Abstract
We describe the use of small interfering RNAs (siRNAs) to down-regulate H- and L-ferritin levels in HeLa cells. siRNAs repressed H- and L-ferritin expression to about 20% to 25% of the background level in both stable and transient transfections. HeLa cells transfected with H- and L-ferritin cDNAs were analyzed in parallel to compare the effects of ferritin up- and down-regulation. We found that large modifications of L-ferritin levels did not affect iron availability in HeLa cells but positively affected cell proliferation rate in an iron-independent manner. The transient down-regulation of H-ferritin modified cellular iron availability and resistance to oxidative damage, as expected. In contrast, the stable suppression of H-ferritin in HeLa cell clones transfected with siRNAs did not increase cellular iron availability but made cells less resistant to iron supplementation and chelation. The results indicate that L-ferritin has no direct effects on cellular iron homeostasis in HeLa cells, while it has new, iron-unrelated functions. In addition, they suggest that H-ferritin function is to act as an iron buffer.
Introduction
Ferritins are 24-mer proteins that play a central role in regulating cellular iron availability and toxicity by readily interacting with Fe(II), oxidizing and sequestering it inside their large cavity.1,2 In mammals, the cytosolic ferritins are ubiquitous and made of 2 subunit types, the H- and L-chains, with about 50% sequence identity and very similar 3-dimensional (3D) structures.3 A third ferritin subunit, specifically targeted to the mitochondria, has been recently identified and has tissue-specific expression.4,5 H- and L-chain expression is under independent transcriptional regulation, so that the H/L proportion is tissue specific.2,3 In addition, they are under the same iron-dependent posttranscriptional regulation mediated by the iron-responsive element–iron-regulatory protein (IRE-IRP) machinery.6,7 H-chains have ferroxidase activity, which accelerates Fe(II) oxidation, the rate-limiting step of ferritin iron incorporation, in a reaction that consumes 1 dioxygen molecule per 2 Fe(II) ions with the production of hydrogen peroxide.8 The catalytic centers are buried inside the protein fold and consist of di-iron binding sites coordinated by atoms of 7 residues that are conserved in most ferritins from animals, plants, bacteria, and mitochondria.3,9 The L-subunit has no catalytic activity on its own, but it assists the activity of the H-subunits by offering sites for iron nucleation and mineralization and increasing the turnover at the ferroxidase centers.10 The in vitro mechanism of ferritin iron uptake is reviewed by Harrison and Arosio.3
Cell transfection has been used to study the biologic role of ferritin. In initial studies COS cells were transiently transfected with the cDNAs for H- and L-ferritins, but this had no evident effect on cellular iron homeostasis despite high levels of expression.11 Strong effects were observed when H-ferritins were stably expressed in cells either by using a construct in which the IRE was inactivated and ferritin constitutively up-regulated12 or by placing the cDNA under the control of the inducible tetracycline promoter.13 H-chain overexpression determined a reduction of labile iron pool and an increase of IRP activity. This was accompanied by a reduction of cell proliferation rate and by an increased resistance to the oxidative stress caused by H2O2, effects that could be reversed either by prolonged iron supplementation to the cells or by inactivating the ferroxidase center of the overexpressed H-ferritin.13 Opposite effects were obtained when ferritin expression was down-regulated by antisense oligonucleotides: The labile iron pool expanded, and transferrin-bound iron uptake was increased, probably consequent to a down-regulation of IRP activity.14 The inhibition of ferritin expression did not influence long-term cell proliferation but facilitated growth resumption of cells arrested at G1/S phase.15 These results suggest that cellular iron availability is a limiting factor for cell proliferation possibly affecting the activity of cyclin-dependent kinases.16 In addition, it was shown that H-ferritin expression has an antiapoptotic effect apparently unrelated to its iron-binding capacity.17
The biologic role of L-ferritin is less clear. Clinical evidence indicates that its constitutive overexpression in subjects with the hereditary hyperferritinemia cataract syndrome has negligible effects on body iron handling.18 The disorder is caused by mutations in the IRE sequence of L-ferritin messenger that cause a 10-fold to 20-fold increase of L-ferritin levels in tissues and serum,19 but it does not determine evident body iron load or decompartmentalization. It is often associated with early onset of bilateral cataract, likely caused by protein aggregation in the lens.20 These data are in partial contradiction with indications that HeLa cells transfected with L-ferritin cDNA have a reduced reactive oxygen species (ROS) production21 and that antisense L-chain inhibition expanded the labile iron pool similarly to H-ferritin inhibition.14,15 Thus, L-chain may participate in the regulation of cellular iron availability, because it was proposed by the early findings that the L/H ratio is strictly regulated in a tissue-specific manner22 and that it changes in iron-loaded organs.23
Here we report the use of small interfering RNAs (siRNAs) as a recent tool to down-regulate ferritin levels in HeLa cells more efficiently than antisense DNAs and evaluate their effects in comparison with those obtained by up-regulating the ferritin via cDNA transfection. The results show that (1) the modifications of L-ferritin levels do not affect iron availability in HeLa cells but positively affect cell proliferation rates in an iron-independent manner; (2) the transient down-regulation of H-ferritin modifies cellular iron availability and resistance to oxidative damage, as expected; and (3) the stable reduction of H-ferritin in HeLa cell clones transfected with siRNAs did not increase cell iron availability but made cells less resistant to iron supplementation and chelation, consistent with the notion that H-ferritin acts as an iron buffer.
Materials and methods
Production of siRNAs
To produce double-stranded siRNAs by in vitro transcription we followed the procedure described by Donze and Picard.24 Briefly, we produced desoxy-oligonucleotides in which 20 nucleotides (nt) of sense or antisense sequence was flanked at 3′ by the TATAGTGAGTCGTATTA sequence, complementary to T7 promoter, and at 5′ by an overhang of 2 A. The sense sequences were the following: H-siRNA1 (at +29 nt from start codon), GCCAGAACTACCACCAGGAC; H-siRNA2 (at +129 nt), GTGGCTTTGAAGAACTTTGC; H-siRNA3 (at +333 nt), GAATCAGTCACTACTGGAAC; H-siRNA4 (at +501 nt), GGAATATCTCTTTGACAAGC; L-siRNA1 (at +17 nt from start codon), GTCAGAATTATTCCACCGAC; L-siRNA2 (at +259 nt), GAAGATGAGTGGGGTAAAAC; L-siRNA3 (at +310 nt), GAGAAAAAGCTGAACCAGGC; L-siRNA4 (at +380 nt), GTGACTTCCTGGAGACTCAC. All the sequences terminated with G and C, necessary for T7 polymerase activity. The oligonucleotides were annealed with the primer for T7 polymerase, and the enzyme was added. The synthesized RNAs were annealed, analyzed on polyacrylamide electrophoresis, and used for transfection. The construct for stable transfection encoded a short-hairpin RNA and was obtained by cloning an oligonucleotide made of the sense and antisense H-siRNA3 sequences separated by a small loop sequence into the pSilencer 1.0-U6 vector (Ambion, Huntingdon, United Kingdom), which contains the murine U6 promoter. The DNA insert includes 4 nucleotide overhangs complementary to the ApaI and EcoRI restriction sites. The construct was named pS-H3.
HeLa cell transfection
Different amounts of double-stranded (ds)–siRNAs (0.4 to 3.2 μg) produced by in vitro transcription were transfected in 3.5 × 105 HeLa cells (Clontech, Palo Alto, CA) with Lipofectamine 2000 (Invitrogen, Paisley, United Kingdom) (1:2.5 ratio DNA/Lipofectamine) following manufacturer's instruction. The cells were grown in Dulbecco modified Eagle medium (DMEM) (Life Technologies, Invitrogen), 10% fetal bovine serum (Clontech), 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM l-glutamine, and then were harvested and analyzed. To verify transfection efficiency, HeLa cells were transfected under the same conditions with control (nonsilencing) ds-siRNA (Qiagen-Xeragon, Germantown, MD) labeled with rhodamine. After transfection, the cells were fixed in 3% paraformaldehyde and inspected under the fluorescent microscope. Costaining with Hoechst dye showed an efficiency of more than 95% (not shown). To produce stable clones, HeLa cells were transfected with 1.8 μg of the plasmid pS-H3 together with 0.5 μg of the pTK-Hyg plasmid (5:1 molar ratio) (Clontech) by Lipofectamine transfection. The colonies were grown in DMEM with hygromycin D (Clontech) (150 μg/mL), and the ones surviving after 25 days were analyzed for H-ferritin content by enzyme-linked immunosorbent assay (ELISA).
Cell culture and analysis
The stable HeLa cell H- and L-clones that express ferritin H– and L–wild-type under the control of the tetracycline promoter have been described.13,17 In the presence of 2 ng/mL doxycycline (Sigma, St Louis, MO) (dox+) the exogenous ferritin expression was repressed, whereas in the absence it was induced (dox-). To evaluate the cell proliferation rate, the HeLa cells were grown for 40 hours, counted, and 104 cells were plated in 96-well culture plates (Greiner, Longwood, FL). They were grown for another 18 hours at 37°C in 0.1 mL medium, and then 10 μL methyl-thiazol-tetrazolium (MTT) solution (5 mg/mL in phosphate-buffered saline) was added, and after 3 hours the developed color was read at 570 mm,13 following manufacturer's instruction (Sigma). For measurement of 3H-thymidine uptake, 4 × 104 cells were plated in 48-well culture plates (Greiner) and incubated with 1 μCi (37 KBq) methyl-3H-thymidine per well for 18 hours at 37°C. The cells were washed 2 times with phosphate-buffered saline and 3 times with ice-cold 10% trichloroacetic acid and incubated with 1 M NaOH for 30 minutes at 37°C. Incorporated 3H was determined using a liquid scintillation counter (Packard, Palo Alto, CA).25
The analysis of the cytotoxic effect of H2O2 was performed as described by Cozzi et al.13 Briefly, 104 cells were grown for 18 hours and then washed and treated with different concentrations of H2O2 for 2 hours in serum-free medium. The plates were washed twice, and cellular vitality was measured by incubation with MTT for 3 hours, as above. In the experiments in which the effect of ferric ammonium citrate (FAC) and desferrioxamine (DFO) was analyzed, 104 or 2 × 105 cells were plated in 96- or 6-well culture plates with 1 mM FAC or 0.1 mM DFO and grown for 18 hours and 40 hours, respectively. Then the cells were washed with phosphate-buffered saline and further incubated with MTT for 3 hours, as above, or the cell mortality was monitored by trypan blue exclusion method.
Metabolic labeling and immunoprecipitation
HeLa cells were metabolically labeled as described by Corsi et al.11 Briefly, 5 × 105 cells were grown for 1 hour in DMEM without methionine and cysteine (ICN Biomedicals, Costa Mesa, CA), 0.5% bovine serum albumin, and then added with 50 μCi (1850 KBq)/mL 35S-methionine (ICN Biomedicals) and grown for another 18 hours. After lysis of the cells with 0.5 mL of lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]–HCl pH 8.0, 200 mM LiCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 0.5% Nonidet P-40), the total radioactivity associated to the soluble proteins was determined by trichloroacetic acid precipitation. Cytosolic lysates (4 × 106 cpm) were immunoprecipitated as described by Corsi et al11 by first adding antiferritin antibodies and then protein A–Sepharose 50% vol/vol (Sigma). The immunobeads were collected, loaded on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and exposed to autoradiography.
Biochemical and immunologic methods
Ferritin levels were determined with ELISA using the monoclonal antibody rH02 (specific for the H-ferritin) or L03 (specific for the L-ferritin) calibrated on the corresponding recombinant homopolymer.26 Protein concentration was evaluated by the bicinchoninic acid (BCA) method (Pierce, Cheshire, United Kingdom) calibrated on bovine serum albumin. In immunoblot experiments 20 μg of soluble proteins was loaded on 12% SDS-PAGE. After transfer, the nitrocellulose filters were incubated with mouse anti-TfR1 antibody (1:500) (Zymed, St Francisco, CA), with rabbit anti–poly(adenosine diphosphate-ribose) polymerase (anti-PARP) p85 fragment (1:500) (Promega, Madison, WI), or with mouse anti–H-ferritin subunit (HS59) (1:500) 27 followed by secondary peroxidase-labeled antibody (Sigma). Bound activity was revealed by enhanced chemiluminescence (ECL) (Amersham, Uppsala, Sweden). IRP activity was evaluated by electromobility shift assays as described by Corsi et al.11 Cells (2 × 105) were grown for 18 hours, lysed, and 2-μg samples of soluble proteins were incubated with a molar excess of 32P-labeled H-ferritin IRE probe, RNase T1, and heparin in the presence or the absence of 2% 2-mercaptoethanol. RNA protein complexes were separated on 6% nondenaturing PAGE and exposed to autoradiography. The intensity of IRE-IRP band was quantified by densitometry in the linear range.
Results
Production and analysis of siRNAs for H- and L-ferritins
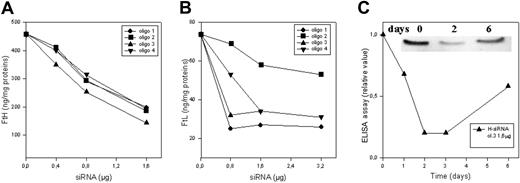
We produced double-stranded (ds)–siRNAs targeting 4 different regions of the coding regions of H-mRNA and of L-ferritin mRNA. They were chosen manually for being 19 nt long, having 5′G and 3′C termini, for a GC content less than 60%, and for being as separated as possible within the coding sequence. In basic local alignment sequence tool (BLAST) analysis on human genome, the sequences hit only the corresponding ferritin gene and some ferritin pseudogenes but not the mitochondrial ferritin sequence. The RNAs were transcribed in vitro, annealed, and used for transfection as described by Donze and Picard.24 HeLa cells were transfected with various amounts of ds-siRNAs, harvested 40 hours later, and H- and L-ferritin content was analyzed by specific ELISA assays. The 4 ds-siRNAs for H-ferritin reduced H-ferritin levels in a dose-dependent manner to about 20% to 25% of the background level (Figure 1A), and this was accompanied by a dose-dependent increase of L-ferritin to about 3-fold over background (Table 1). The 4 ds-siRNAs for L-ferritin reduced L-ferritin level in a dose-dependent manner; one was less effective (up to 60% reduction) while the other 3 behaved similarly with a reduction to about 20% to 25% of the background (Figure 1B) without affecting H-ferritin level (Table 1). Using at least 1.6 μg siRNAs, H-ferritin level decreased from about 390 ng/mg protein to about 100 ng/mg and L-ferritin level from about 75 to about 16 ng/mg (Table 1). Maximum inhibition occurred between days 2 and 3 after transfection, and then it increased (Figure 1C), as already reported for other proteins.28 The use of fluorescent-labeled control (nonsilencing) siRNA showed that our conditions yielded a transfection efficiency above 95%, in agreement with reported data.29 The results indicate that siRNAs are effective in reducing ferritin content and that they are specific because they down-regulate only the targeted ferritins.
Ferritin repression in HeLa cells transfected with siRNAs. HeLa cells were transfected with various amounts of 4 different H-siRNAs (A) and 4 different L-siRNAs (B) (described in “Materials and methods”), harvested 40 hours later, and analyzed for ferritin content by ELISA specific for H-ferritin (A) and L-ferritin (B). (C) Time course analysis of ferritin expression of HeLa cells transfected with 1.6 μg H-siRNA (oligo 3). As shown in the insert, the cell homogenates (20 μg total proteins) were loaded on 12% SDS-PAGE for the indicated time and immunoblotted with a monoclonal anti–H-ferritin subunit antibody (HS-59). Representative of 3 independent experiments with similar results.
Ferritin repression in HeLa cells transfected with siRNAs. HeLa cells were transfected with various amounts of 4 different H-siRNAs (A) and 4 different L-siRNAs (B) (described in “Materials and methods”), harvested 40 hours later, and analyzed for ferritin content by ELISA specific for H-ferritin (A) and L-ferritin (B). (C) Time course analysis of ferritin expression of HeLa cells transfected with 1.6 μg H-siRNA (oligo 3). As shown in the insert, the cell homogenates (20 μg total proteins) were loaded on 12% SDS-PAGE for the indicated time and immunoblotted with a monoclonal anti–H-ferritin subunit antibody (HS-59). Representative of 3 independent experiments with similar results.
Ferritin content in transfected HeLa cells
Cells . | H-ferritin, ng/mg total protein . | L-ferritin, ng/mg total protein . |
|---|---|---|
| HeLa control | 393 ± 57 | 74 ± 6 |
| HeLa + H-siRNA | 102 ± 36 | 222 ± 4 |
| HeLa + L-siRNA | 384 ± 21 | 16 ± 6 |
| HeLa + H/L-siRNA | 95 ± 5 | 24 ± 3 |
| Clone ΔH1 | 98 ± 44 | 97 ± 7 |
| L-clone dox+ | 438 ± 40 | 82 ± 5 |
| L-clone dox– | 434 ± 34 | 215 ± 55 |
| HeLa control + FAC 1 mM | 2225 ± 270 | 600 ± 31 |
| Clone ΔH1 + FAC 1 mM | 310 ± 20 | 1540 ± 63 |
Cells . | H-ferritin, ng/mg total protein . | L-ferritin, ng/mg total protein . |
|---|---|---|
| HeLa control | 393 ± 57 | 74 ± 6 |
| HeLa + H-siRNA | 102 ± 36 | 222 ± 4 |
| HeLa + L-siRNA | 384 ± 21 | 16 ± 6 |
| HeLa + H/L-siRNA | 95 ± 5 | 24 ± 3 |
| Clone ΔH1 | 98 ± 44 | 97 ± 7 |
| L-clone dox+ | 438 ± 40 | 82 ± 5 |
| L-clone dox– | 434 ± 34 | 215 ± 55 |
| HeLa control + FAC 1 mM | 2225 ± 270 | 600 ± 31 |
| Clone ΔH1 + FAC 1 mM | 310 ± 20 | 1540 ± 63 |
H-and L-ferritin levels are shown in the siRNA-transfected HeLa cells and control cells, in the clone ΔH1, and in the repressed (dox+) and derepressed (dox–) L-clone. The HeLa cells and the clone ΔH1 were evaluated also after treatment with 1 mM FAC for 18 hours. Ferritin content was determined using specific ELISA assays. Data are expressed as nanograms of ferritin per milligram of total proteins. Means and SD from at least 3 independent experiments are shown.
Modulation of L-ferritin expression
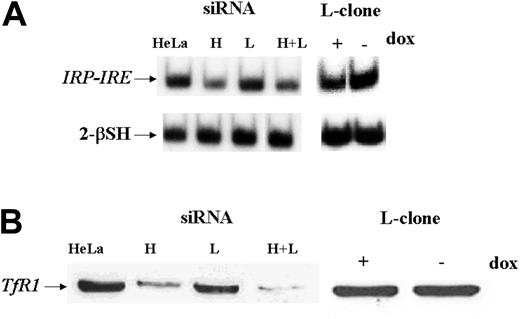
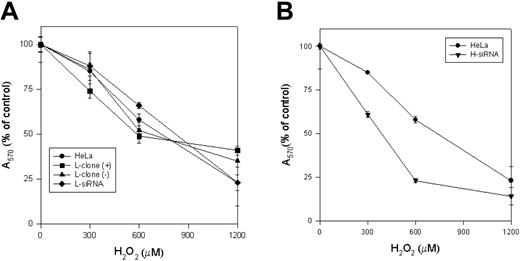
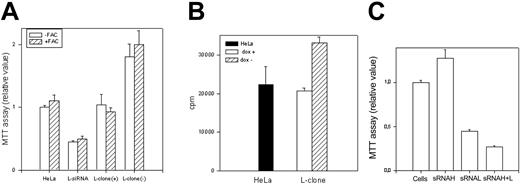
As an approach to study L-ferritin biologic functions, we used L-siRNA1 and a HeLa cell clone (L-clone) transfected with L-ferritin cDNA under the control of the inducible tetracycline promoter.17 In the presence of the suppressor doxycycline, L-ferritin level was analogous to that of the untransfected parent cells, while after withdrawal of it, L-ferritin level increased about 3-fold (Table 1). Thus, the transfections with L-siRNA and with L-cDNA modified L-ferritin from 16 to 215 ng/mg of total proteins, more than 13-fold range. However, H-ferritin remained at a level of about 400 ng/mg proteins after both modifications, as in the nontransfected parent cells (Table 1). Also, IRP activity, determined by electromobility shift assay (EMSA) (Figure 2A), and TfR1 level, determined by blotting (Figure 2B), were analogous in the control, L-siRNA–transfected cells and in the induced L-clone. Thus, the variation of L-ferritin content did not modify iron availability indices. Next, we analyzed cell resistance to H2O2 and cellular proliferation rates, which are strongly affected by H-ferritin expression.13,15 MTT assay showed that the viability of cells exposed to H2O2 over the range 0 to 1200 μM was analogous in the control, L-siRNA–transfected cells and in the induced L-clone (Figure 3A). In contrast, cell proliferation rate, measured by MTT assay (Figure 4A) or by thymidine incorporation (Figure 4B), was about 1.5-fold higher in the induced L-clone than in the control. Opposite, L-siRNA transfection caused a decrease of proliferation rate to about 50% (Figure 4A). The observation was confirmed by an alternative method: Total cellular protein after growing 105 cells for 18 hours was about 50% higher in the induced L-clone than in the controls, while it was about 40% lower in the L-siRNA–transfected cells. In addition, we found that transfection with H-siRNA alone caused an about 20% increase of MTT assay, while the cotransfection with H- and L-siRNAs caused an about 80% reduction of it, which is even higher than that caused by L-siRNA alone (Figure 4C). The inhibitory proliferation effect of H-ferritin overexpression is iron mediated and is abolished by prolonged iron supplementation.13 Thus, we incubated the cells with 0.1 mM FAC for 24 hours before MTT assay, as before.13 However, the treatment did not modify cell proliferation in the control, L-siRNA–transfected, uninduced and induced L-clone (Figure 4A). In conclusion, we found that L-ferritin level does not affect any of the major parameters of iron availability but regulates cell proliferation in a direction and with a mechanism that is different from that caused by the variation of H-ferritin levels.
IRP activity and TfR1 expression in L-clone and HeLa cells transfected with siRNAs. HeLa cells were transfected with 1.6 μg H-siRNA (H), L-siRNA (L), or the 2 together (H+L) and analyzed 40 hours later, while L-clone was grown in the presence (dox+) or absence (dox-) of doxycycline for 7 days before analysis. (A) Samples of 2 μg of total soluble protein extracts were incubated with a 32P-labeled IRE H-ferritin probe in the absence or presence of 2% 2-mercaptoethanol (2-β–SH), and the RNA-protein complexes were separated on nondenaturing gel electrophoresis and exposed to autoradiography. (B) Thirty micrograms of cellular extracts were loaded on 12% SDS-PAGE, blotted with a mouse antihuman transferrin receptor antibody (α-TfR1), and developed by ECL. Representative of 3 independent experiments with similar results.
IRP activity and TfR1 expression in L-clone and HeLa cells transfected with siRNAs. HeLa cells were transfected with 1.6 μg H-siRNA (H), L-siRNA (L), or the 2 together (H+L) and analyzed 40 hours later, while L-clone was grown in the presence (dox+) or absence (dox-) of doxycycline for 7 days before analysis. (A) Samples of 2 μg of total soluble protein extracts were incubated with a 32P-labeled IRE H-ferritin probe in the absence or presence of 2% 2-mercaptoethanol (2-β–SH), and the RNA-protein complexes were separated on nondenaturing gel electrophoresis and exposed to autoradiography. (B) Thirty micrograms of cellular extracts were loaded on 12% SDS-PAGE, blotted with a mouse antihuman transferrin receptor antibody (α-TfR1), and developed by ECL. Representative of 3 independent experiments with similar results.
Effect of H2O2. The L-clone and HeLa cells transfected with L-siRNA (A) and H-siRNA (B) for 40 hours were plated (104 for well) and incubated for 2 hours with the indicated concentration of H2O2 in serum free-medium. After washing, the cellular vitality was measured by MTT assay. Values were plotted as percentage of the absorbance of the untreated control cells. Data are the means ± SD of 3 independent experiments, each in 8 replicates.
Effect of H2O2. The L-clone and HeLa cells transfected with L-siRNA (A) and H-siRNA (B) for 40 hours were plated (104 for well) and incubated for 2 hours with the indicated concentration of H2O2 in serum free-medium. After washing, the cellular vitality was measured by MTT assay. Values were plotted as percentage of the absorbance of the untreated control cells. Data are the means ± SD of 3 independent experiments, each in 8 replicates.
Cell growth. The L-clone (dox+ and dox-) and HeLa cells transfected with the L-siRNA were grown in absence or presence of 0.1 mM ferric ammonium citrate (-/+FAC) for 24 hours. (A) Cell proliferation was evaluated by MTT assay (104 cells); the values of dox- cells were statistically different from those of dox+ cells (P < .001, n = 24). (B) Cell proliferation was evaluated by 3H-thymidine incorporation analysis (4 × 104 cells); the values of dox- cells were statistically different from control (P < .001, n = 12). (C) MTT assay of cells transiently transfected with H-siRNA, L-siRNA, and the 2 together. Data are the means ± SD of 3 independent experiments.
Cell growth. The L-clone (dox+ and dox-) and HeLa cells transfected with the L-siRNA were grown in absence or presence of 0.1 mM ferric ammonium citrate (-/+FAC) for 24 hours. (A) Cell proliferation was evaluated by MTT assay (104 cells); the values of dox- cells were statistically different from those of dox+ cells (P < .001, n = 24). (B) Cell proliferation was evaluated by 3H-thymidine incorporation analysis (4 × 104 cells); the values of dox- cells were statistically different from control (P < .001, n = 12). (C) MTT assay of cells transiently transfected with H-siRNA, L-siRNA, and the 2 together. Data are the means ± SD of 3 independent experiments.
Modulation of H-ferritin expression
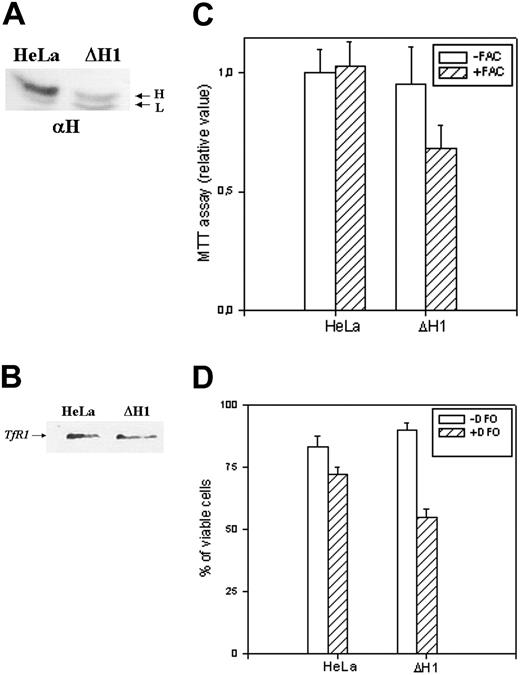
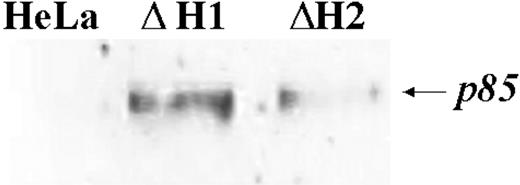
We first studied the properties of HeLa cells transiently transfected with H-siRNAs. H-ferritin inhibition was accompanied by about 3-fold increases of L-ferritin levels (Table 1), an about 50% reduction of IRP activity (Figure 2A), and an about 80% decrease of TfR1 level (Figure 2B). The additional cotransfection with L-siRNA1 inhibited L-ferritin (Table 1), modified only marginally IRP activity, while further decreasing TfR1 expression (Figure 2). Next we analyzed the resistance to H2O2 by MTT assay (Figure 3B), and we found it to be largely decreased, as expected, an effect particularly evident at 600 μM H2O2, the condition in which H-ferritin overexpression was the most protective.13 Altogether the findings confirm that H-ferritin levels regulate iron availability.13,15 Then, we tried to produce clones in which H-ferritin was stably suppressed. To this aim we cloned the sequences of the H-siRNA3 in a hairpin structure into the vector pSilencer 1.0-U6 and verified that its transfection caused a significant reduction of H-ferritin (not shown). The plasmid (1.8 μg) was cotransfected with 0.5 μg pTK-Hyg plasmid (5:1 molar ratio) carrying hygromycin resistance, and clones were selected after growth in 150 μg/mL hygromycin. ELISA assay identified 26 clones with low H-ferritin levels, and 2 of them, ΔH1 and ΔH2, were chosen for further analysis. The H-ferritin concentration was reduced to about 25% of the control, as in the H-ds-siRNA–transfected cells (Table 1). Surprisingly, the stable H-ferritin reduction in ΔH1 clone was accompanied by a marginal 20% increase of L-ferritin, while the transient H-ferritin reduction caused a much higher, 3-fold, induction of L-ferritin (Table 1). ELISA assays showed analogous levels of H- and L-ferritins in the ΔH1 clone (98 and 97 ng/mg protein, respectively, Table 1), and immunoprecipitation experiments of metabolically labeled cells confirmed that ferritin in the clone was composed by equal amounts of H- and L-subunit (Figure 5A). In addition, we found that TfR1 levels (Figure 5B) and MTT proliferation rate of the clone (Figure 5C) were analogous to the values of the untransfected parent cells. Thus, the stable H-ferritin suppression did not cause the expected increase of iron cellular availability. The only abnormal biochemical finding in ΔH1 and ΔH2 clones was the spontaneous accumulation of p85 PARP peptide, a product of caspase activity considered an index of apoptosis (Figure 6). This was absent in the untransfected parent cells, although it can be strongly activated by apoptotic stimuli such as tumor necrosis factor-α (TNF-α) plus actinomycin D.17 However, cells of ΔH1 and ΔH2 clones did not show morphologic signs of apoptosis, and their proliferation rate was analogous to that of the control cells (Figure 5C).
Analysis of the stable clone. (A) Immunoprecipitation experiments on untrasfected (HeLa) and ΔH1 homogenate cells. The cells were metabolically labeled as described in “Materials and methods,” and 4 × 106 cpm of the cytosolic lysates were precipitated with saturating amounts of anti–H-ferritin antibody (αH). The precipitates were analyzed on 12% SDS-PAGE and exposed to autoradiography. Representative of 2 independent experiments with similar results. (B) Cellular extracts of untrasfected cells and ΔH1 clone were separated on 12% SDS-PAGE and blotted with an anti-TfR1 antibody. Representative of 2 independent experiments with similar results. (C) Analysis of the cells' vitality by MTT assay after growth in the presence or absence of 1 mM ferric ammonium citrate (+/-FAC) for 18 hours. (D) Cell mortality was monitored by trypan blue exclusion method after growing the cells without or with 0.1 mM DFO for 2 days. The data are expressed as percentage of the unstained cells to the number of cells counted. Data of panel C and D are means and SD of 3 independent experiments in octaplicate and triplicate, respectively.
Analysis of the stable clone. (A) Immunoprecipitation experiments on untrasfected (HeLa) and ΔH1 homogenate cells. The cells were metabolically labeled as described in “Materials and methods,” and 4 × 106 cpm of the cytosolic lysates were precipitated with saturating amounts of anti–H-ferritin antibody (αH). The precipitates were analyzed on 12% SDS-PAGE and exposed to autoradiography. Representative of 2 independent experiments with similar results. (B) Cellular extracts of untrasfected cells and ΔH1 clone were separated on 12% SDS-PAGE and blotted with an anti-TfR1 antibody. Representative of 2 independent experiments with similar results. (C) Analysis of the cells' vitality by MTT assay after growth in the presence or absence of 1 mM ferric ammonium citrate (+/-FAC) for 18 hours. (D) Cell mortality was monitored by trypan blue exclusion method after growing the cells without or with 0.1 mM DFO for 2 days. The data are expressed as percentage of the unstained cells to the number of cells counted. Data of panel C and D are means and SD of 3 independent experiments in octaplicate and triplicate, respectively.
PARP blotting. The fraction of apoptotic cells was determined by blotting analysis of cell homogenates of 2 different stable clones with anti-p85 PARP antibody after running on 12% SDS-PAGE. The H-ferritin content in the 2 clones, ΔH1 and ΔH2, are 98 and 190 ng/mg of total proteins, respectively. Representative of 2 independent experiments with similar results.
PARP blotting. The fraction of apoptotic cells was determined by blotting analysis of cell homogenates of 2 different stable clones with anti-p85 PARP antibody after running on 12% SDS-PAGE. The H-ferritin content in the 2 clones, ΔH1 and ΔH2, are 98 and 190 ng/mg of total proteins, respectively. Representative of 2 independent experiments with similar results.
To have insight on the H-ferritin role under conditions of altered iron availability, we incubated the cells with 1 mM FAC for 18 hours, conditions that are marginally toxic for HeLa cells. MTT assay showed that the treatment did not modify significantly the viability of untransfected HeLa cells, while it decreased to about 30% that of ΔH1 clone (Figure 5C). The treatment caused an increase of H- (6-fold) and L-ferritin (8-fold) in the control cells, while in ΔH1 clone the increase was of 3- and 16-fold, respectively (Table 1). Next, we incubated the cells with 0.1 mM DFO for 2 days, conditions that cause 10% mortality in untransfected HeLa cells, as monitored by trypan blue exclusion (Figure 5D). In contrast, cell mortality was about 40% in the ΔH1 clone (Figure 5D). Thus, stable H-ferritin suppression decreased cell resistance to iron excess and chelation.
Discussion
Functionality of the siRNAs
We found that 7 of 8 siRNAs analyzed were effective in reducing the expression of the targeted ferritin down to about 20% to 25% of the background level. Specificity was assessed by the lack of inhibition of the nontargeted ferritin. The same repression level was obtained with other 3 siRNAs complementary for the 5′–untranslated region (5′UTR) of L-ferritin designed to inhibit specifically the endogenous protein but not the transfectant one, which lacks the 5′UTR (not shown). A similar repression level was obtained in the stably transfected ΔH1 clone, suggesting that the effectiveness of the siRNAs reaches saturation at about 80% repression. This finding contrasts with a higher level of siRNA-dependent suppression (more than 90%) observed for other genes30 and may be related to the relative abundance and/or to the high stability of H- and L-ferritin mRNAs.31 Alternatively, siRNAs might target only the free and not the IRP-bound ferritin mRNAs.28 However, this hypothesis is not supported by the finding that iron supplementation, which decreases the IRP-bound mRNA pool, stimulated H-ferritin expression in the ΔH1 clone (about 3-fold increase) even less than in control HeLa cells (about 5-fold increase) (Table 1). Altogether the results suggest that ferritin siRNA silencing is more efficient than antisense oligonucleotide suppression, which accounted for 50% to 60% inhibition,14,15 although the data were obtained on different cells lines and a direct comparison may be difficult.
Role of L-ferritin
L-ferritin is ubiquitous in mammalian cells and is the major constituent of serum ferritin. Its role in regulating iron homeostasis and availability is unclear. To study it, we used transfection with siRNAs and cDNA to modify L-ferritin accumulation over a 13-fold range (Table 1). L-ferritin suppression did not affect the levels of the partner H-ferritin, demonstrating the specificity of L-siRNAs. More important, L-ferritin modulation in either direction did not affect TfR1 levels, IRP activity (Table 1 and Figure 2), and cell resistance to H2O2 damage (Figure 3A). The simplest interpretation of these findings is that L-ferritin levels do not modify the size of the labile iron pool, which is known to regulate the 3 proteins and the resistance to H2O2.13 A minor additional repression of TfR1 was observed when L-ferritin was suppressed together with H-ferritin (Figure 2B), and this is consistent with in vitro evidence that L-chain assists H-chain in increasing ferritin iron incorporation.10,13 In fact, it was already reported that the corepression of H- and L-ferritins by antisense oligonucleotides produced stronger cellular effects than the repression by single H-antisense.15 The lack of an evident role of L-ferritin in the regulation of iron metabolism, indicated by our cellular models, is in agreement with the clinical data showing that the systemic overexpression of L-ferritin in hereditary hyperferritinemia cataract syndrome has no evident effect on iron homeostasis.32 It is also consistent with recent findings that the constitutive down-regulation of L-ferritin in a subject with a disabling mutation in the L-chain start codon had no evident effect on iron metabolism.33 However, a rare and dominant disorder named neuroferritinopathy was recently described.34 It is associated with an adenine insertion at position 460-461 in the coding region of L-ferritin, and the affected subjects show late-onset movement disorders and iron deposition in the brain basal ganglia. This suggests that changes in L-ferritin modify iron metabolism in cells of the central nervous system (CNS), although this is more likely caused by abnormal properties of the mutant protein than by haploinsufficiency.
A new and unexpected finding was that the HeLa cell proliferation rate was positively modulated by L-ferritin levels. It increased about 1.5-fold in the L-clone and was halved after L-siRNA silencing (Figure 4A-B). This is intriguing because the H-ferritin level also affects cell proliferation, but it does it in the opposite direction, possibly by limiting iron availability to cyclin-dependent kinases or enzymes of DNA synthesis.13,15 In fact, this H-ferritin activity was abolished by inactivating its ferroxidase center or by supplementing the cells with iron.13 On the other hand, L-ferritin has no ferroxidase activity, did not modify cellular iron availability, and its proproliferative effect was not modified by cellular iron supplementation. Thus, H- and L-ferritins act on cell proliferation with different mechanisms. The finding that the 2 ferritins have opposite effects may help to explain why iron supplementation, which induces both of them, did not modify cell proliferation in HeLa cells (eg, the control of Figure 5C). However, one may expect that the 2 act in concert when the level of only 1 ferritin type is modified. For example, transient transfection with H-siRNA increased by about 20% MTT proliferation (Figure 4C), an effect that we mainly attributed to the indirect L-chain up-regulation. In fact, the cotransfection with L-siRNA not only completely abolished it but also reduced MTT proliferation to levels even lower than those obtained with L-siRNA (Figure 4C). This suggests that L-ferritin down-regulation may contribute to the repression of cell proliferation caused by H-ferritin up-regulation.13 This finding may be biologically relevant, because TNF-α and other cytokines modulate specifically H-ferritin,35 and L-ferritin expression varies during monocyte differentiation.36 One must remember that a stimulatory effect on cell proliferation following L-rich ferritin addition to cultured cells has been observed before.37-39 Iron incorporation is the only L-ferritin function so far established by in vitro study,3 but this does not seem to be involved in the proproliferation activity. This suggests that L-ferritin affects some cellular pathways that remain to be identified.
Role of H-ferritin
The use of siRNAs to transiently repress H-ferritin confirmed the data previously obtained by up-regulating H-ferritin with cDNA transfection in HeLa cells11 and were in large agreement with those obtained by suppressing the protein with antisense desoxy-oligonucleotides in K562 and in HEK-293 cells.14,37 Namely, H-ferritin level through its ferroxidase activity regulates primarily cell iron availability, and this has secondary effects on the resistance to the oxidative damage induced by H2O2.13 An interesting advantage of siRNAs is the possibility to down-regulate various genes at the same time: we demonstrated it on H- and L-ferritins, and preliminary data indicate that it can be extended to TfR1 and other proteins of iron metabolism. So, this technology may be used to pinpoint the specific role of various proteins in regulating iron homeostasis. We previously observed that transient and stable H-ferritin cDNA transfections produce results dramatically different11,13 ; therefore, we worked to produce cell lines stably transfected with H-siRNA. We succeeded in obtaining such clones, and we analyzed the ones with the lowest level of H-ferritin. Surprisingly, the major parameters of iron availability—L-ferritin (Table 1) and TfR1 levels (Figure 5B)—were essentially unchanged in the clones compared with control. This contrasted with the transiently transfected cells, where the same degree of H-ferritin suppression modified the indices and increased iron availability. The finding indicates the activation of long-term mechanisms that compensate for the stable reduction of H-ferritin ferroxidase activity. The existence of such compensatory mechanisms has already been suggested by the study of cells transfected with IRP1 cDNA.40 The finding may go some way to explain why different tissues and cell lines maintain normal iron availability even with different levels of H-ferritin. For example, heterozygous Fth (+/-) mice have normal iron metabolism despite a large reduction of systemic H-ferritin level, and the only observed phenotype was an L-ferritin increase,41 compatible with that we observed in the ΔH1 clone.
A novel indication of the biologic role of H-ferritin comes from the finding that ΔH1 clone showed a reduced resistance to both iron supplementation and chelation (Figure 5C-D). This might be due to the modification of ferritin stability caused by altered iron availability.42 Alternatively, and more likely, it can be interpreted as H-ferritin acting as an iron buffer: low concentrations are sufficient to regulate iron availability under stationary conditions, but they reduce the cell capacity to respond to fast modifications of iron availability. This may occur because the ferroxidase activity of H-chain becomes insufficient to incorporate and detoxify the iron in excess and because the reduced ferritin level cannot supply sufficient iron in response to chelation. Based on the available data, we postulate that cellular H-ferritin level is regulated on the basis of the needs to respond to fast modifications of iron homeostasis and should be maintained below a (unphysiologic) threshold above which apparent iron deficiency occurs, as in the H-clone.13 The hypothesis predicts that erythroid cells and macrophages, which are exposed to fast iron influxes, are rich in H-ferritin, as it occurs. However, other factors should be taken into account; for example, low levels of H-ferritin seem to make ΔH1 cells more sensitive to apoptosis, indicated by the signs of activated caspases and by the higher killing effects of chelators, which are proapoptotic agents.43
In conclusion, present analysis shows that L-ferritin levels do not affect iron availability in HeLa cells, while H-ferritin levels regulate and buffer cellular iron availability. In addition, the data suggest a role for the L-ferritin in promoting cell growth, which needs additional work to be clarified.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-06-1842.
Supported in part by Telethon-Italy grant GP-0075Y01 (S.L.), CNR-Agenzia 2000 (P.A., S.L.), MIUR-Cofin grants (P.A.), and MIUR-Firb grants (P.A., S.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal