Patient no. 5 (56) | 0 | 2500 | 0 |
| Median HBV viral load* | 0 | 2500 ± 108 | 0 ± 6 × 108 |
| Alanine aminotransferase | |||
| Median ALT in patient nos. 1 to 5* | 19 ± 14 × 103 | 95 ± 78 × 103 | 24 ± 12 × 103 |
| Median ALT in 56 individuals without reactivation* | 29 ± 41 × 103 | 101 ± 136 × 103 | 33 ± 75 × 103 |
. | Before chemotherapy . | Maximum during chemotherapy . | After chemotherapy . |
|---|---|---|---|
| HBV viral load (age, y) | |||
| Patient no. 1 (41) | 0 | 2.3 × 109 | 1.4 × 109 |
| Patient no. 2 (36) | 0 | 2500 | 0 |
| Patient no. 3 (38) | 0 | 7500 | 0 |
| Patient no. 4 (41) | 0 | 1150 | 45 350 |
| Patient no. 5 (56) | 0 | 2500 | 0 |
| Median HBV viral load* | 0 | 2500 ± 108 | 0 ± 6 × 108 |
| Alanine aminotransferase | |||
| Median ALT in patient nos. 1 to 5* | 19 ± 14 × 103 | 95 ± 78 × 103 | 24 ± 12 × 103 |
| Median ALT in 56 individuals without reactivation* | 29 ± 41 × 103 | 101 ± 136 × 103 | 33 ± 75 × 103 |
HBV viral load is measured in copies/mL, and ALT in U/L.
Medians ± standard deviations are shown
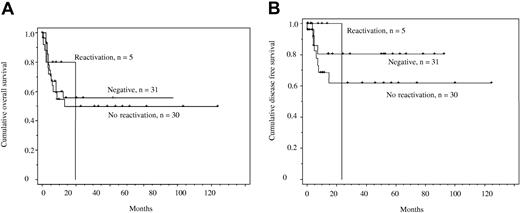
We also compared overall survival and disease-free survival for 3 patient groups described herein: (1) those who were HBV core-antibody negative (n = 31), (2) those who were core-antibody positive without evidence of HBV reactivation (n = 25), and (3) individuals who were core-antibody positive and reactivated (n = 5). Figure 1A demonstrates overall survival and Figure 1B, disease-free survival: there were no significant differences between the groups. The vertical drop in the group with HBV reactivation is indicative of the fact that patient 2 died with the longest follow-up after ARL diagnosis.
Kaplan Meier overall and disease-free survival curves. (A) Overall survival in individuals with ARL who were core-antibody negative, those who were core-antibody positive with no evidence of reactivation, and those who were core-antibody positive with evidence of reactivation. The y-axis shows the probability of survival against time. No comparisons were significant (P > .9). (B) Disease-free survival in individuals with ARL who were core-antibody negative, those who were core-antibody positive with no evidence of reactivation, and those who were core-antibody positive with evidence of reactivation. No comparisons were significant (P > .5).
Kaplan Meier overall and disease-free survival curves. (A) Overall survival in individuals with ARL who were core-antibody negative, those who were core-antibody positive with no evidence of reactivation, and those who were core-antibody positive with evidence of reactivation. The y-axis shows the probability of survival against time. No comparisons were significant (P > .9). (B) Disease-free survival in individuals with ARL who were core-antibody negative, those who were core-antibody positive with no evidence of reactivation, and those who were core-antibody positive with evidence of reactivation. No comparisons were significant (P > .5).
We show in a high-risk cohort that reactivation, as measured by HBV DNA increases during chemotherapy for ARL, is uncommon and does not adversely affect outcome. The frequency of reactivation reported in previous studies varies substantially due to differing definitions of reactivation. Some studies have defined reactivation as an increase in ALT, whereas others have used virologic parameters.5-7 This study has investigated quantitative measures of viral replication as a marker of true reactivation in a well-characterized patient group. Here, all patients selected for investigation of increases in HBV DNA were HBV core-antibody positive and surface-antigen negative prior to chemotherapy.
Despite the patients here testing negative for HBV surface antigen and HBV DNA prior to chemotherapy, a frequency of reactivation higher than that observed may have been expected due to (1) the high frequency (30/61) of core antibody positivity, (2) underlying HIV and malignancy-induced immunosuppression, and (3) the use of steroids in chemotherapeutic regimens shown to increase the frequency of reactivation.8 As only 2 individuals became positive for HBV surface antigen, this study also demonstrates that it is difficult to establish without HBV DNA quantification the precise frequency of reactivation. Simple serologic tests such as detection of core antibody or surface antigen do not suffice in specifically indicating those HIV-1–positive individuals with ARL who are likely to reactivate. If patients are surface-antigen negative prior to chemotherapy, the risk of hepatitis due to HBV reactivation appears low. This is in contrast to previous work that has investigated reactivation in those who were HBV surface-antigen carriers. In these studies, the rates of reactivation varied between 10% and 40%, with up to 50% of those who reactivated developing severe hepatitis.1-3,5,6,9,10
Future studies should investigate HBV DNA increases in HIV-1–infected patients who are surface-antigen positive. Such individuals are significantly less common in Europe, and ongoing collaborative studies would benefit from involvement in the Far East, where hepatitis B carriage is more common.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal