Abstract
The responsiveness and diversity of peripheral B-cell repertoire decreases with age, possibly because of B-cell clonal expansions, as suggested by the incidence of serum monoclonal immunoglobulins and of monoclonal chronic lymphocytic leukemia (CLL)–like B lymphocytes in clinically silent adults. We phenotyped peripheral blood cells from 500 healthy subjects older than 65 years with no history or suspicion of malignancies and no evidence of lymphocytosis. In 19 cases (3.8%) a κ/λ ratio of more than 3:1 or less than 1:3 was found: 9 were CD5+, CD19+, CD23+, CD20low, CD79blow, sIglow (classic CLL-like phenotype); 3 were CD5+, CD19+, CD23+, CD20high, CD79blow, sIglow (atypical CLL-like), and 7 were CD5-, CD19+, CD20high, CD23-, CD79bbright, FMC7+, sIgbright (non–CLL-like). In 2 subjects, 2 phenotypically distinct unrelated clones were concomitantly evident. No cases were CD10+. Polymerase chain reaction (PCR) analysis demonstrated a monoclonal rearrangement of IgH genes in 15 of 19 cases. No bcl-1 or bcl-2 rearrangements were detected. Using a gating strategy based on CD20/CD5/CD79 expression, 13 additional CLL-like B-cell clones were identified (cumulative frequency of classic CLL-like: 5.5%). Thus, phenotypically heterogeneous monoclonal B-lymphocyte expansions are common among healthy elderly individuals and are not limited to classic CLL-like clones but may have the phenotypic features of different chronic lymphoproliferative disorders, involving also CD5- B cells.
Introduction
It is now well established that with aging both humans and mice tend to produce an antibody response of limited heterogeneity with respect to isotype, antigen-binding affinity, idiotype, and heavy chain variable (VH) gene use.1 The cause for these phenomena is so far unknown. They are not due to a primary defect of B-cell precursors nor to abnormalities of bone marrow (BM) environment, as indicated by BM transplantation experiments in mice.2 They may reflect, at least in part, the decreased number of B-cell precursors that are observed within the BM of aging humans and mice,3-5 though B-cell generation continues unabated during adulthood, and can also be ascribed to altered helper T-cell activity.6,7 One intriguing possibility is that the age-related limited diversity of the B-cell repertoire may be accounted for by the appearance of B-cell clonal expansions. The most striking example is provided by the age-associated increasing incidence of serum monoclonal immunoglobulins, which occurs in both mice and humans (monoclonal gammopathies of undetermined significance [MGUS]).8,9 In addition, the observation that expanded clones of Ly-1+ B cells are universally detectable in senescent normal mice1,10,11 is mirrored by the recent finding that monoclonal CD5+ B lymphocytes are present in a relevant number of clinically silent adults.12 The phenotype of circulating monoclonal cells (CD5+, CD19+, CD23+, CD20low, CD79blow, sIglow) resembles very closely that of chronic lymphocytic leukemia (CLL). These clones have been detected with increased frequency among relatives of patients with CLL.13 In this context it is of interest that first degree relatives of patients with CLL have a risk of developing CLL, as well as other lymphoid malignancies, which is more than 3 times greater than the risk of the general population.14
To approach the problem of the relationship between age-related progressive restriction of the B-cell repertoire and the development of B-cell malignancies, we aimed at defining whether clonal expansions of B-lymphocyte subsets different from the CD5+ “classic CLL-like” cells might be present in the elderly. To this end, we studied by immunoglobulin (Ig) light chain restriction and IgH–polymerase chain reaction (PCR) analysis the peripheral blood (PB) of 500 healthy individuals older than 65 years. Our study indicates that monoclonal expansions of B lymphocytes are common among elderly patients who have an otherwise normal blood cell count. They are not only represented by classic CLL-like CD5+ clones but also by CD5- clones resembling other chronic lymphoproliferative disorders. The presence of such clones suggests that B-cell monoclonality may be a phenomenon of lymphoid senescence more generalized than previously described whose clinical significance needs wider prospective studies.
Materials and methods
Blood samples
EDTA (ethylenediaminetetraacetic acid) PB samples were obtained from 500 individuals (269 women and 231 men) older than 65 years (mean age, 73.7 years), the oldest being 98 years old. Individuals were selected among outpatients from 3 different facilities in the countryside outside the Turin, Italy, metropolitan area, referred for common routine blood test (eg, blood glucose, blood lipids). All individuals were interviewed prior to blood collection. Those who had a history or a suspicion of malignancy were excluded from the study. All individuals included in the study had a normal blood cell count, with no evidence of lymphocytosis at the routine blood test. The study was performed over 20 months, encompassing all different year periods, in order to exclude any seasonal bias. All samples were processed within 24 hours after blood withdrawal.
Cell preparation, staining, and FACS analysis
Leukocytes were obtained from PB, washed 3 times in phosphate-buffered saline (PBS) plus bovine serum albumin (BSA) 0.3% to remove serum, incubated with the proper antibodies, washed 2 times in PBS+BSA, incubated with NH4Cl (8.6 g/L in distilled water), and finally washed 2 times in PBS+BSA. Cells were incubated with either one of the 2 following antibody mixes: (1) tricolor (TC)–conjugated anti-CD5, allophycocyanin (APC)–conjugated anti-CD19, fluorescein isothiocyanate (FITC)–conjugated F(ab)2–anti-κ and phycoerythrin (PE)–anti-λ light chain (Dako Cytomation, Carpinteria, CA) for all the 500 samples; (2) TC-labeled anti-CD5, FITC-labeled anti-CD20, PE-labeled anti-CD79b (Becton Dickinson, San Jose, CA), APC-labeled anti-CD19 (Caltag Laboratories, San Francisco, CA) for 350 of 500 samples.
For each sample at least 200 000 events were acquired on a FACSCalibur equipped with a 488 argon ion laser and 635 red diode laser (Becton Dickinson) and analyzed with the CellQuest software system (Becton Dickinson) according to 2 different gating strategies. In Ab mix (1), low forward and side scatter (FSC/SSC), CD19+ cells were gated and further divided into CD5- and CD5+ subsets and the percentage of κ+ and λ+ events was evaluated in both populations. The κ/λ ratio was considered abnormal when it was more than 3:1 or less than 1:3. In Ab mix (2), we followed the sequential gating strategy described in Rawstron et al12 in order to identify CLL-like cells (ie, CD20low, CD5high, CD79blow cells).
When clonal B-cell populations were identified based on the presence of an abnormal κ/λ ratio (1) or of CLL-like phenotype (2), they were further characterized by staining with PE-conjugated anti-CD10, anti-CD20, anti-CD23, anti-CD79b, anti-IgM, anti-IgD, anti-IgG, and FITC-conjugated anti-FMC7.
DNA extraction and PCR amplification for IgVH, bcl-1 and bcl-2 genes
Genomic DNA was extracted from whole blood using the QIAmp blood kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. To detect the presence of monoclonal IgH rearrangements, DNA was amplified by PCR using a seminested protocol.15 Two different parallel reactions were carried out containing FRII consensus and FRIII consensus 5′ primers, respectively, together with a JH consensus 3′ primer, for the first amplification reaction. In the following seminested reactions the JH 3′ primers were substituted with a more internal one. PCR products were separated onto 10% (FrII) or 15% (FrIII) polyacrylamide gels. The expected molecular weight of a specific band was 240 to 260 (FRII) and 80 to 120 (FRIII) base pairs.
PCR amplification at the major and minor breakpoint region of the bcl-2/IgH translocation was performed using nested oligonucleotides as previously described.16
PCR-mediated detection of the t(11;14) involving the bcl-1 major translocation cluster was performed using a nested protocol17 consisting of a first PCR reaction containing a mixture of a bcl-1–specific 5′ primer (GATGGGCTTCTCTCACCTACTA) and a JH3-specific 5′ primer. The second nested reaction was performed using a mixture of a more internal bcl-1–specific 5′ primer (GGTTAGACTGTGATTAGC) and a JH4-specific 5′ primer. Both reactions were carried out for 30 cycles as follows: denaturation at 94°C for 30 seconds; annealing at 60°C for 30 seconds; extension at 72°C for 30 seconds.
For both bcl-1 and bcl-2 reactions, PCR products were separated onto 2% agarose gels.
Results
Light chain restriction among circulating B lymphocytes in the blood of otherwise healthy elderly subjects
We first aimed at defining the frequency of an unbalanced ratio between κ and λ light chain–expressing B cells in the blood of the elderly, as a sign of the presence of a monoclonal B-cell population. We examined, by cytofluorograph analysis, blood samples from 500 individuals over the age of 65, using a 4-color staining with anti-CD19, anti-CD5–anti-κ light chain, and anti-λ light chain. In all cases the κ/λ ratio was analyzed within the total CD19+ lymphocytes, as well as within the CD19+ CD5- and the CD19+ CD5+ subpopulations. Light chain restriction was observed in 19 cases (9 women and 10 men; 3.8% of the total) in whom a perturbation of the κ/λ ratio was either more than 3:1 or less than 1:3 (Figure 1A). In 16 of 19 cases, B lymphocytes showed a κ light chain restriction which was more than 10:1 in 9 cases, more than 4:1 in 5 cases, and more than 3:1 in the 2 remaining cases. In 3 of 19 cases, a λ light chain restriction was evident and was more than 10:1 in 1 case, more than 4:1 in 1 case, and more than 3:1 in the third case. The light chain restriction was found among CD19+ CD5- cells in 7 cases and among CD19+ CD5+ cells in 12 cases.
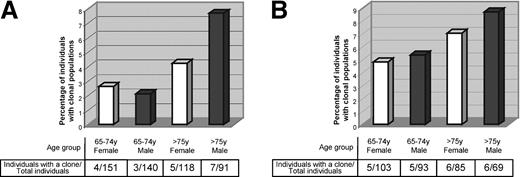
Monoclonal B cells are frequent in the blood of otherwise healthy elderly people, and their frequency increases with age. (A) The graphic shows the percentage of all (both CD5+ and CD5-) monoclonal B cells present in the blood of people older than 65 years, detected by an unbalanced κ/λ light chain ratio on the surface of blood B cells. Individuals are grouped according to age (65-74 years, and older than 75 years) and gender (white and black bars show the prevalence of B-cell clones among women and men, respectively). Numbers of female and male individuals with monoclonal populations within the total number of female and male individuals analyzed are shown. (B) The graphic shows the percentage of CD5+ classic CLL-like B-cell clones either detected by the unbalanced κ/λ light chain ratio or by a sequential gating strategy in the blood of people older than 65 years. Individuals are grouped according to age (65-74 years, and older than 75 years) and gender (white and black bars show the prevalence of B-cell clones among women and men, respectively). Numbers of female or male individuals with classic CLL-like clones within the total number of female and male individuals analyzed are shown.
Monoclonal B cells are frequent in the blood of otherwise healthy elderly people, and their frequency increases with age. (A) The graphic shows the percentage of all (both CD5+ and CD5-) monoclonal B cells present in the blood of people older than 65 years, detected by an unbalanced κ/λ light chain ratio on the surface of blood B cells. Individuals are grouped according to age (65-74 years, and older than 75 years) and gender (white and black bars show the prevalence of B-cell clones among women and men, respectively). Numbers of female and male individuals with monoclonal populations within the total number of female and male individuals analyzed are shown. (B) The graphic shows the percentage of CD5+ classic CLL-like B-cell clones either detected by the unbalanced κ/λ light chain ratio or by a sequential gating strategy in the blood of people older than 65 years. Individuals are grouped according to age (65-74 years, and older than 75 years) and gender (white and black bars show the prevalence of B-cell clones among women and men, respectively). Numbers of female or male individuals with classic CLL-like clones within the total number of female and male individuals analyzed are shown.
The individuals carrying a monoclonal population showed neither an altered PB cell count nor a lymphocytosis (lymphocytes mean value, 2.083 × 109/L [2083/μL]; range, 1.225-3.977 × 109/L [1225-3977/μL]). Between 3.4% and 30% of all lymphocytes were actually CD19+ B cells, at a mean concentration of 0.293 × 109/L (293/μL) (range, 0.061-1.193 × 109/L [61-1193/μL]). Monoclonal B cells represented a variable proportion of total CD19+ B cells, with a mean of 39% and a range of 5% to 83% of total B lymphocytes.
The prevalence of monoclonal B cells increased with age, being 2.4% among individuals younger than 75 years of age as compared with 5.7% of individuals older than 75 years. In the latter age group a male prevalence was also evident (7.7% versus 4.2% in females) (Figure 1A).
Monoclonal B-cell populations in otherwise healthy elderly individuals are phenotypically heterogeneous
All cases carrying a CD19+ B-cell population with a light chain restriction were further phenotyped (Table 1).
Clone type and case no. . | Age, y . | Sex . | L-chain . |
|---|---|---|---|
| Classic CLL-like (CD20low, CD23+, CD5bright, FMC7-, CD10-, IgMlow, CD79blow) | |||
| 1* | 70 | F | κ |
| 2* | 85 | F | κ |
| 3* | 79 | F | κ |
| 4 | 91 | M | κ |
| 5* | 68 | F | κ |
| 6 | 68 | M | κ |
| 7 | 71 | M | κ |
| 8 | 86 | M | κ |
| 9† | 76 | F | λ |
| Atypical CLL-like (CD20high, CD23+, CD5bright, FMC7-, CD10-, IgMlow, CD79blow) | |||
| 10 | 78 | M | κ |
| 11 | 65 | F | κ |
| 12‡ | 68 | F | κ |
| Non-CLL-like (CD20high, CD23-, CD5-, FMC7+, CD10-, IgM1+low, CD79b+) | |||
| 13§ | 78 | F | κ |
| 14 | 78 | F | λ |
| 15∥¶ | 76 | M | κ |
| 16¶ | 80 | M | λ |
| 17 | 81 | M | κ |
| 18# | 86 | M | κ |
| 19** | 74 | M | κ |
Clone type and case no. . | Age, y . | Sex . | L-chain . |
|---|---|---|---|
| Classic CLL-like (CD20low, CD23+, CD5bright, FMC7-, CD10-, IgMlow, CD79blow) | |||
| 1* | 70 | F | κ |
| 2* | 85 | F | κ |
| 3* | 79 | F | κ |
| 4 | 91 | M | κ |
| 5* | 68 | F | κ |
| 6 | 68 | M | κ |
| 7 | 71 | M | κ |
| 8 | 86 | M | κ |
| 9† | 76 | F | λ |
| Atypical CLL-like (CD20high, CD23+, CD5bright, FMC7-, CD10-, IgMlow, CD79blow) | |||
| 10 | 78 | M | κ |
| 11 | 65 | F | κ |
| 12‡ | 68 | F | κ |
| Non-CLL-like (CD20high, CD23-, CD5-, FMC7+, CD10-, IgM1+low, CD79b+) | |||
| 13§ | 78 | F | κ |
| 14 | 78 | F | λ |
| 15∥¶ | 76 | M | κ |
| 16¶ | 80 | M | λ |
| 17 | 81 | M | κ |
| 18# | 86 | M | κ |
| 19** | 74 | M | κ |
CD23 staining was not performed
CD10 staining was not performed
FMC7, CD10, and CD79b stainings were not performed
CD79b staining was not performed
CD23 staining was not performed
FMC7 staining was not performed
Contained a second coexisting clone, atypical CLL-like
Contained a second coexisting clone, classic CLL-like
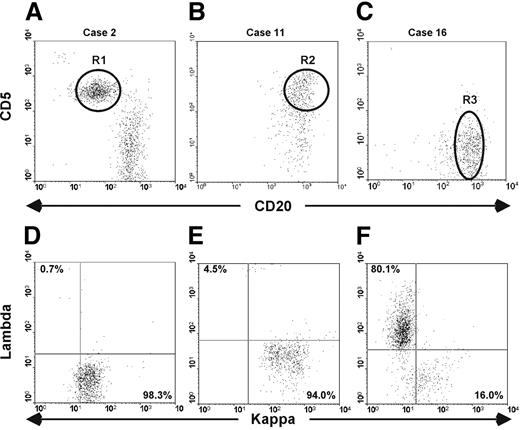
Nine cases (8 κ+ and 1 λ+) were CD5+ and CD23+, and had a low expression of CD20, CD79b, and sIg. This phenotype resembled that of CLL and therefore these cases were defined as classic CLL-like12 (Table 1 and Figure 2A,D).
Monoclonal B cells present in the blood of elderly people are phenotypically heterogeneous. Cytofluorimetric analyses of whole blood from 3 patients carrying either a classic CLL-like (A,D), or an atypical CLL-like (B,E), or a non–CLL-like (C,F) B-cell clone are presented. (A-C) Dot plots were obtained by gating lymphocytes (identified on side-scatter and forward-scatter profiles) expressing CD19. The intensity of CD20 and CD5 is shown on the x-axis and y-axis, respectively. R1, R2, and R3 gates indicate the clones. (D-F) Dot plots show κ and λ light chain expression of the B-cell clones as identified on panels A, B, and C.
Monoclonal B cells present in the blood of elderly people are phenotypically heterogeneous. Cytofluorimetric analyses of whole blood from 3 patients carrying either a classic CLL-like (A,D), or an atypical CLL-like (B,E), or a non–CLL-like (C,F) B-cell clone are presented. (A-C) Dot plots were obtained by gating lymphocytes (identified on side-scatter and forward-scatter profiles) expressing CD19. The intensity of CD20 and CD5 is shown on the x-axis and y-axis, respectively. R1, R2, and R3 gates indicate the clones. (D-F) Dot plots show κ and λ light chain expression of the B-cell clones as identified on panels A, B, and C.
Three cases, all with κ LC restriction, also expressed CD5 and CD23 and low levels of CD79b and sIg. However, in contrast to the classic CLL-like subtype, monoclonal cells showed a bright expression of CD20. We defined this phenotype as atypical CLL-like (Table 1 and Figure 2B,E).
In the remaining 7 cases (5 κ+ and 2 λ+) monoclonal cells were strongly positive for CD20, CD79b, and sIg but were negative for CD5 expression, had variable levels of FMC7, and in most cases did not express CD23 (Table 1 and Figure 2C,F). We defined these cases as non–CLL-like.
Among all 500 individuals analyzed in our study, none was CD10+, excluding the presence of germinal center (GC)–derived cells. In addition, no cases were concomitantly positive for CD5 and negative for CD23, thereby making a mantle cell origin unlikely.
PCR analysis for IgH rearrangements and translocations of the monoclonal B populations
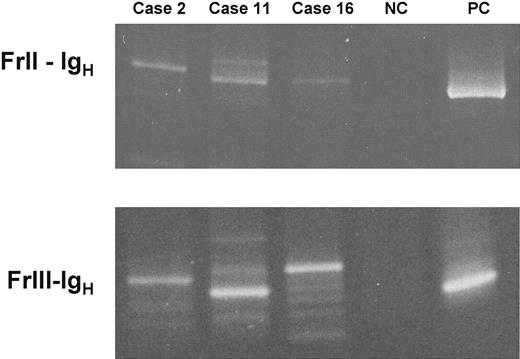
In 15 of 19 cases carrying a monoclonal B-cell population, a genomic PCR analysis of the IgH genes was performed, using consensus primers to FrII or FrIII and JH. A monoclonal rearrangement was demonstrated in 14 of 15 amplifiable cases (Figure 3). In the same samples, we performed a nested PCR analysis to detect bcl-1/JH and bcl-2/JH rearrangements. All 15 cases analyzed were negative for both tests.
PCR analysis confirms the presence of monoclonal IgH rearrangements. Two seminested PCR reactions were performed to detect FrII-IgH and FrIII-IgH rearrangements on genomic DNA. PB DNA was obtained from 3 cases carrying a classic CLL-like (case 2), an atypical CLL-like (case 11), and a non–CLL-like (case 16) monoclonal B-cell population. PCR products have been visualized under transillumination after separation onto 10% (for FrII-IgH) and 15% polyacrylamide gels (for FrIII-IgH). NC and PC indicate negative and positive control (MEC-1 B-cell line30 ).
PCR analysis confirms the presence of monoclonal IgH rearrangements. Two seminested PCR reactions were performed to detect FrII-IgH and FrIII-IgH rearrangements on genomic DNA. PB DNA was obtained from 3 cases carrying a classic CLL-like (case 2), an atypical CLL-like (case 11), and a non–CLL-like (case 16) monoclonal B-cell population. PCR products have been visualized under transillumination after separation onto 10% (for FrII-IgH) and 15% polyacrylamide gels (for FrIII-IgH). NC and PC indicate negative and positive control (MEC-1 B-cell line30 ).
Twenty-four consecutive samples chosen among the individuals of our study who carried no monoclonal B-cell populations were used as controls for all the 3 assays. They showed neither distinct monoclonal IgH rearrangements nor bcl-1/JH or bcl-2/JH rearrangements.
More B-cell clones can be identified using a sequential gating strategy
In order to confirm the high frequency of circulating cells with a CLL phenotype in healthy individuals12 and to exclude any possible bias in selecting our study population, we screened PB from 350 samples of our cohort with a 4-color staining including CD19, CD5, CD20, and CD79b. Using the gating strategy previously described,12 we were able to identify classic CLL-like B-cell clones in 13 additional individuals, which were undetectable by the classic κ/λ ratio likely due to the tiny size of the clone. CLL-like B-cells actually represented a negligible proportion of total CD19+ B cells, with a mean of 1.8% and a range of 0.7% to 4% of total B lymphocytes, the mean value of total CD19+ B cells being 0.165 × 109/L (165/μL) (range, 0.085-0.264 × 109/L [85/μL-264/μL]) and representing a percentage of 4% to 20% of all lymphocytes. None of these 13 individuals showed an altered PB cell count or a lymphocytosis (lymphocytes mean value, 2.052 × 109/L [2052/μL]; range, 1.120-2.940 × 109/L [1120/μL-2940/μL]).
All 13 clones had an LC restriction, 9 cases being κ+ and 4 λ+. An extended phenotype analysis was performed in 10 of 13 cases and revealed that monoclonal cells were CD23+, CD10-, FMC7-, sIglow confirming the similarity with classic CLL cells.
In 10 of 13 cases a genomic IgH-PCR analysis was performed and a monoclonal rearrangement was demonstrated in 6 of 10 cases. The success rate is low but similar to that reported in the literature,12,18 likely because of the minimal size of the clone. In the same samples, we performed a nested PCR analysis to detect bcl-1/JH and bcl-2/JH rearrangements. All 10 cases analyzed were negative for both tests.
Considering all together the classic CLL-like clones observed with the light chain restriction criteria (9/500) and those identified through the CLL-specific gating strategy (13/350), we were able to detect 22 cases carrying cells with CLL phenotype (11 men and 11 women; Figure 1B). This gives a cumulative frequency of classic CLL-like clones of 5.5% in the population above 65 years of age.
Two distinct B-cell clones can coexist in the same blood sample
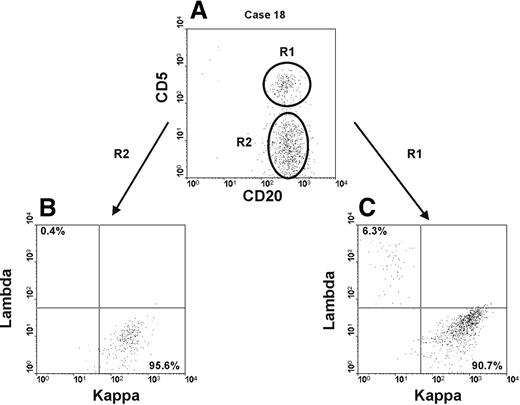
Interestingly, in 2 different blood samples it was possible to identify the presence of 2 distinct B-cell clones with an unrelated phenotype (Table 1 and Figure 4). In one case, the prevalent clone (57% of all CD19+ B cells) was a non–CLL-like clone (κ+) (Figure 4 and Table 1, case 18) which coexisted with a smaller atypical CLL-like clone (κ+) (20% of total CD19+ B cells) (Figure 4 and Table 1, case 18). In a second case, a non–CLL-like clone (κ+) was again prevalent being 56% of total CD19+ B cells (Table 1, case 19) and was found together with a classic CLL-like clone (κ+) of more limited size (8% of all CD19+ B cells) (Table 1, case 19). In both individuals, the 2 distinct clones could be easily distinguished by the differential expression of CD5 that was bright in the classic and atypical CLL-like cells and negative in the non–CLL-like cells. For all the 4 clones, the κ/λ ratio was more than 10:1.
Two distinct B-cell clones can coexist in the same blood sample. Cytofluorimetric analyses of whole blood from one individual (case 18) carrying 2 distinct B-cell clones is presented. (A) Dot plot was obtained by gating lymphocytes (identified on SSC and FSC profiles) expressing CD19. The intensity of CD20 and CD5 is shown on the x-axis and y-axis, respectively. R1 and R2 identify an atypical CLL-like clone and a non–CLL-like clone, respectively. (B-C) Dot plots show κ and λ light chain expression, on the x-axis and y-axis, respectively, of the atypical CLL-like clone (R1) in panel B and of the non–CLL-like clone (R2) in panel C.
Two distinct B-cell clones can coexist in the same blood sample. Cytofluorimetric analyses of whole blood from one individual (case 18) carrying 2 distinct B-cell clones is presented. (A) Dot plot was obtained by gating lymphocytes (identified on SSC and FSC profiles) expressing CD19. The intensity of CD20 and CD5 is shown on the x-axis and y-axis, respectively. R1 and R2 identify an atypical CLL-like clone and a non–CLL-like clone, respectively. (B-C) Dot plots show κ and λ light chain expression, on the x-axis and y-axis, respectively, of the atypical CLL-like clone (R1) in panel B and of the non–CLL-like clone (R2) in panel C.
Discussion
This study demonstrates by Ig light chain restriction and confirms by IgH-PCR analysis the presence of monoclonal B-cell populations in the blood of 19 (3.8%) of 500 otherwise healthy individuals over the age of 65 years. The prevalence of monoclonal B cells increased with age, being higher in individuals above 75 years of age. Also, a male prevalence was manifest, especially among the older age group (Figure 1A).
Monoclonal B cells were identified in both the CD5+ and the CD5- subsets of CD19+ B lymphocytes. Extended cytofluorograph analysis showed quite a heterogeneous phenotype of monoclonal B cells that allowed the classification of circulating clones in classic CLL-like (9 cases), atypical CLL-like (3 cases), and non–CLL-like (7 cases) cells (Table 1 and Figure 2). In addition, the screening of 350 of 500 cases with a CD19/CD20/CD5/CD79b staining and a previously described gating strategy12 revealed 13 more cases carrying circulating cells with a classic CLL-like phenotype. Due to their low number, these cells were undetectable with the light chain restriction method. All together, the 22 cases fulfilling the phenotypic characteristics of classic CLL cells12 made an overall frequency of 5.5% in the total population studied (Figure 1B). This value overlaps the 5% frequency originally reported (22/442) in a slightly younger population (> 60 years).12 The study population where we confirm the high frequency of CLL-like clones among otherwise healthy aging people is geographically unrelated to the population studied by Rawstron et al12 and is also different in terms of selection (primary care versus hospital outpatients) eliminating the possibility of ethnic or social bias, thereby confirming the widespread essence of the phenomenon. It has to be underscored that the previous study12 detected the presence (though at lower frequencies) of such clonal populations also among younger individuals (> 40 years < 65 years), indicating that this situation is not restricted to, although it is more common in, older people. In addition, the fact that our study was carried out over 20 months helps to rule out the possibility of any seasonal bias that could sustain the reported phenomenon.
A striking and rather unexpected finding was the overall high number of non–CLL-like clones (7 cases) detected in our population with a technique as simple as the unbalance of the κ/λ ratio among CD19+ B lymphocytes. It is even possible that the frequency of circulating non–CLL-like clones may be higher than reported here. It has been previously shown that a disease-specific gating assay such as that used to detect CLL cells19 is more sensitive than the light chain restriction method. Unfortunately a similar assay is not currently available for all B-cell neoplasms. One can reasonably predict that a more-specific staining technique, when available, will allow the detection of an even higher number of cases of otherwise healthy individuals with circulating monoclonal CD5- non–CLL-like cells.
Of interest is that no clones of putative GC or mantle zone (MZ) origin could be detected in the population studies. This striking lack of detection could be due merely to a “topographical” reason, since both GC and MZ cells are centered in secondary lymphoid organs, with a weak propensity to invade the periphery.20 It would obviously be of great interest to examine “normal” lymph nodes in a large cohort of aging people to evaluate the presence of light chain restriction, as a possible sign of lymphoid senescence. Alternatively, the lack of GC and MZ B-cell clones in the elderly could be due to a “functional” reason. Both GC B cells and naive MZ B cells are short lived, do not persist long in the body, and likely do not undergo a real senescence, as experienced memory B cells do.21 By contrast, for example, CLL malignant B cells are considered to be activated and likely antigen experienced.22-25
On the basis of light chain restriction analysis, the number of clinically silent CD5- non–CLL-like B-cell clones present in the blood of the elderly is similar to that of CD5+ classic CLL-like cases (9 versus 7, respectively). Therefore, CD5+ B cells do not appear to be more prone to display monoclonality with aging than CD5- B lymphocytes. The observation that B-cell clones of different phenotype and cellular origin are detectable in the blood of otherwise healthy elderly subjects brings the issue of circulating monoclonal B cells into a more general perspective. In essence, it indicates that the progressive alteration and restriction of the B-cell repertoire in the elderly may be a phenomenon of lymphoid senescence more generalized than previously assumed, as also suggested by the concomitant presence in the PB of 2 distinct circulating clones (Table 1 and Figure 4). Even more important, it raises the question of whether these populations merely reflect a physiologic aspect of senescence or whether any of them can progress to overt malignancy. Several “markers” of malignancy including the bcl-2/IgH rearrangement for follicular lymphoma, not to mention the Philadelphia (Ph) chromosome for chronic myeloid leukemia, can be detected in the PB of a variable proportion of healthy subjects.26-28 It is presently unknown to what extent their presence may influence the subsequent development of an overt malignancy. Likewise, only a proportion of subjects presenting with MGUS will actually have their disease evolve into a full-blown multiple myeloma.29 Following this line of reasoning, one has to remark that, despite the similar number of malignancy-like clones detected in the blood of healthy subjects, CD5+ B-cell malignancies are more frequent than CD5- diseases.20 This could indicate that the progression of a single “aging” clone toward a clinically evident disease may be due to the likelihood of successive transforming events, rather than to the monoclonal status only. The higher frequency of CLL in the general population suggests either that the possibility of oncogenic “hits” must be more frequent for CLL precursor cells or that CLL precursor cells are intrinsically more prone to transformation.
The question we are facing is whether the presence of monoclonal B cells in the PB of otherwise healthy subjects may have a clinical bearing and if so, to what extent. Experimental approaches including cell purification with cytogenetic and genomic studies coupled to animal experiments can be devised to try to answer this question in the near future. In the meantime, the results of the present study call for increased caution in interpreting cytofluorimetric results in a clinical setting. The widespread use of the evaluation of the κ/λ ratio, during common diagnostic procedures, suggests that clinically silent circulating B-cell clones may be rather easily reported during routine controls, bringing along the difficulty of the interpretation in terms of clinical prognosis. Prospective studies are definitely needed in order to define the features, if any, that can discriminate between “benign B-cell clones” and “progressive B-cell clones” as well as to identify those individuals who would benefit from clinical follow up. The experience with MGUS suggests that this may be a clinical result quite difficult to reach.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-09-3277.
Supported in part by Associazione Italiana per la Ricerca sul Cancro (AIRC), Progetto Oncologia CNR-MIUR, and MURST 40%. C.S. was supported by the “Comitato G. Ghirotti,” Torino, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge the experienced advice and the fruitful discussion received from Dr Carlo Senore, Servizio di Epidemiologia, ASL 1, Torino, Italy. We also thank the health personnel of ASL 8, distretto di Carmagnola, and in particular Dr Dario Filiberto and Dr Nicola Siclari, who made this work possible.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal