Abstract
Phospholipase D (PLD) regulates the polymorphonuclear leukocyte (PMN) functions of phagocytosis, degranulation, and oxidant production. Ceramide inhibition of PLD suppresses PMN function. In streptolysin O–permeabilized PMNs, PLD was directly activated by guanosine 5′-[gamma-thio]triphosphate (GTPγS) stimulation of adenosine diphosphate (ADP)–ribosylation factor (ARF) and Rho, stimulating release of lactoferrin from specific granules of permeabilized PMNs; PLD activation and degranulation were inhibited by C2-ceramide but not dihydro-C2-ceramide. To investigate the mechanism of ceramide's inhibitory effect on PLD, we used a cell-free system to examine PLD activity and translocation from cytosol to plasma membrane of ARF, protein kinase C (PKC)α and β, and RhoA, all of which can activate PLD. GTPγS-activated cytosol stimulated PLD activity and translocation of ARF, PKCα and β, and RhoA when recombined with cell membranes. Prior incubation of PMNs with 10 μM C2-ceramide inhibited PLD activity and RhoA translocation, but not ARF1, ARF6, PKCα, or PKCβ translocation. However, in intact PMNs stimulated with N-formyl-1-methionyl-1-leucyl-1-phenylalamine (FMLP) or permeabilized PMNs stimulated with GTPγS, C2-ceramide did not inhibit RhoA translocation. Exogenous RhoA did not restore ceramide-inhibited PLD activity but bound to membranes despite ceramide treatment. These observations suggest that, although ceramide may affect RhoA in some systems, ceramide inhibits PLD through another mechanism, perhaps related to the ability of ceramide to inhibit phosphatidylinositol-bisphosphate (PIP2) interaction with PLD.
Introduction
Polymorphonuclear leukocytes (PMNs) serve an important role in host defense by ingestion (phagocytosis) and destruction of microbes. Destruction is accomplished by release of reactive oxygen intermediates and granule enzymes to the phagosome. We and others have demonstrated that PMN phagocytosis, degranulation, and oxidant production are regulated by phospholipase D (PLD).1-7 PLD produces phosphatidic acid (PA) which may act directly as a second messenger or be converted to diacylglycerol (DAG) by phosphatidate phosphohydrolase. DAG activates protein kinase C (PKC)8 and may contribute to granule-membrane fusion.9 Two mammalian PLDs, PLD1 and PLD2, have been described10 ; however, recent PMN studies have only begun to distinguish between the 2 isoforms. PLD1 activation is dependent on the small guanosine triphosphateases (GTPases) adenosine diphosphate (ADP)–ribosylation factor (ARF) and RhoA, one or more PKC isoforms, and phosphatidylinositol-bisphosphate (PIP2), whereas PLD2 activity appears not to involve the GTPases but is primarily stimulated by PIP2.10 Activation of PLD in PMN and HL-60 cells is dependent on ARF and RhoA,11,12 suggesting that PLD1 is the primary isozyme being measured in previous PMN studies. Both ARF1 and ARF6 have been implicated in PLD activation in phagocytic cells.13,14
Sphingolipid signaling is involved in cell growth, differentiation, and apoptosis.15 During PMN phagocytosis, plasma membrane-associated neutral sphingomyelinase is activated, resulting in accumulation of ceramide within the cell.3,16 Significant ceramide accumulation occurs concurrent with a decline in the rate of phagocytosis, suggesting that ceramide is a negative regulator of PMN function. The exogenous addition of the cell-permeable analog C2-ceramide has been shown to inhibit phagocytosis, degranulation, and oxidant production in PMNs through its inhibition of PLD activity.1,3 The inhibition of PLD by ceramide is well established17-19 ; however, the mechanism of inhibition has not been defined. Others have found C2-ceramide to inhibit ARF and RhoA translocation to the plasma membrane in HL-60 cells.19 Other lipids found to inhibit PLD include the polyisoprenol phosphate, presqualene diphosphate, which directly inhibits PLD1b and superoxide generation,20 and certain lysophospholipids which inhibit rat brain PLD.21
In this study we hypothesized that direct activation of PLD by way of guanosine 5′-[gamma-thio]triphosphate (GTPγS) mobilization of ARF and RhoA would allow evaluation of ceramide effects that could not be observed with receptor activation. We used 2 methods to bypass receptor activation of PLD, streptolysin-O permeabilization and a cell-free system. By using each method we could activate PLD and inhibit activation with C2-ceramide. In permeabilized PMNs we stimulated degranulation with GTPγS through the same pathway.
Patients, materials, and methods
Materials
D-erythro-C2-ceramide, D-erythro-C6-ceramide, and D-erythro-dihydro-C2-ceramide were purchased from Matreya (Pleasant Gap, PA). Antibodies against lactoferrin were a gift from Dr Niels Borregaard, State University Hospital, Copenhagen, Denmark. Antibodies against ARF1 and ARF6 were kindly provided by Dr Sylvain Bourgoin, Université Laval, Québec, Canada. The antibody against RhoA was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Isozyme-specific PKC antibodies were obtained from BD Transduction Laboratories, San Diego, CA. Recombinant RhoA was purchased from Calbiochem (San Diego, CA).
Cells
PMNs were isolated from peripheral venous blood from healthy volunteers as previously described.3 Volunteers gave informed consent according to the Helsinki protocol. Data reported herein are pooled from experiments using several different donors. For experiments in which cells were disrupted, PMNs were pretreated with 5 mM diisopropyl fluorophosphate on ice for 5 minutes, then washed 3 times with phosphate-buffered saline (PBS).
Lactoferrin release
PMNs were first incubated with the indicated concentrations of lipids for 30 minutes at 22°C. In later experiments it was determined that preincubation was not necessary for inhibition when PMNs were permeabilized, so ceramide was added along with the other reagents. PMNs (20 × 106 cells/mL final) in Dulbecco phosphate buffer (50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 100 mM KCl, 20 mM NaCl, 2 mM MgCl2, 1 mM EGTA (ethyleneglycoltetraacetic acid), and 1 g/L dextrose) with 1 mg/mL bovine serum albumin (BSA) were added to tubes containing (final concentrations) 75 U/mL streptolysin O (Sigma, St Louis, MO), GTPγS as indicated in Figure 1, 1 mM adenosine triphosphate (ATP), and 3 μM Ca++. Samples were incubated for 10 minutes at 37°C, then placed on ice 3 minutes. PMNs were removed by centrifugation, and supernatants were assayed for lactoferrin by enzymelinked immunosorbent assay (ELISA) as previously described.22 All data were analyzed using 2-sided Student t tests.
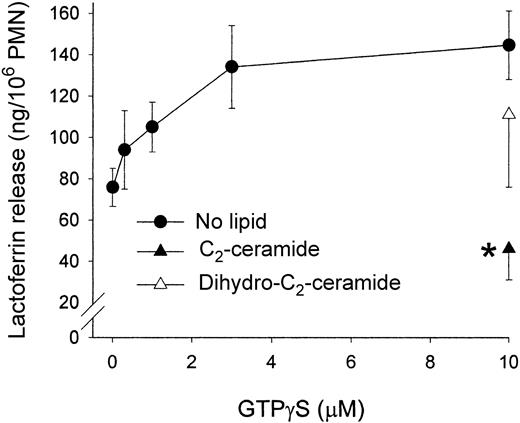
GTPγS stimulation of lactoferrin release from streptolysin O–permeabilized PMNs: inhibition by C2-ceramide. PMNs were first incubated with 50 μM lipid, then added to tubes containing (final concentrations) 75 U/mL streptolysin O, GTPγS as indicated, 1 mM ATP, and 3 μM Ca++. Samples were incubated for 10 minutes at 37°C. PMNs were removed by centrifugation, and supernatants were assayed for lactoferrin by ELISA. Data shown are the mean ± SEM of 4 experiments. *Significantly different from stimulated control; P < .001; dihydro-C2-ceramide not significantly different from control at P = .05.
GTPγS stimulation of lactoferrin release from streptolysin O–permeabilized PMNs: inhibition by C2-ceramide. PMNs were first incubated with 50 μM lipid, then added to tubes containing (final concentrations) 75 U/mL streptolysin O, GTPγS as indicated, 1 mM ATP, and 3 μM Ca++. Samples were incubated for 10 minutes at 37°C. PMNs were removed by centrifugation, and supernatants were assayed for lactoferrin by ELISA. Data shown are the mean ± SEM of 4 experiments. *Significantly different from stimulated control; P < .001; dihydro-C2-ceramide not significantly different from control at P = .05.
PLD activity
PMNs were first labeled with 1-O-[3H]-octadecyl-sn-glycero-3 phosphocholine ([3H]-lyso-PAF) (Amersham, Arlington Heights, IL) as previously described.3 Incubations were carried out as described for lactoferrin experiments except for the addition of 1% ethanol for 5 minutes at 37°C prior to the stimulation/permeabilization step. Cell pellets were extracted according to the method of Van Veldhoven and Bell.23 Lipids were separated in a thin-layer chromatography (TLC) system as previously described,2 and the plate was exposed to film. Radioactive phosphatidylethanol bands were scraped for scintillation counting.
PLD activity in cell-free system
PMNs were first [3H]-labeled as described in “PLD activity.” PMNs (2 × 106/mL) were incubated with 10 μM C2-ceramide, dihydro-C2-ceramide, or no lipid (control cells) for 30 minutes at 22°C. Cells were then suspended at 1 × 108/mL in disruption buffer (100 mM KCl, 3 mM NaCl, 10 mM piperazine diethanesulfonic acid [pH 7.0], 3.5 mM MgCl2, 1 mM PMSF (phenylmethylsulfonyl fluoride), 100 μg/mL soybean trypsin inhibitor, 10 μM pepstatin, and 10 μg/mL each of aprotinin and leupeptin) and probe sonicated twice for 15 seconds. Cytosol and plasma membrane fractions were separated as described previously.1 Briefly, samples were ultracentrifuged over discontinuous 15% and 35% sucrose gradients. Plasma membrane fractions were taken from the 15% 35% interface, then pelleted. Membranes were homogenized and assayed for protein with a bicinchoninic acid (BCA) assay. Equal amounts of membrane protein were used for each sample, and cytosol from only control cells was used. Before activation, samples were incubated with 1% ethanol. In some experiments, recombinant RhoA was then added to the cytosol. Cytosol was activated with 10 μM GTPγS (5 minutes, 37°C) then combined with membrane protein isolated from controls or cells pretreated with lipid (10 minutes, 37°C). Samples were extracted and TLC was performed as described in “PLD activity.”
Protein translocation in cell-free system
Aliquots were taken from samples in PLD experiments in the reconstituted system. These aliquots were ultracentrifuged, and the pellets were solubilized in sample buffer to run 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred, and Western blots were performed with antibodies against ARF1, ARF6, PKCα and β, and RhoA as described previously.24 Scanning densitometry was performed to quantify ARF1 and RhoA.
Protein translocation in intact and permeabilized PMNs
PMNs were incubated with C2-ceramide, dihydro-C2-ceramide, or buffer for 30 minutes at 22°C. Intact cells were then incubated with 100 nM N-formyl-1-methionyl-1-leucyl-1-phenylalamine (FMLP) for 10 minutes at 37°C. Alternatively, PMNs were streptolysin O–permeabilized, then stimulated as described in “PLD activity in cell-free system” with 10 μM GTPγS for 10 minutes at 37°C. Cells were probe sonicated, and cytosol was separated as described in “PLD activity in cell-free system,” then samples were prepared for 12.5% SDS-PAGE and Western blotting with antibody against RhoA.
Measurement of C2-ceramide in cell fractions
Results
Previous studies by us and others1,3,19 have demonstrated C2-ceramide inhibition of PLD activity. However, many of these studies involved cell-free systems, wherein some functions cannot be assayed, or receptor-mediated PLD activation, in which parallel pathways may also be activated and confound the signaling model. We permeabilized PMNs with streptolysin O to introduce GTPγS and, thus, directly activate PLD and monitored PMN activation by evaluating lactoferrin release from specific granules. GTPγS stimulated the release of lactoferrin in a dose-dependent manner and reached maximum effect at a concentration of 10 μM. C2-ceramide (50 μM) significantly inhibited lactoferrin release, whereas the inactive analog dihydro-C2-ceramide did not (Figure 1). Some lactoferrin release occurred in unstimulated controls as a result of cell warming and handling, and ceramide inhibition was observed even at that low level of release. During streptolysin-O permeabilization, PMNs were at a cell density of 20 × 106/mL, in contrast to our previous studies, in which PMNs were suspended at 2 × 106/mL. In the latter studies, 10 μM C2-ceramide was adequate to inhibit degranulation.1 In the permeabilized PMNs, 50 μM C2-ceramide was required. To evaluate possible nonspecific interactions between C2-ceramide and streptolysin O, we measured C2-ceramide in membrane and cytosol fractions of intact and streptolysin O–permeabilized PMNs after incubations. Streptolysin O did not prevent ceramide association with the membrane, and ceramide concentration did not significantly affect the percentage of the total associated with the membrane (data not shown). To inhibit function, intact PMNs required preincubation with C2-ceramide,3 but permeabilized PMNs did not, suggesting that ceramide entered the cell through the pores created by the toxin.
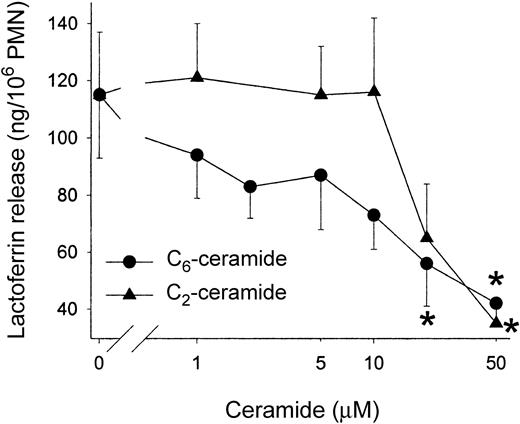
Lactoferrin release was also measured in the presence of C6-ceramide. C6-ceramide inhibited lactoferrin release more potently than C2-ceramide at the lower doses tested. At 20 μM, C6-ceramide inhibited release significantly, whereas C2-ceramide did not (Figure 2).
C6-ceramide inhibits lactoferrin release more potently than C2-ceramide. PMNs were incubated with lipids, permeabilized, and stimulated as described for Figure 1. Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .05.
C6-ceramide inhibits lactoferrin release more potently than C2-ceramide. PMNs were incubated with lipids, permeabilized, and stimulated as described for Figure 1. Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .05.
To determine whether ceramide had a direct effect on lactoferrin catalysis or regulation, we incubated PMNs with C2- or C6-ceramide and measured the total lactoferrin in detergent-lysed whole-cell preparations. Ceramide incubation had no effect on total lactoferrin present (data not shown).
PLD activity was also tested in streptolysin O–permeabilized PMNs, under the same conditions as lactoferrin release. GTPγS stimulated PLD in a dose-dependent fashion, peaking at 10 μM with a 10-fold increase over baseline (Figure 3). GTPγSat100 μM did not stimulate PLD significantly more than did 10 μM (data not shown). C2-ceramide (50 μM) significantly inhibited PLD activity, whereas dihydro-C2-ceramide treatment enhanced PLD activity. These results demonstrate that ceramide inhibits PLD activity and PLD-dependent functions in cells directly stimulated with GTPγS, as it does in receptor-stimulated PMNs.1
PLD activity in streptolysin O–permeabilized GTPγS-stimulated PMNs: inhibition by C2-ceramide. PMNs were first labeled with [3H]-lyso-PAF. Incubations were carried out as described in Figure 1 except for the addition of 1% ethanol for 5 minutes at 37°C prior to the stimulation-permeabilization step. PMNs were pelleted and extracted with chloroform and methanol. TLC was performed, and the plate was exposed to film. Radioactive bands were scraped for scintillation counting. Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .001. NS indicates not significantly different from stimulated control; dpm, disintegrations per minute; and PEt, phosphatidylethanol.
PLD activity in streptolysin O–permeabilized GTPγS-stimulated PMNs: inhibition by C2-ceramide. PMNs were first labeled with [3H]-lyso-PAF. Incubations were carried out as described in Figure 1 except for the addition of 1% ethanol for 5 minutes at 37°C prior to the stimulation-permeabilization step. PMNs were pelleted and extracted with chloroform and methanol. TLC was performed, and the plate was exposed to film. Radioactive bands were scraped for scintillation counting. Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .001. NS indicates not significantly different from stimulated control; dpm, disintegrations per minute; and PEt, phosphatidylethanol.
To further evaluate the mechanisms underlying PLD activation, we used a reconstituted cell-free system in which [3H]-lyso-PAF–labeled PMNs were treated with C2-ceramide, then disrupted, and cytosol was separated from plasma membrane. The cytosol was activated with 10 μM GTPγS, then the membrane fraction from control or ceramide-treated cells was added to the cytosol to reconstitute PLD activity. PLD activity increased about 7-fold with stimulation (Figure 4). Activity was inhibited significantly by C2-ceramide but not by dihydro-C2-ceramide, demonstrating results similar to those observed in permeabilized PMNs. As ceramide was not added after cell disruption, inhibition suggests that ceramide remains bound to cell membranes during the disruption and fractionation processes.
C2-ceramide treatment of intact PMNs: inhibition of PLD activity in a reconstituted system. PMNs were [3H]-labeled, incubated with 10 μM C2-ceramide, dihydro-C2-ceramide, or no lipid (control cells) for 30 minutes at 22°C, then disrupted by probe sonication. Cytosol and plasma membrane fractions were separated by ultracentrifugation over sucrose gradients. Samples were incubated with 1% ethanol. Cytosol from control cells only was activated with GTPγS, then combined with equal amounts of membrane protein from control or ceramide-pretreated PMNs (10 minutes, 37°C). Samples were extracted, and TLC was performed as described in “PLD activity in cell-free system.” Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .05.
C2-ceramide treatment of intact PMNs: inhibition of PLD activity in a reconstituted system. PMNs were [3H]-labeled, incubated with 10 μM C2-ceramide, dihydro-C2-ceramide, or no lipid (control cells) for 30 minutes at 22°C, then disrupted by probe sonication. Cytosol and plasma membrane fractions were separated by ultracentrifugation over sucrose gradients. Samples were incubated with 1% ethanol. Cytosol from control cells only was activated with GTPγS, then combined with equal amounts of membrane protein from control or ceramide-pretreated PMNs (10 minutes, 37°C). Samples were extracted, and TLC was performed as described in “PLD activity in cell-free system.” Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .05.
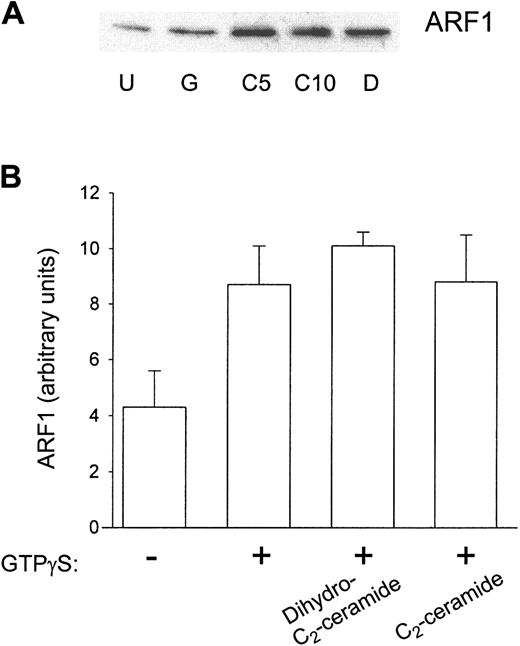
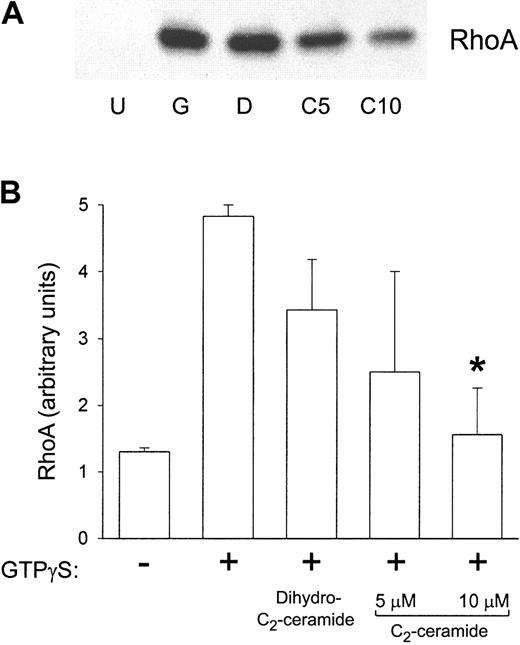
Others have found that ARF and RhoA translocation to the plasma membrane are partially inhibited by 50 μM C2-ceramide in HL-60 cells.19 To address the effect of ceramide in PMN function, first we used the reconstituted cell-free system of GTPγS activation. After incubating cytosol with plasma membrane, samples from the cell-free system were centrifuged again to separate the fractions. These fractions were Western blotted and probed for ARF1, ARF6, and RhoA. ARF1 association with the plasma membrane was not significantly affected by prior C2-ceramide treatment (Figure 5), suggesting that ceramide does not prevent GTPγS from interacting with proteins in this system. Similarly, the amount of ARF6 associated with the plasma membrane was not affected by C2-ceramide (data not shown). However, RhoA translocation was significantly inhibited by prior treatment with 10 μM C2-ceramide, whereas it was not inhibited by dihydro-C2-ceramide (Figure 6), suggesting that the mechanism of PLD inhibition by ceramide would be a failure of RhoA translocation to PMN plasma membranes containing PLD. To further test this hypothesis, we evaluated RhoA translocation to the membrane in intact PMNs and in streptolysin O–permeabilized PMNs. In contrast to the cell-free system, translocation was not inhibited by C2-ceramide (Figure 7), suggesting that the inhibition of PLD by ceramide is not necessarily mediated through RhoA.
ARF translocation to the plasma membrane in the reconstituted system was not inhibited by prior C2-ceramide treatment. Aliquots were taken from samples in experiments described in Figure 4. These aliquots were ultracentrifuged, and the pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against ARF1. (A) A representative Western blot. U indicates unstimulated; G, GTPγS; C, C2-ceramide (5 and 10 μM); D, dihydro-C2-ceramide. (B) Scanning densitometry of the ARF1 band in Western blots. Data shown are the mean ± SEM of 5 experiments.
ARF translocation to the plasma membrane in the reconstituted system was not inhibited by prior C2-ceramide treatment. Aliquots were taken from samples in experiments described in Figure 4. These aliquots were ultracentrifuged, and the pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against ARF1. (A) A representative Western blot. U indicates unstimulated; G, GTPγS; C, C2-ceramide (5 and 10 μM); D, dihydro-C2-ceramide. (B) Scanning densitometry of the ARF1 band in Western blots. Data shown are the mean ± SEM of 5 experiments.
RhoA translocation to the plasma membrane in the reconstituted system was inhibited by prior C2-ceramide treatment. Aliquots were taken from samples in experiments described in Figure 4. These aliquots were ultracentrifuged, and the pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against RhoA. (A) A representative Western blot. U indicates unstimulated; G, GTPγS; D, dihydro-C2-ceramide; C, C2-ceramide (5 and 10 μM). (B) Scanning densitometry of the RhoA band in Western blots. Data shown are the mean ± SEM of 4 experiments. *Significantly different from stimulated control; P < .05.
RhoA translocation to the plasma membrane in the reconstituted system was inhibited by prior C2-ceramide treatment. Aliquots were taken from samples in experiments described in Figure 4. These aliquots were ultracentrifuged, and the pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against RhoA. (A) A representative Western blot. U indicates unstimulated; G, GTPγS; D, dihydro-C2-ceramide; C, C2-ceramide (5 and 10 μM). (B) Scanning densitometry of the RhoA band in Western blots. Data shown are the mean ± SEM of 4 experiments. *Significantly different from stimulated control; P < .05.
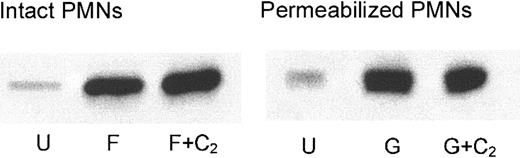
RhoA translocation to the plasma membrane in intact and streptolysin O–permeabilized PMNs treated with C2-ceramide (C2). Intact PMNs were activated with 100 nM FMLP (F), whereas permeabilized PMNs were treated with 10 μM GTPγS (G). Cells were disrupted by probe sonication, and cytosol was separated from membranes by ultracentrifugation. Membrane pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against RhoA. U indicates unstimulated. Results are representative of 4 experiments.
RhoA translocation to the plasma membrane in intact and streptolysin O–permeabilized PMNs treated with C2-ceramide (C2). Intact PMNs were activated with 100 nM FMLP (F), whereas permeabilized PMNs were treated with 10 μM GTPγS (G). Cells were disrupted by probe sonication, and cytosol was separated from membranes by ultracentrifugation. Membrane pellets were solubilized in sample buffer to run 12.5% SDS-PAGE. Proteins were transferred, and Western blots were performed with antibody against RhoA. U indicates unstimulated. Results are representative of 4 experiments.
If ceramide were to block the interaction between RhoA and PLD, addition of exogenous RhoA might overcome ceramide inhibition. In the cell-free system, the addition of up to 32 μg/mL (approximately 1.4 μM) exogenous RhoA did not restore PLD activity (Figure 8A). However, the exogenous RhoA, initially added to the cytosolic fraction, was capable of binding to the membrane fraction in the presence of C2-ceramide (Figure 8B), providing more evidence that ceramide does not only inhibit PLD through an effect on RhoA.
Effect of exogenous RhoA on PLD activity and RhoA translocation in a PMN reconstituted system. (A) PMNs were labeled with [3H]-lyso-PAF. PMNs were incubated with 10 μM C2-ceramide (C2), then disrupted, and membrane was separated from cytosol as in Figure 4. Samples were incubated with 1% ethanol in the presence of 100 μM GTPγS and increasing concentrations of recombinant His-tagged RhoA (rRhoA). [3H]Phosphatidylethanol was separated by using thin-layer chromatography. U indicates unstimulated. (B) Exogenous RhoA translocation to the plasma membrane was determined by Western blot of the membrane pellets with antibody against RhoA in untreated cells (U), control cells with exogenous RhoA (rRhoA), and C2-ceramide (C2)–treated cells with exogenous RhoA. nRhoA indicates native RhoA. Results are representative of 3 experiments.
Effect of exogenous RhoA on PLD activity and RhoA translocation in a PMN reconstituted system. (A) PMNs were labeled with [3H]-lyso-PAF. PMNs were incubated with 10 μM C2-ceramide (C2), then disrupted, and membrane was separated from cytosol as in Figure 4. Samples were incubated with 1% ethanol in the presence of 100 μM GTPγS and increasing concentrations of recombinant His-tagged RhoA (rRhoA). [3H]Phosphatidylethanol was separated by using thin-layer chromatography. U indicates unstimulated. (B) Exogenous RhoA translocation to the plasma membrane was determined by Western blot of the membrane pellets with antibody against RhoA in untreated cells (U), control cells with exogenous RhoA (rRhoA), and C2-ceramide (C2)–treated cells with exogenous RhoA. nRhoA indicates native RhoA. Results are representative of 3 experiments.
PKCα, and likely PKCβ, are involved in positive regulation of PLD1.26 The PKCs translocate to the plasma membrane on cell stimulation where they can interact with PLD. Thus, we evaluated samples from the cell-free system for PKC translocation. Prior treatment with C2-ceramide had no effect on the amount of PKCα or β associated with the plasma membrane (data not shown), as we observed previously in intact PMNs.1
Phosphorylation processes appear to be involved with PLD activation; for example, tyrosine kinase inhibitors reduce PLD activation and membrane recruitment of ARF, RhoA, and PKCα.27 However, ARF1 and RhoA translocation were not affected by using apyrase to hydrolyze the ATP in a cell-free system from HL-60 leukemic cells.19 We incubated the fractions in our cell-free system with 5 U/mL apyrase for 10 minutes at 37°C before GTPγS activation. Neither PLD activity nor RhoA translocation was affected by apyrase treatment (data not shown), indicating that activation and translocation were not energy-dependent.
Discussion
In this study we demonstrated functional activity in streptolysin O–permeabilized PMNs by release of lactoferrin from specific granules. We determined that activation of ARF and RhoA by GTPγS in permeabilized PMNs led to both activation of PLD and degranulation. Permeabilization allowed GTPγS to enter PMNs, bypassing receptor activation. Lactoferrin release and PLD activation responded to similar doses of GTPγS and were both inhibited by treatment with C2-ceramide, consistent internally and with previous studies.1 Enhancement of PLD in permeabilized PMNs by the generally inactive dihydro-C2-ceramide may have occurred because of potential metabolism of dihydroceramide to sphingosine. C6-ceramide was more potent than C2-ceramide in inhibiting lactoferrin release, suggesting endogenous ceramide with its increased carbon chain length would be even more potent in inhibiting function in vivo. In the cell-free system, PLD was similarly activated by GTPγS and inhibited by C2-ceramide. In the cell-free system we observed inhibition of RhoA translocation to the plasma membrane by ceramide pretreatment. However, ceramide did not block RhoA translocation in intact or permeabilized PMNs.
In other cell systems, translocation of ARF and RhoA to the cell membrane is inhibited by 20 to 50 μM C2-ceramide, 2- to 5-fold more than was needed to block RhoA, but not ARF, in the cell-free system in this study.19,28 Inhibition of RhoA translocation by ceramide in the cell-free system was specific, as RhoA was inhibited by a concentration of 10 μM C2-ceramide but not the inactive analog dihydro-C2-ceramide. This concentration of C2-ceramide was previously shown not to result in significant damage to PMNs as assessed by lactate dehydrogenase release, while specifically inhibiting phagocytosis, degranulation, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, but not chemotaxis.1,3 In contrast to the cell-free system, in the intact PMN, cytochalasin B treatment by itself led to RhoA association with the membrane which was not affected by ceramide treatment.1 Similarly, in intact PMNs incubated with FMLP alone, or in permeabilized PMNs activated with GTPγS, RhoA association with the membrane was not affected by ceramide. Addition of exogenous RhoA was unable to overcome ceramide inhibition of PLD in the cell-free system, despite binding to the membrane fraction where PLD is located.
The discrepancy in Rho translocation between cell-free and permeabilized systems may indicate a more complex interaction between ceramide, PLD, and G proteins. For example, in the cell-free system, ceramide-treated membranes may be remodeled such that ceramide affects Rho binding to PLD but not to the membrane itself. Ceramide treatment of intact and permeabilized PMNs may inhibit PLD through another mechanism, such as disruption of the interaction of PLD with PIP2 as initially suggested by Singh et al,29 Using a cell-free system of dimethyl sulfoxide (DMSO)–differentiated (PMN-like) HL-60 cells, Singh et al29 found that PLD activation is inhibited by ceramide at low concentrations of PIP2. This inhibition is antagonized by excess PIP2, consistent with the idea that ceramide inhibits a PLD-PIP2 interaction. We attempted these experiments in our PMN cell-free system, applying PIP2 and ceramide in liposomes of phosphatidylcholine and phosphatidylethanolamine to recombined cytosol and membrane fractions.29 However, we were unable to replicate the HL-60 results, finding no PIP2 enhancement of PLD activity in the presence or absence of ceramide (data not shown). The PLD in PMN is primarily localized in secretory vesicles,30 which fractionate with the plasma membrane in the method we used. The PLD in these PMN membrane fraction vesicles may be unavailable to the exogenous PIP2, in contrast to the PLD of HL-60 cells which is in the plasma membrane. Singh et al29 also observed that the activities of truncated mutants of PLD1 are inhibited by ceramide. These data together suggest that ceramide interacts with the catalytic core of PLD and that ceramide inhibits PLD more effectively when PIP2 concentrations are rate limiting.29 Our data do not preclude the possibility of direct ceramide interaction with PLD. Additionally, overexpression of RhoA or its downstream effector Rho kinase increases activity of phosphoinositide 4-phosphate (PI4-P) 5-kinase and resulting levels of PIP2,31 suggesting RhoA could affect PLD both directly as a cofactor and indirectly by regulating PIP2, another cofactor. We measured PI4-P 5-kinase activity by the phosphorylation of PI4-P using the method of Oude Weernink et al31 but found no inhibition by ceramide (data not shown).
Ceramide inhibition of RhoA translocation in the cell-free system could be related to phosphorylation/dephosphorylation events. Ceramide begins to accumulate after cell activation, at a time early enough to indicate a negative regulatory role.3,32 Ceramide activates several protein kinases and phosphatases15,33,34 ; thus, ceramide possibly could affect RhoA function indirectly through stimulation of phosphorylation or dephosphorylation. Phosphorylation of RhoA by protein kinase A enhances its dissociation from the membrane,35,36 suggesting RhoA association with the membrane may be regulated by phosphorylation. To evaluate whether a phosphorylation process was required to mobilize RhoA, we treated the PMNs with apyrase to deplete the cells of ATP. We found that apyrase treatment to hydrolyze endogenous ATP did not affect RhoA translocation. Other studies suggested that PKC regulates translocation of RhoA in an ATP-independent manner,10 consistent with our observations. However, PKC did not appear to be involved in translocation of RhoA in the cell-free system, based on ceramide inhibition of RhoA translocation but not translocation of PKCα or β.
In summary, our finding of ceramide inhibition of RhoA translocation in the cell-free system does not preclude the direct interaction of ceramide and PLD. Ceramide may interfere with binding of cofactors to PLD or production of PIP2 through Rho kinase effects on a phosphatidylinositol kinase.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2002-11-3341.
Supported by grants from the National Institutes of Health (grants AI20065 [L.A.B.], DK41487, and DK39255 [J.A.S.]).
Presented in abstract form at the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 10, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. PLD activity in streptolysin O–permeabilized GTPγS-stimulated PMNs: inhibition by C2-ceramide. PMNs were first labeled with [3H]-lyso-PAF. Incubations were carried out as described in Figure 1 except for the addition of 1% ethanol for 5 minutes at 37°C prior to the stimulation-permeabilization step. PMNs were pelleted and extracted with chloroform and methanol. TLC was performed, and the plate was exposed to film. Radioactive bands were scraped for scintillation counting. Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .001. NS indicates not significantly different from stimulated control; dpm, disintegrations per minute; and PEt, phosphatidylethanol.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2002-11-3341/6/m_zh80060458160003.jpeg?Expires=1767720379&Signature=rs2w~izMdTuof~LW4zaXswwp8emyhWAktbMSYXfh9V69QoP3e3QVhV9B1P~4Ic2-b6B3xoPaIWXiDUr6HGhw5RFfV3B79GK8wDvHhd-YcAmK8HB9vvhgqEFGrIT22AsLYdjid3YgiLmnxWVESlNtHg8GCOb0hraICeZgKg0sBWC9v3sSTzFBHmp~-FTfd~uAvA561WmmXcGvl6Z5hAOL9DOhW3iyrCMbHhUwxGB5CVloSk~LOA-ubHPJ~MkTRcU3q5Qq-EdH5Jg6yW1u7rARgT1RpPyT9~0B7MAqqw3p7KJytqUoVClfqTT1IqE7kdYhQCM6tZhSoNmzygQNvg2o5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. C2-ceramide treatment of intact PMNs: inhibition of PLD activity in a reconstituted system. PMNs were [3H]-labeled, incubated with 10 μM C2-ceramide, dihydro-C2-ceramide, or no lipid (control cells) for 30 minutes at 22°C, then disrupted by probe sonication. Cytosol and plasma membrane fractions were separated by ultracentrifugation over sucrose gradients. Samples were incubated with 1% ethanol. Cytosol from control cells only was activated with GTPγS, then combined with equal amounts of membrane protein from control or ceramide-pretreated PMNs (10 minutes, 37°C). Samples were extracted, and TLC was performed as described in “PLD activity in cell-free system.” Data shown are the mean ± SEM of 5 experiments. *Significantly different from stimulated control; P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2002-11-3341/6/m_zh80060458160004.jpeg?Expires=1767720379&Signature=WE-rnxEIzRJ0KV45nmoTbkqK8T3p94X21WeiDAgECuaygVnCk-Vi5Sa0zaRxB5qlBZVLn9C~dkN0WibbL9WHKwJHfEOB1tYTswVSA4i59yGrG7rZrBhuMuq4YzYVKv-fg05-XKHoHxykF-ue6-suxpBcxcsA9REHUmn-wYiAOUqULBsiXtGGAXEhTxpOe-hBZcaaWh-1MKlLfiBQLgVkIp1qENgLdLJVAV9ebn~OYnRuMtJT8QxAYUK-7yIpa8W0w~cRK3mAn2sjVnQX-wZuBHrUovEN8uYP5I0imLVb7yc3TkhRsfshs9QSvHWJyVjUQQpTd4qCEIwXPNP5mqw-tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 8. Effect of exogenous RhoA on PLD activity and RhoA translocation in a PMN reconstituted system. (A) PMNs were labeled with [3H]-lyso-PAF. PMNs were incubated with 10 μM C2-ceramide (C2), then disrupted, and membrane was separated from cytosol as in Figure 4. Samples were incubated with 1% ethanol in the presence of 100 μM GTPγS and increasing concentrations of recombinant His-tagged RhoA (rRhoA). [3H]Phosphatidylethanol was separated by using thin-layer chromatography. U indicates unstimulated. (B) Exogenous RhoA translocation to the plasma membrane was determined by Western blot of the membrane pellets with antibody against RhoA in untreated cells (U), control cells with exogenous RhoA (rRhoA), and C2-ceramide (C2)–treated cells with exogenous RhoA. nRhoA indicates native RhoA. Results are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2002-11-3341/6/m_zh80060458160008.jpeg?Expires=1767720379&Signature=Amgy~l3ToGxjXjp1dzFoQ5RFfqxLwer5blKzeCE8uE-C1c~5h5tb0reD-uX5qqlmMD7CXykSflBaG~hMNuVtFi-ezZ6K471ZtAejcDxJcrn0ljupmN9832AmkL9v8zfPx5Ricxjjf7ZTG~2e9eCdKWj2teCiELfXS79zuhAA7DhRfpc5lzz4T0d3T6lj5FfTZs~Z-6SQ8aKrz21X1cRj3qLOXG~JemmKoxovsMgSSfcLZXrUYCMty9d6Tqd8pew4-DaY8if8wZp0aCCVRmU3yvB8dHsVWRCfdfceN42tY0JPcdhiJwExXrk4NMqZBKZwI9eWn8guYEImVnb3g5abIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal