Abstract
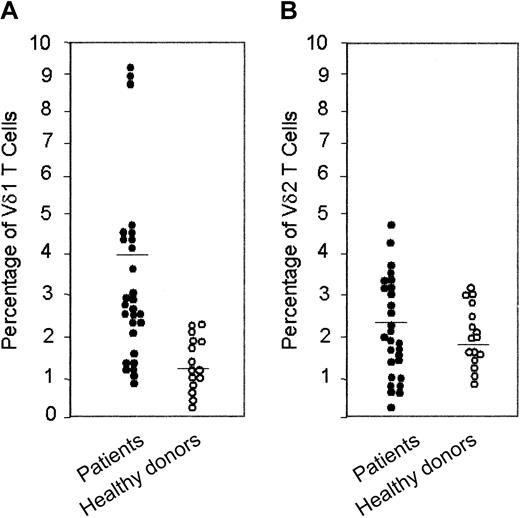
We show that HIV-1–infected patients have increased concentrations of circulating Vδ1 T cells (2.2%-9.0% of T lymphocytes; healthy donors, 1.0%-2%) and, in some instances, Vδ2 T cells (3.5%-4.8% vs 2.0%-3.3%). In these patients, both Vδ1 and Vδ2 T cells are CXCR3+CXCR4+, whereas in healthy donors CXCR4 was preferentially expressed on Vδ1 T lymphocytes. γδ T cells transmigrated across endothelial monolayers, in response to interferon-γ–inducing protein-10 (IP-10/CXCL10), stromal cell–derived factor-1 (SDF-1/CXCL12), or both, according to the expression of the specific receptors CXCR3 and CXCR4. Interestingly, 6Ckine/SLC/CCL21 was more effective than IP-10/CXCL10 on Vδ1 CXCR3+ cells, whereas Vδ2 CXCR3+ cells were driven more efficiently by IP-10/CXCL10. IP-10/CXCL10– and SDF-1/CXCL12–induced transmigration was dependent on phosphoinositide-3 kinase (PI-3K), as demonstrated by the use of the specific blockers wortmannin and LY294002 and by the activation of the downstream serine kinase Akt/PKB on ligation of CXCR3 and CXCR4. Occupancy of CXCR3, but not of CXCR4, led to CAMKII activation; accordingly, the CAMKII inhibitors KN62 and KN93 decreased IP-10/CXCL10– but not SDF-1/CXCL12–driven transmigration. Finally, HIV-1 Tat, which is present in the serum of HIV-1–infected patients, interferes with the chemotactic activity of these chemokines because of the cysteine-rich domain of the protein, which contains CXC and CC chemokine–like sequences.
Introduction
γδ T lymphocytes represent a relevant proportion of the mucosa-associated lymphoid tissue known to be deeply involved in the first-line defense against several pathogens and tumors.1-3 Among γδ T cells, the Vδ2 subset represents most circulating γδ lymphocytes, whereas Vδ1 T cells are resident within epithelial tissues, and it is still unclear whether and how they recirculate.1-3 In AIDS patients, Vδ1 T lymphocytes are increased in the bloodstream and decreased in duodenal mucosa,4,5 suggesting that this subpopulation can recirculate under certain pathologic conditions.
On the other hand, in the acute phase of multiple sclerosis, we reported that the Vδ2 γδ T-cell subset is expanded in the blood of patients. These cells display a high transendothelial migratory capability, apparently in the absence of chemotactic stimuli, and use natural killer receptor protein 1a (NKRP1a/CD161) for this function, through the activation of calcium-calmodulin kinase II (CAMKII).6,7 In contrast, Vδ1 T lymphocytes do not bear NKRP1a and selectively express the platelet-endothelial cell adhesion molecule-1 (PECAM-1), which is involved in their transendothelial migration, leading to the engagement of phosphoinositide-3 kinase (PI-3K) and the activation of Akt/PKB.6,7
Migration of lymphocytes, including γδ T cells, depends not only on the combined actions of various adhesion molecules,8,9 it is controlled by the interactions between chemokines and their receptors.10-14 In human CXC chemokines, 2 of 4 conserved cysteines are separated by an amino acid X, whereas CC chemokines display 2 cysteines side by side.12,13 On the basis of their physiologic features, one can distinguish between “inflammatory” (or inducible) chemokines, which include I-309 (CCL1) and interferon-inducible protein 10 (IP-10, CXCL10), and “homeostatic” (or constitutive) chemokines, such as stromal cell-derived factor-1 (SDF-1, CXCL12) and 6Ckine/SLC (CCL21).13,15,16 Functional studies indicate that circulating γδ T cells migrate in response to chemokine regulated upon activation normal T-cell expressed and secreted (RANTES), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and IP-10.8,14 In terms of chemokine receptors, Vδ2 T lymphocytes express CCR5 (receptor for RANTES, MIP-1α, MIP-1β) and CXCR3 (whose ligands are represented by IP-10/CXCL10 and 6Ckine/SLC/CCL21),13,15,16 which is not always found on the Vδ1 T-cell surface.14
In the present work, we show that (1) in HIV-1–infected patients, a population of Vδ1 T cells coexpressing CXCR4 and CXCR3 is increased in peripheral blood; (2) Vδ1 and Vδ2 γδ T-cell subsets transmigrate in response to the homeostatic or inflammatory chemokines, IP-10/CXCL10 and SDF-1/CXCL12, according to the expression of their specific receptors CXCR3 or CXCR4; (3) IP-10/CXCL10– and 6Ckine/CCL21-induced transmigration are dependent on PI-3K and CAMKII, activated on the ligation of CXCR3, whereas SDF-1/CXCL12 is dependent only on PI-3K; (4) HIV-1 Tat, present in the serum of HIV-1–infected patients, interferes with the chemotactic activity of IP-10/CXCL10, I-309/CCL1, and SDF-1/CXCL12 because of the cysteine-rich domain of the protein, which contains CXC and CC chemokine–like sequences.16-18
Patients, materials, and methods
Patients
Twenty-eight HIV-1–infected patients provided informed consent and were consecutively enrolled in this study conducted at the Infectious Diseases Department (Department of Internal Medicine, University of Genoa). The stage of disease was defined according to the Centers for Disease Control and Prevention (CDC) criteria. Eight patients underwent naive antiretroviral treatment (clinical features, including CD4 and CD8 counts of all patients, are summarized in Table 1). Fifteen seronegative healthy subjects, matched for sex and age, were also studied. HIV-1 RNA was quantitated using the commercial branched DNA (bDNA ultrasensitive assay; Chiron, Amsterdam, Netherlands) with a lower limit of detection of 50 RNA copies mL–1.
Characteristics of HIV-1—infected patients
Patient . | Sex . | Age, y . | HIV-1+ year . | CD4+, % . | CD4+ cells, μL-1 . | CD8+, % . | CD8+ cells, μL-1 . | CD4/CD8 ratio . | Therapy . | Stage* . | HIV RNA, copies/mL-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 32 | 2001 | 36 | 450 | 27 | 340 | 1.3 | No | A | < 80 |
| 2 | F | 31 | 2002 | 24 | 327 | 43 | 473 | 0.7 | No | A | 27 000 |
| 3 | F | 38 | 1988 | 43 | 1159 | 40 | 1080 | 0.9 | No | A | 9000 |
| 4 | F | 55 | 1997 | 41 | 510 | 39 | 487 | 1.0 | No | A | 8100 |
| 5 | M | 42 | 2000 | 22 | 582 | 43 | 1075 | 0.5 | No | A | 17 000 |
| 6 | F | 55 | 2003 | 40 | 783 | 40 | 788 | 1.0 | No | A | 6800 |
| 7 | F | 43 | 1987 | 26 | 378 | 62 | 909 | 0.4 | No | A | < 80 |
| 8 | F | 36 | 1994 | 10 | 55 | 54 | 313 | 0.2 | No | A | 24 000 |
| 9 | F | 69 | 1996 | 15 | 449 | 45 | 1402 | 0.3 | D4T, DDI, EFV | B | < 80 |
| 10 | M | 67 | 1996 | 49 | 981 | 26 | 525 | 1.9 | D4T, 3TC, EFV | A | < 80 |
| 11 | M | 38 | 2001 | 24 | 469 | 42 | 1178 | 0.4 | D4T, DDI, EFV | A | < 80 |
| 12 | M | 62 | 2001 | 34 | 698 | 40 | 800 | 0.9 | AZT, 3TC, ABC | A | < 80 |
| 13 | M | 37 | 2000 | 32 | 662 | 36 | 740 | 0.9 | AZT, 3TC, ABC | A | < 80 |
| 14 | M | 33 | 2001 | 38 | 736 | 36 | 696 | 1.0 | AZT, 3TC, ABC | A | < 80 |
| 15 | M | 73 | 2001 | 31 | 727 | 42 | 966 | 0.7 | AZT, 3TC, EFV | A | < 80 |
| 16 | M | 45 | 2002 | 26 | 294 | 41 | 451 | 0.6 | D4T, DDI, EFV | A | < 80 |
| 17 | M | 46 | 1988 | 29 | 698 | 51 | 1224 | 0.6 | EFU, 3TC, LPN | A | < 80 |
| 18 | F | 59 | 1996 | 13 | 182 | 42 | 588 | 0.3 | D4T, DDI, EFV | C | < 80 |
| 19 | M | 43 | 2000 | 36 | 524 | 31 | 451 | 1.1 | D4T, DDI, EFV | C | < 80 |
| 20 | M | 27 | 2001 | 50 | 1311 | 32 | 836 | 1.6 | AZT, 3TC, ABC | A | < 80 |
| 21 | F | 43 | 2000 | 39 | 667 | 40 | 676 | 0.6 | AZT, 3TC, EFV | A | < 80 |
| 22 | M | 37 | 1985 | 40 | 899 | 36 | 816 | 1.1 | D4T, DDI, EFV | A | < 80 |
| 23 | M | 41 | 1985 | 38 | 452 | 28 | 339 | 1.3 | AZT, 3TC, DDI | A | < 80 |
| 24 | M | 30 | 2000 | 27 | 686 | 16 | 405 | 1.7 | AZT, 3TC, EFV | C | < 80 |
| 25 | F | 34 | 1994 | 47 | 920 | 12 | 235 | 3.9 | D4T, DDI, EFV | A | < 80 |
| 26 | F | 35 | 2000 | 40 | 1295 | 33 | 1076 | 1.2 | AZT, 3TC, EFV | A | < 80 |
| 27 | F | 31 | 1996 | 44 | 921 | 39 | 822 | 1.1 | NVL, SQN-sg | A | < 80 |
| 28 | F | 56 | 2003 | 2 | 8 | 43 | 172 | 0 | AZT, 3TC, LPN, R | A | 5000 |
Patient . | Sex . | Age, y . | HIV-1+ year . | CD4+, % . | CD4+ cells, μL-1 . | CD8+, % . | CD8+ cells, μL-1 . | CD4/CD8 ratio . | Therapy . | Stage* . | HIV RNA, copies/mL-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 32 | 2001 | 36 | 450 | 27 | 340 | 1.3 | No | A | < 80 |
| 2 | F | 31 | 2002 | 24 | 327 | 43 | 473 | 0.7 | No | A | 27 000 |
| 3 | F | 38 | 1988 | 43 | 1159 | 40 | 1080 | 0.9 | No | A | 9000 |
| 4 | F | 55 | 1997 | 41 | 510 | 39 | 487 | 1.0 | No | A | 8100 |
| 5 | M | 42 | 2000 | 22 | 582 | 43 | 1075 | 0.5 | No | A | 17 000 |
| 6 | F | 55 | 2003 | 40 | 783 | 40 | 788 | 1.0 | No | A | 6800 |
| 7 | F | 43 | 1987 | 26 | 378 | 62 | 909 | 0.4 | No | A | < 80 |
| 8 | F | 36 | 1994 | 10 | 55 | 54 | 313 | 0.2 | No | A | 24 000 |
| 9 | F | 69 | 1996 | 15 | 449 | 45 | 1402 | 0.3 | D4T, DDI, EFV | B | < 80 |
| 10 | M | 67 | 1996 | 49 | 981 | 26 | 525 | 1.9 | D4T, 3TC, EFV | A | < 80 |
| 11 | M | 38 | 2001 | 24 | 469 | 42 | 1178 | 0.4 | D4T, DDI, EFV | A | < 80 |
| 12 | M | 62 | 2001 | 34 | 698 | 40 | 800 | 0.9 | AZT, 3TC, ABC | A | < 80 |
| 13 | M | 37 | 2000 | 32 | 662 | 36 | 740 | 0.9 | AZT, 3TC, ABC | A | < 80 |
| 14 | M | 33 | 2001 | 38 | 736 | 36 | 696 | 1.0 | AZT, 3TC, ABC | A | < 80 |
| 15 | M | 73 | 2001 | 31 | 727 | 42 | 966 | 0.7 | AZT, 3TC, EFV | A | < 80 |
| 16 | M | 45 | 2002 | 26 | 294 | 41 | 451 | 0.6 | D4T, DDI, EFV | A | < 80 |
| 17 | M | 46 | 1988 | 29 | 698 | 51 | 1224 | 0.6 | EFU, 3TC, LPN | A | < 80 |
| 18 | F | 59 | 1996 | 13 | 182 | 42 | 588 | 0.3 | D4T, DDI, EFV | C | < 80 |
| 19 | M | 43 | 2000 | 36 | 524 | 31 | 451 | 1.1 | D4T, DDI, EFV | C | < 80 |
| 20 | M | 27 | 2001 | 50 | 1311 | 32 | 836 | 1.6 | AZT, 3TC, ABC | A | < 80 |
| 21 | F | 43 | 2000 | 39 | 667 | 40 | 676 | 0.6 | AZT, 3TC, EFV | A | < 80 |
| 22 | M | 37 | 1985 | 40 | 899 | 36 | 816 | 1.1 | D4T, DDI, EFV | A | < 80 |
| 23 | M | 41 | 1985 | 38 | 452 | 28 | 339 | 1.3 | AZT, 3TC, DDI | A | < 80 |
| 24 | M | 30 | 2000 | 27 | 686 | 16 | 405 | 1.7 | AZT, 3TC, EFV | C | < 80 |
| 25 | F | 34 | 1994 | 47 | 920 | 12 | 235 | 3.9 | D4T, DDI, EFV | A | < 80 |
| 26 | F | 35 | 2000 | 40 | 1295 | 33 | 1076 | 1.2 | AZT, 3TC, EFV | A | < 80 |
| 27 | F | 31 | 1996 | 44 | 921 | 39 | 822 | 1.1 | NVL, SQN-sg | A | < 80 |
| 28 | F | 56 | 2003 | 2 | 8 | 43 | 172 | 0 | AZT, 3TC, LPN, R | A | 5000 |
HIV-1+ year was year of seroconversion.
D4T indicates stavudine; DDI, didanosine; EFV, efavirenz; 3TC, lamivudine; AZT, zidovudine; ABC, abaqavir; NVL, nelfinavir; SQN-sg, saquinavir soft gel; LPN, lopinavir; and R, ritonavir.
Stage was defined according to CDC criteria.
Monoclonal antibodies and reagents
The anti-Vδ1 monoclonal antibody (mAb) A13 and the anti-Vδ2 mAb BB3 (both immunoglobulin G1 [IgG1]) were prepared as described.19 Phycoerythrin (PE)–conjugated anti-CXCR3 (IgG1) or anti-CXCR4 (IgG2a) mAbs were from BD PharMingen Europe (Milan, Italy). The anti-αβ TCR BMA031 and the anti-Vδ1 mAb MCA2080 were obtained from Serotec (Cergy Saint-Christophe, France). Recombinant interleukin-2 (IL-2) was from Pepro Tec EC (London, United Kingdom); SDF-1, I-309, and IP-10 were from R&D Systems Europe (Oxon, United Kingdom); and 6Ckine was from Biosource International (Camarillo, CA). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and with glutamine and penicillin-streptomycin (Biochrom, Berlin, Germany). Phytohemagglutinin (PHA), the PI-3K inhibitors wortmannin and LY294002, goat antimouse (GAM) and murine immunoglobulin were from Sigma Chemical (St Louis, MO), and the CAMKII inhibitors KN62 or KN93 or the inactive compound KN92 were from Calbiochem-Merck (KgaA, Darmstadt, Germany). Anti-Tat rabbit antiserum, synthetic Tat, and the overlapping Tat-derived peptides Tat20-39, Tat24-51, Tat46-60, Tat56-70, and Tat65-80 were purchased from Tecnogen (Piana di Monteverna, Italy); the mutated Tat24-51(25-27Ala) or Tat24-51(30-31Ala) were from PRIMM (Milan, Italy). Biotinylated rabbit anti-Tat IgG was from DBA (Milan, Italy).
Isolation and culture of γδ T-cell populations
Peripheral blood mononuclear cells (PBMCs) from HIV-1–infected patients and healthy donors were isolated using Ficoll-Hypaque gradient. Highly purified CD3+ γδ+ T cells were obtained from PBMCs by negative immunodepletion with anti-CD14–, anti-CD4–, and anti-CD8–coated magnetic microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany). To obtain Vδ1 or Vδ2 T-cell lines, CD4–CD8– cells were seeded under limiting-dilution conditions in 96-well, U-bottomed microplates (Greiner Laborteckinc, Nurtingen, Germany) and were cultured in RPMI 1640 medium supplemented with 200 mM L-glutamine, 10% FCS, 1 μg/mL PHA, 25 U/mL rIL-2, and 105 irradiated feeder cells (3000 rad [3 Gy]) as described.6 Vδ1 cell lines were A13+MCA2080+, Vδ2 cell lines were BB3+, and all were BMA031-negative (not shown).
Immunofluorescence and cytofluorometric analysis
Direct immunofluorescence on peripheral whole blood of healthy donors and HIV-1–infected patients was performed with the PE-conjugated anti-CXCR3 or CXCR4 mAbs and the anti-TCRVδ1 MCA2080 or the A13 or BB3 mAbs labeled with the Zenon Alexafluor 488 mouse IgG1 Labeling Kit (Molecular Probes Europe BV, Leiden, Netherlands). Immunofluorescence staining of cultured cells was performed as described elsewhere.6 Control aliquots were stained with fluorochrome-conjugated, isotype-matched, irrelevant mAbs. Samples were analyzed on a flow cytometer (FACSort; Becton Dickinson) equipped with an argon ion laser exciting Alexafluor 488 and PE at 488 nm. Data were analyzed using Lysis II (version 1.1; Becton Dickinson) and are expressed as Log red fluorescence intensity (arbitrary units [au]) versus Log green fluorescence intensity (au) or compared with number of cells. Calibration was assessed with Calibrite particles using the AutoCOMP computer program (Becton Dickinson).
Transendothelial migration assay
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described6,7 and were used within 4 passages. Endothelial confluent monolayers were tested for their integrity before the migration assay, as described, using Transwell cell culture chambers (polycarbonate filters, 3-μ pore size; Costar, Cambridge, MA).6,7 Vδ1 or Vδ2 T cells were recovered, washed twice, and exposed to 50 ng/mL IP-10/CXCL10, I-309/CCL1, SDF-1/CXCL12, or C6Kine/SCL/CCL21 added to the lower chamber during transmigration. In some experiments, Vδ1 or Vδ2 T-cell lines were preincubated for 10 minutes at 4°C with 100 nM Tat or Tat peptides or for 30 minutes with wortmannin (1-100 nM) or LY294002 (0.2-20 μM), KN62 or KN93 or KN92 (0.1-10 μM) and were washed before the transmigration assay. In other experiments, cells from HIV-1–infected patients were incubated with autologous serum (1:2 dilution), in the absence or presence of the rabbit anti-Tat antiserum (Tecnogen) used at 1:400 dilution. To quantitatively express the results of transmigration assays, Vδ1 or Vδ2 T-cell lines were labeled with chromium Cr 51 (51Cr; sodium chromate; NEN, Boston, MA) and were added to the upper compartment of the Transwell chamber, as described.6,7 At different time points, migrated cells were recovered from the lower compartment of the chamber and were lysed with 100 mM Tris-HCl (pH 7.4) 0.1% Triton X-100. The radioactivity of the samples was measured in a gamma-counter. Results are expressed as the percentage of migrating cells calculated as described.6,7 Statistical analysis was performed using the Student t test.
Measurement of CAMKII or PI-3K activation
CAMKII activation in γδ cells was tested on cross-linking of CXCR3, CXCR4, γδ T-cell receptor (TCR) obtained with the specific mAbs (5 μg/mL) followed by GAM, in the absence or presence of the CAMKII inhibitor KN62 or with the inactive compound KN92. CAMKII was measured with the CAMKII assay kit, using the specific substrate and 32P-γ adenosine triphosphate (ATP), after immunoprecipitation with the specific anti-CAMKII antibody (Upstate Biotechnology, Lake Placid, NY) and chromatography.7 Results are expressed as cpm × 10–3 and are the mean ± SD of triplicate samples. PI-3K activity was tested indirectly by analyzing the phosphorylation of the downstream serine/threonine kinase Akt1/PKBα (p-Akt) in cell lysates of γδ T cells, assessed with the enzyme-linked immunosorbent assay (ELISA) kit (BioSource Europe, Nivelles, Belgium) on the ligation of CXCR3 or CXCR4, in the presence or absence of the PI-3K inhibitor LY294002. As a control, cells were exposed to murine immunoglobulin plus GAM. The same samples were analyzed for the content of total Akt with a specific ELISA assay kit (BioSource). Results are expressed as the percentage of p-Akt, normalized for total Akt (units/106 cells), and are the mean ± SD of 3 independent experiments.
Measurement of Tat in patient sera
Tat was measured in the serum of HIV-1 patients by ELISA using a rabbit anti-Tat antiserum (10 μg/mL), directed to synthetic Tat, as capture antibody and a biotinylated rabbit anti-Tat antiserum (1 μg/mL), raised against recombinant Tat, as detection antibody, followed by avidin–horseradish peroxidase (Av-HRP, 1:2000; Sigma) and by the substrate (2,2′-azino-bis(3-ethylbenzthyazoline-6-sulfonic acid); Sigma). Sera from HIV-1–HBV+/HCV+ patients or healthy donors were analyzed for comparison. Optical density (OD) of each sample was measured at 450 nm, and results expressed in nanograms per milliliter were referred to synthetic Tat used as the standard.
Results
Expression of CXCR4 and CXCR3 on Vδ1 or Vδ2 T-cell subsets in HIV-1–infected patients and healthy donors
Cytofluorometric analysis of peripheral whole blood of 28 HIV-1–infected patients showed that 19 of 28 patients have increased levels of circulating Vδ1 T cells that usually belong to the “resident” γδ T-cell population, ranging between 2.2% and 9.0% of T lymphocytes (healthy donors, 0.4%-2.3%; Figure 1A; Table 2). Five of these patients also displayed an increased percentage of Vδ2 T cells (3.2%-4.8% vs 0.8%-3.1% in healthy donors; Figure 1B; Table 2).
Percentage of Vδ1 and Vδ2 T cells in peripheral blood of HIV-1–infected patients and healthy donors. Ex vivo analysis of circulating lymphocytes from HIV-1–infected patients compared with healthy donors, as indicated: direct immunofluorescence of lymphocytes in whole blood, with Alexafluor 488–labeled anti-Vδ1 (A) or anti-Vδ2 (B) mAbs. Samples were analyzed using FACS gated to lymphocytes, and results are expressed as percentage of positive cells.
Percentage of Vδ1 and Vδ2 T cells in peripheral blood of HIV-1–infected patients and healthy donors. Ex vivo analysis of circulating lymphocytes from HIV-1–infected patients compared with healthy donors, as indicated: direct immunofluorescence of lymphocytes in whole blood, with Alexafluor 488–labeled anti-Vδ1 (A) or anti-Vδ2 (B) mAbs. Samples were analyzed using FACS gated to lymphocytes, and results are expressed as percentage of positive cells.
Expression of CXCR3 or CXCR4 on Vδ1 or Vδ2 T cells in peripheral blood of HIV-1—infected patients and healthy donors
. | Vδ2+ . | Vδ2+ CXCR3+ . | Vδ2+ CXCR4+ . | Vδ1+ . | Vδ1+ CXCR3+ . | Vδ1+ CXCR4+ . |
|---|---|---|---|---|---|---|
| Patients | ||||||
| 1 | 1.0 | 0.9 | 0.9 | 1.0 | 0.8 | 1.0 |
| 2 | 1.6 | 1.5 | 1.6 | 1.2 | 1.0 | 1.2 |
| 3 | 0.7 | 0.6 | 0.7 | 1.4 | 1.3 | 1.2 |
| 4 | 1.9 | 1.8 | 1.7 | 2.2 | 1.8 | 2.0 |
| 5 | 0.7 | 0.7 | 0.7 | 4.2 | 3.2 | 3.6 |
| 6 | 2.7 | 2.8 | 2.3 | 4.1 | 2.0 | 3.9 |
| 7 | 0.3 | 0.2 | 0.3 | 1.0 | 0.9 | 1.1 |
| 8 | 3.8 | 2.8 | 3.1 | 2.2 | 1.6 | 1.8 |
| 9 | 0.4 | 0.3 | 0.4 | 2.3 | 2.0 | 1.9 |
| 10 | 2.4 | 2.3 | 2.6 | 2.9 | 1.9 | 2.9 |
| 11 | 0.3 | 0.2 | 0.3 | 8.8 | 4.3 | 8.6 |
| 12 | 1.3 | 1.3 | 1.2 | 2.7 | 1.7 | 1.4 |
| 13 | 1.1 | 1.0 | 1.3 | 2.3 | 1.6 | 2.0 |
| 14 | 3.0 | 1.9 | 1.6 | 1.2 | 1.0 | 1.2 |
| 15 | 1.0 | 0.9 | 0.7 | 2.2 | 1.3 | 2.2 |
| 16 | 3.2 | 2.4 | 2.0 | 4.1 | 2.3 | 3.9 |
| 17 | 3.5 | 2.2 | 1.0 | 4.2 | 2.0 | 1.7 |
| 18 | 4.0 | 3.3 | 1.1 | 9.2 | 2.9 | 4.8 |
| 19 | 2.0 | 1.9 | 1.0 | 0.9 | 0.6 | 0.4 |
| 20 | 1.8 | 1.4 | 1.3 | 4.4 | 3.3 | 3.3 |
| 21 | 3.4 | 2.9 | 2.6 | 9.3 | 7.3 | 6.4 |
| 22 | 1.4 | 1.4 | 1.6 | 2.7 | 1.7 | 2.7 |
| 23 | 0.4 | 0.2 | 0.2 | 2.0 | 0.8 | 0.6 |
| 24 | 4.8 | 3.8 | 4.8 | 3.0 | 2.9 | 2.0 |
| 25 | 3.3 | 2.5 | 3.2 | 1.8 | 1.5 | 1.7 |
| 26 | 2.2 | 0.8 | 0.4 | 3.6 | 1.2 | 0.7 |
| 27 | 2.0 | 2.0 | 2.2 | 2.7 | 1.7 | 2.2 |
| 28 | 1.4 | 0.7 | 1.4 | 4.0 | 2.8 | 3.9 |
| Healthy donors | ||||||
| 1 | 0.9 | 0.8 | 0.2 | 1.9 | 0.8 | 1.6 |
| 2 | 1.3 | 1.2 | 0.3 | 1.2 | 0.6 | 1.2 |
| 3 | 3.1 | 2.8 | 0.7 | 0.9 | 0.6 | 0.8 |
| 4 | 2.9 | 2.2 | 0.6 | 0.6 | 0.2 | 0.5 |
| 5 | 0.8 | 0.6 | 0.1 | 0.5 | 0.2 | 0.5 |
| 6 | 1.4 | 1.0 | 0.3 | 2.3 | 0.9 | 2.1 |
| 7 | 2.6 | 2.2 | 0.6 | 0.8 | 0.2 | 0.6 |
| 8 | 2.0 | 1.9 | 0.2 | 0.2 | 0.1 | 0.1 |
| 9 | 2.3 | 2.0 | 0.3 | 2.2 | 1.0 | 2.1 |
| 10 | 1.6 | 1.2 | 0.9 | 2.0 | 0.9 | 1.9 |
| 11 | 2.8 | 2.0 | 0.3 | 0.8 | 0.4 | 0.7 |
| 12 | 1.7 | 1.3 | 0.2 | 1.8 | 1.0 | 1.7 |
| 13 | 1.8 | 1.0 | 1.1 | 1.7 | 0.9 | 0.6 |
| 14 | 1.9 | 1.8 | 0.5 | 1.1 | 0.5 | 0.9 |
| 15 | 2.4 | 1.6 | 0.2 | 0.4 | 0.2 | 0.4 |
. | Vδ2+ . | Vδ2+ CXCR3+ . | Vδ2+ CXCR4+ . | Vδ1+ . | Vδ1+ CXCR3+ . | Vδ1+ CXCR4+ . |
|---|---|---|---|---|---|---|
| Patients | ||||||
| 1 | 1.0 | 0.9 | 0.9 | 1.0 | 0.8 | 1.0 |
| 2 | 1.6 | 1.5 | 1.6 | 1.2 | 1.0 | 1.2 |
| 3 | 0.7 | 0.6 | 0.7 | 1.4 | 1.3 | 1.2 |
| 4 | 1.9 | 1.8 | 1.7 | 2.2 | 1.8 | 2.0 |
| 5 | 0.7 | 0.7 | 0.7 | 4.2 | 3.2 | 3.6 |
| 6 | 2.7 | 2.8 | 2.3 | 4.1 | 2.0 | 3.9 |
| 7 | 0.3 | 0.2 | 0.3 | 1.0 | 0.9 | 1.1 |
| 8 | 3.8 | 2.8 | 3.1 | 2.2 | 1.6 | 1.8 |
| 9 | 0.4 | 0.3 | 0.4 | 2.3 | 2.0 | 1.9 |
| 10 | 2.4 | 2.3 | 2.6 | 2.9 | 1.9 | 2.9 |
| 11 | 0.3 | 0.2 | 0.3 | 8.8 | 4.3 | 8.6 |
| 12 | 1.3 | 1.3 | 1.2 | 2.7 | 1.7 | 1.4 |
| 13 | 1.1 | 1.0 | 1.3 | 2.3 | 1.6 | 2.0 |
| 14 | 3.0 | 1.9 | 1.6 | 1.2 | 1.0 | 1.2 |
| 15 | 1.0 | 0.9 | 0.7 | 2.2 | 1.3 | 2.2 |
| 16 | 3.2 | 2.4 | 2.0 | 4.1 | 2.3 | 3.9 |
| 17 | 3.5 | 2.2 | 1.0 | 4.2 | 2.0 | 1.7 |
| 18 | 4.0 | 3.3 | 1.1 | 9.2 | 2.9 | 4.8 |
| 19 | 2.0 | 1.9 | 1.0 | 0.9 | 0.6 | 0.4 |
| 20 | 1.8 | 1.4 | 1.3 | 4.4 | 3.3 | 3.3 |
| 21 | 3.4 | 2.9 | 2.6 | 9.3 | 7.3 | 6.4 |
| 22 | 1.4 | 1.4 | 1.6 | 2.7 | 1.7 | 2.7 |
| 23 | 0.4 | 0.2 | 0.2 | 2.0 | 0.8 | 0.6 |
| 24 | 4.8 | 3.8 | 4.8 | 3.0 | 2.9 | 2.0 |
| 25 | 3.3 | 2.5 | 3.2 | 1.8 | 1.5 | 1.7 |
| 26 | 2.2 | 0.8 | 0.4 | 3.6 | 1.2 | 0.7 |
| 27 | 2.0 | 2.0 | 2.2 | 2.7 | 1.7 | 2.2 |
| 28 | 1.4 | 0.7 | 1.4 | 4.0 | 2.8 | 3.9 |
| Healthy donors | ||||||
| 1 | 0.9 | 0.8 | 0.2 | 1.9 | 0.8 | 1.6 |
| 2 | 1.3 | 1.2 | 0.3 | 1.2 | 0.6 | 1.2 |
| 3 | 3.1 | 2.8 | 0.7 | 0.9 | 0.6 | 0.8 |
| 4 | 2.9 | 2.2 | 0.6 | 0.6 | 0.2 | 0.5 |
| 5 | 0.8 | 0.6 | 0.1 | 0.5 | 0.2 | 0.5 |
| 6 | 1.4 | 1.0 | 0.3 | 2.3 | 0.9 | 2.1 |
| 7 | 2.6 | 2.2 | 0.6 | 0.8 | 0.2 | 0.6 |
| 8 | 2.0 | 1.9 | 0.2 | 0.2 | 0.1 | 0.1 |
| 9 | 2.3 | 2.0 | 0.3 | 2.2 | 1.0 | 2.1 |
| 10 | 1.6 | 1.2 | 0.9 | 2.0 | 0.9 | 1.9 |
| 11 | 2.8 | 2.0 | 0.3 | 0.8 | 0.4 | 0.7 |
| 12 | 1.7 | 1.3 | 0.2 | 1.8 | 1.0 | 1.7 |
| 13 | 1.8 | 1.0 | 1.1 | 1.7 | 0.9 | 0.6 |
| 14 | 1.9 | 1.8 | 0.5 | 1.1 | 0.5 | 0.9 |
| 15 | 2.4 | 1.6 | 0.2 | 0.4 | 0.2 | 0.4 |
Direct immunofluorescence on whole blood of HIV-1-infected patients was performed with PE-anti-CXCR3 or -CXCR4 mAbs, and the anti-Vδ1 A13 or the anti-Vδ2 BB3 mAbs were labeled using the Zenon Alexafluor 488 mouse IgG1 Labeling Kit. Samples were analyzed on a flow cytometer equipped with an argon ion laser exciting PE and Alexafluor at 488 nm and gated on lymphocytes. Data are expressed as percentage of positive or double-positive cells.
To understand the mechanisms possibly underlying the increased number of circulating Vδ1 T cells in HIV-1–infected patients, we analyzed the expression of CXCR3 and CXCR4 chemokine receptors on Vδ1 and Vδ2 γδ T cells, in the peripheral blood of healthy donors or HIV-1–infected patients. We found that in 11 of 15 healthy donors, Vδ1 T lymphocytes are CXCR4+ (Figure 2A; Table 2), with 70% of the cells CXCR3+ and 30% CXCR3– (Figure 2B; Table 2). In 4 donors, however, Vδ1 T cells were CXCR4dull or negative (Figure 2C; Table 2). The Vδ2 T-cell subset was CXCR4dull (Figure 2D) or negative in most of the donors (Figure 2F; Table 2); furthermore, Vδ2 T cells were always CXCR3+ (Figure 2E; Table 2).
Expression of CXCR3 and CXCR4 on Vδ1 and Vδ2 γδ T cells in healthy donors. Ex vivo analysis of circulating lymphocytes from healthy donors (2 representative phenotypes of 15 donors): double immunofluorescence of lymphocytes in the whole blood, with Alexafluor 488–labeled anti-Vδ1 (A-C) or anti-Vδ2 (D-F) mAbs compared with PE-conjugated anti-CXCR4 mAb (A,C,D,F) or PE-conjugated anti-CXCR3 mAb (B,E). Samples were analyzed using FACS gated to lymphocytes, and results are expressed as log green (x-axis) compared with log red (y-axis) fluorescence intensity (arbitrary units [au]). Numbers in the quadrants of each panel indicate the percentage of double-positive (upper right) or single-positive (upper left and lower right) cells.
Expression of CXCR3 and CXCR4 on Vδ1 and Vδ2 γδ T cells in healthy donors. Ex vivo analysis of circulating lymphocytes from healthy donors (2 representative phenotypes of 15 donors): double immunofluorescence of lymphocytes in the whole blood, with Alexafluor 488–labeled anti-Vδ1 (A-C) or anti-Vδ2 (D-F) mAbs compared with PE-conjugated anti-CXCR4 mAb (A,C,D,F) or PE-conjugated anti-CXCR3 mAb (B,E). Samples were analyzed using FACS gated to lymphocytes, and results are expressed as log green (x-axis) compared with log red (y-axis) fluorescence intensity (arbitrary units [au]). Numbers in the quadrants of each panel indicate the percentage of double-positive (upper right) or single-positive (upper left and lower right) cells.
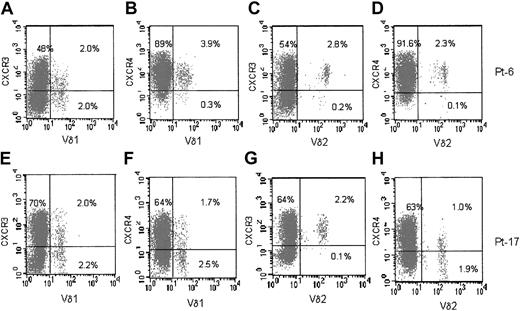
In HIV-1–infected patients, both Vδ1 and Vδ2 T cells were CXCR3+ (Figure 3, panels A and E for Vδ1, panels C and G for Vδ2 subset) and CXCR4+ (panel B for Vδ1, panel D for Vδ2 cells). In some (4 of 28) cases either population was CXCR4dull or negative (Figure 3F, H). Thus, the main difference between healthy donors and HIV-1–infected patients, in addition to the number of circulating Vδ1 T cells, is the expression of CXCR4 on Vδ1 and Vδ2 populations in HIV-1 patients.
Expression of CXCR3 and CXCR4 on Vδ1 and Vδ2 γδ T cells in HIV-1–infected patients. Ex vivo analysis of circulating lymphocytes from HIV-1–infected patients (2 representative phenotypes of 28 patients). (A-D) Patient 6. (E-H) Patient 17. Double immunofluorescence of lymphocytes in the whole blood, with Alexafluor 488–labeled anti-Vδ1 (A,B,E,F) or anti-Vδ2 (C,D,G,H) mAbs compared with PE-conjugated anti-CXCR3 mAb (A,C,E,G) or PE-conjugated anti-CXCR4 mAb (B,D,F,H). Samples were analyzed using FACS gated to lymphocytes, and results are expressed as log green (x-axis) compared with log red (y-axis) fluorescence intensity. Numbers in the quadrants of each panel indicate the percentage of double-positive (upper right) or single-positive (upper left and lower right) cells.
Expression of CXCR3 and CXCR4 on Vδ1 and Vδ2 γδ T cells in HIV-1–infected patients. Ex vivo analysis of circulating lymphocytes from HIV-1–infected patients (2 representative phenotypes of 28 patients). (A-D) Patient 6. (E-H) Patient 17. Double immunofluorescence of lymphocytes in the whole blood, with Alexafluor 488–labeled anti-Vδ1 (A,B,E,F) or anti-Vδ2 (C,D,G,H) mAbs compared with PE-conjugated anti-CXCR3 mAb (A,C,E,G) or PE-conjugated anti-CXCR4 mAb (B,D,F,H). Samples were analyzed using FACS gated to lymphocytes, and results are expressed as log green (x-axis) compared with log red (y-axis) fluorescence intensity. Numbers in the quadrants of each panel indicate the percentage of double-positive (upper right) or single-positive (upper left and lower right) cells.
Migratory response to homeostatic or inflammatory chemokines by Vδ1 or Vδ2 T lymphocytes
We further investigated the ability of the 2 γδ T-cell subsets to transmigrate across endothelial monolayers in response to CXCR3 or CXCR4 chemokines. To this aim, γδ T-cell lines were derived from healthy donors; clones preferentially expressing CXCR3 or CXCR4 or coexpressing the 2 chemokine receptors, obtained from healthy donors, were selected for further functional studies. Fluorescence-activated cell sorter (FACS) analysis of cloned Vδ2 or Vδ1 γδ T lymphocytes obtained from healthy donors showed that both subpopulations are CCR5+ CCR3–, in keeping with previous data,14 and display different phenotypes with regard to CXCR3 or CXCR4 expression or coexpression. Among 40 Vδ2 and 30 Vδ1 clones analyzed, obtained from 6 healthy donors, 24 Vδ2 clones were CXCR3+ CXCR4–, 3 were double negative, 8 were double positive, and 5 were CXCR3– and CXCR4+; on the other hand, 12 Vδ1 clones were CXCR3– and CXCR4+, 4 were double negative, 4 were double positive, and 10 were CXCR3+ CXCR4– (not shown in figures or tables).
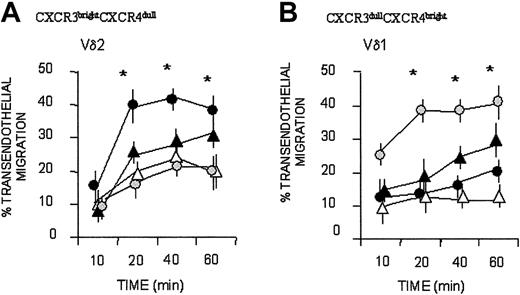
Bulk cultured Vδ2 T cells that were CXCR3bright and CXCR4dull displayed more efficient, faster kinetics of transendothelial migration to IP-10/CXCL10, which binds to CXCR3 (Figure 4A; mean ± SD of 6 cell lines from 6 healthy donors), than to the CXCR4-specific chemokine SDF-1; in turn, SDF-1 was more effective for Vδ1 T cells, expressing CXCR3dull and CXCR4bright (Figure 3B; mean ± SD of 6 cell lines from 6 healthy donors). Both subsets responded to I-309/CCL1 (Figure 3A-B). A certain degree of spontaneous (nonchemokine-driven, Nil in Figure 4) transendothelial migration was observed at 40 minutes, particularly for Vδ2 T lymphocytes, in agreement with our previous reports.6,7
Vδ1 and Vδ2 T-cell migration in response to CXC and CC chemokines. Vδ2 (A) and Vδ1 (B) γδ T-cell lines were assayed for transmigration across HUVEC monolayers at different time points, in the absence (Nil, ▵) or presence of the chemokines SDF-1/CXCL12 ( ), IP-10/CXCL10 (•), I-309/CCL1 (▴), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 6 independent experiments with cell lines from 6 donors. *Student t test; P < .05.
), IP-10/CXCL10 (•), I-309/CCL1 (▴), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 6 independent experiments with cell lines from 6 donors. *Student t test; P < .05.
Vδ1 and Vδ2 T-cell migration in response to CXC and CC chemokines. Vδ2 (A) and Vδ1 (B) γδ T-cell lines were assayed for transmigration across HUVEC monolayers at different time points, in the absence (Nil, ▵) or presence of the chemokines SDF-1/CXCL12 ( ), IP-10/CXCL10 (•), I-309/CCL1 (▴), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 6 independent experiments with cell lines from 6 donors. *Student t test; P < .05.
), IP-10/CXCL10 (•), I-309/CCL1 (▴), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 6 independent experiments with cell lines from 6 donors. *Student t test; P < .05.
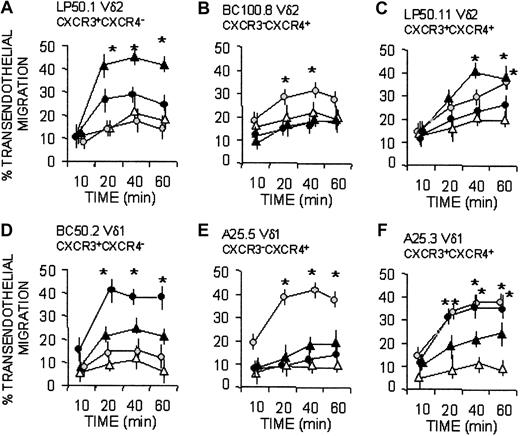
Along this line, CXCR3+ Vδ2 clones (clone LP50.1 and LP50.11 in Figure 5A, C) migrated when challenged with IP-10/CXCL10, whereas BC100.8, which did not respond to this chemokine (Figure 5B), was CXCR3 negative. On the other hand, the CXCR4+ Vδ1 clones (A25.5 and A25.3) migrated in response to SDF-1/CXCL12 (Figure 5E-F), whereas the Vδ1 clone BC50.2, which did not respond to this chemokine (Figure 5D), was CXCR4 negative. Given that Vδ1 T-cell clones expressing CXCR3 (clone BC50.2 and A25.3) were less responsive to IP-10 than CXCR3+ Vδ2 clones (LP50.1 and LP50.11), we used 6CK/SLC/CCL21, a tissue-related, homeostatic chemokine known to interact with CXCR3,13,15,16 in transendothelial migration assays. Interestingly, 6Ckine/SLC/CCL21 was more effective than IP-10/CXCL10 in inducing the transmigration of Vδ1/CXCR3+ clones (Figure 5D, F), at variance with Vδ2/CXCR3+ cells, which were driven more efficiently by IP-10/CXCL10 (Figure 5A, C). Similarly, when Vδ2 T-cell clones expressed CXCR4 (BC100.8 and LP50.11), they showed a slower kinetics of response to SDF-1/CXCL12 (Figure 5B-C) than Vδ1/CXCR4+ clones (Figure 5E-F).
Vδ1 and Vδ2 T-cell migration in response to homeostatic (SDF-1/CXCL12 and 6CK/SLC/CCL21) or inflammatory (IP-10/CXCL10) chemokines. Vδ2 (A-C) and Vδ1 (D-F) γδ T-cell clones, with distinct expression of CXCR3 and CXCR4, as indicated, were assayed for transmigration across HUVEC monolayers at different time points in the absence (Nil, ▵) or presence of the chemokines SDF-1/CXCL12 ( ), IP-10/CXCL10 (▴), or 6CK/SLC/CCL21 (•), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
), IP-10/CXCL10 (▴), or 6CK/SLC/CCL21 (•), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
Vδ1 and Vδ2 T-cell migration in response to homeostatic (SDF-1/CXCL12 and 6CK/SLC/CCL21) or inflammatory (IP-10/CXCL10) chemokines. Vδ2 (A-C) and Vδ1 (D-F) γδ T-cell clones, with distinct expression of CXCR3 and CXCR4, as indicated, were assayed for transmigration across HUVEC monolayers at different time points in the absence (Nil, ▵) or presence of the chemokines SDF-1/CXCL12 ( ), IP-10/CXCL10 (▴), or 6CK/SLC/CCL21 (•), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
), IP-10/CXCL10 (▴), or 6CK/SLC/CCL21 (•), all at 50 ng/mL. Results are expressed as percentage of transendothelial migration, calculated as described in “Patients, materials, and methods” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
Superimposable results were obtained with 14 clones (not shown), derived from 6 donors and expressing the different phenotypes cited in Figure 5. Taken together these data suggest that, in healthy persons, the resident Vδ1 T-cell population preferentially responds to homeostatic, constitutive chemokines, whereas circulating Vδ2 T cells are more sensitive to inflammatory chemokines.
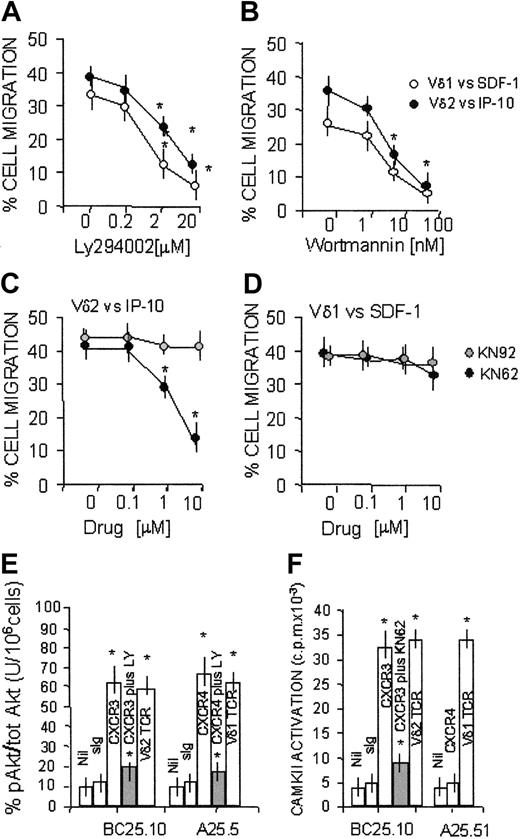
Role of PI-3K and CAMKII in SDF-1 or IP-10/6CKine-driven transmigration of γδ T-cell subsets
It has been reported that CXC chemokines can stimulate PI-3K–dependent chemotaxis of leukocytes20 ; in particular, this enzyme is involved in the SDF-1–induced polarization of lymphocytes21 and sustains prolonged signal transduction through either CXCR4 and CXCR3.23,24 In addition, we reported that the transendothelial migration of Vδ2 lymphocytes requires the activation of CAMKII.7
To determine the role of these kinases in the chemokine-driven migration of Vδ1 or Vδ2 T lymphocytes, these 2 cell subsets were exposed to either PI-3K or CAMKII inhibitors and then were added to endothelial monolayers. These experiments were performed at 60 minutes because at this time point the difference between basal and chemokine-driven migration of both γδ T-cell subsets was maximal (Figure 4). We found that transendothelial migration of Vδ2 or Vδ1 T lymphocytes induced by IP-10 or SDF-1, respectively, was inhibited by the PI-3K blockers LY294002 (Figure 6A) or wortmannin (Figure 6B), in agreement with the finding that CXCR3 and CXCR4 receptors are coupled to this kinase.22,23 Of note, when Vδ2 or Vδ1 T lymphocytes were challenged by IP-10/CXCL10 (Figure 6C) or by 6CK/SLC/CCL21 (not shown), respectively, both acting on CXCR3, transendothelial migration of the 2 γδ T-cell subsets was inhibited by the CAMKII-specific inhibitor KN62. By contrast, KN62 was not effective on cell migration induced by SDF-1/CXCL12 on the Vδ1 subpopulation expressing the specific CXCR4 receptor (Figure 6D). Similar results were obtained with another CAMKII blocker, KN93 (not shown), but not with the inactive compound KN92 (Figure 6C-D). Thus, at variance with CXCR4, CXCR3 appears to be coupled to PI-3K and CAMKII signal transduction pathways.
Transmigration of Vδ1 and Vδ2 T cells to CXCR3 or CXCR4-directed chemokine dependency on PI-3K and CAMKII. Migration across HUVEC monolayers of Vδ2 and Vδ1 γδ T-cell lines, at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 or SDF-1/CXCL12 as described in “Patients, materials, and methods.” (A-B) Effect of the PI-3K inhibitors LY294002 (20-0.2 μM; panel A) or wortmannin (1-100 nM; panel B). ○ indicates Vδ1 cells with SDF-1/CXCL12; •, Vδ2 cells with IP-10/CXCL10. (C-D) Effect of the CAMKII blocker KN62 (10-0.1 μM; •) or the inactive compound KN92 (10-0.1 μM,  on Vδ2 cells with IP-10 (C) or Vδ1 cells with SDF-1 (D). Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). In the absence of chemokines, at 60 minutes the transendothelial migration of Vδ2 or Vδ1 T cells was 15% ± 5% or 10% ± 3%, respectively (not shown). *Student t test; P < .05. (E-F) CXCR4, CXCR3, or TCR was engaged on Vδ1 or Vδ2 T cells using the specific mAbs (5 μg/mL) followed by GAM (100 μg/mL). Nil indicates GAM alone; sIg, murine immunoglobulin followed by GAM. (E) PI-3K activity tested by analyzing the phosphorylation of Akt1/PKB (pAkt) in cell lysates, using ELISA for phosphorylated Akt, normalized for total Akt, in the absence or presence of the PI-3K inhibitor LY294002 (20μM; ▦). Results are expressed as percentage units of pAkt compared with total Akt/106 cells (mean ± SD of 3 independent experiments). (F) CAMKII activity assessed using the specific substrates and 32P-γ ATP after lysis of Vδ2 or Vδ1 T cells, untreated or pretreated with the specific CAMKII blocker KN62 (10 μM; ▦) and immunoprecipitation with anti-CAMKII–specific mAbs. Results are expressed as cpm × 10–3 and are the mean ± SD from 3 independent experiments with cell lines from 3 different donors. *Student t test; P < .05.
on Vδ2 cells with IP-10 (C) or Vδ1 cells with SDF-1 (D). Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). In the absence of chemokines, at 60 minutes the transendothelial migration of Vδ2 or Vδ1 T cells was 15% ± 5% or 10% ± 3%, respectively (not shown). *Student t test; P < .05. (E-F) CXCR4, CXCR3, or TCR was engaged on Vδ1 or Vδ2 T cells using the specific mAbs (5 μg/mL) followed by GAM (100 μg/mL). Nil indicates GAM alone; sIg, murine immunoglobulin followed by GAM. (E) PI-3K activity tested by analyzing the phosphorylation of Akt1/PKB (pAkt) in cell lysates, using ELISA for phosphorylated Akt, normalized for total Akt, in the absence or presence of the PI-3K inhibitor LY294002 (20μM; ▦). Results are expressed as percentage units of pAkt compared with total Akt/106 cells (mean ± SD of 3 independent experiments). (F) CAMKII activity assessed using the specific substrates and 32P-γ ATP after lysis of Vδ2 or Vδ1 T cells, untreated or pretreated with the specific CAMKII blocker KN62 (10 μM; ▦) and immunoprecipitation with anti-CAMKII–specific mAbs. Results are expressed as cpm × 10–3 and are the mean ± SD from 3 independent experiments with cell lines from 3 different donors. *Student t test; P < .05.
Transmigration of Vδ1 and Vδ2 T cells to CXCR3 or CXCR4-directed chemokine dependency on PI-3K and CAMKII. Migration across HUVEC monolayers of Vδ2 and Vδ1 γδ T-cell lines, at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 or SDF-1/CXCL12 as described in “Patients, materials, and methods.” (A-B) Effect of the PI-3K inhibitors LY294002 (20-0.2 μM; panel A) or wortmannin (1-100 nM; panel B). ○ indicates Vδ1 cells with SDF-1/CXCL12; •, Vδ2 cells with IP-10/CXCL10. (C-D) Effect of the CAMKII blocker KN62 (10-0.1 μM; •) or the inactive compound KN92 (10-0.1 μM,  on Vδ2 cells with IP-10 (C) or Vδ1 cells with SDF-1 (D). Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). In the absence of chemokines, at 60 minutes the transendothelial migration of Vδ2 or Vδ1 T cells was 15% ± 5% or 10% ± 3%, respectively (not shown). *Student t test; P < .05. (E-F) CXCR4, CXCR3, or TCR was engaged on Vδ1 or Vδ2 T cells using the specific mAbs (5 μg/mL) followed by GAM (100 μg/mL). Nil indicates GAM alone; sIg, murine immunoglobulin followed by GAM. (E) PI-3K activity tested by analyzing the phosphorylation of Akt1/PKB (pAkt) in cell lysates, using ELISA for phosphorylated Akt, normalized for total Akt, in the absence or presence of the PI-3K inhibitor LY294002 (20μM; ▦). Results are expressed as percentage units of pAkt compared with total Akt/106 cells (mean ± SD of 3 independent experiments). (F) CAMKII activity assessed using the specific substrates and 32P-γ ATP after lysis of Vδ2 or Vδ1 T cells, untreated or pretreated with the specific CAMKII blocker KN62 (10 μM; ▦) and immunoprecipitation with anti-CAMKII–specific mAbs. Results are expressed as cpm × 10–3 and are the mean ± SD from 3 independent experiments with cell lines from 3 different donors. *Student t test; P < .05.
on Vδ2 cells with IP-10 (C) or Vδ1 cells with SDF-1 (D). Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). In the absence of chemokines, at 60 minutes the transendothelial migration of Vδ2 or Vδ1 T cells was 15% ± 5% or 10% ± 3%, respectively (not shown). *Student t test; P < .05. (E-F) CXCR4, CXCR3, or TCR was engaged on Vδ1 or Vδ2 T cells using the specific mAbs (5 μg/mL) followed by GAM (100 μg/mL). Nil indicates GAM alone; sIg, murine immunoglobulin followed by GAM. (E) PI-3K activity tested by analyzing the phosphorylation of Akt1/PKB (pAkt) in cell lysates, using ELISA for phosphorylated Akt, normalized for total Akt, in the absence or presence of the PI-3K inhibitor LY294002 (20μM; ▦). Results are expressed as percentage units of pAkt compared with total Akt/106 cells (mean ± SD of 3 independent experiments). (F) CAMKII activity assessed using the specific substrates and 32P-γ ATP after lysis of Vδ2 or Vδ1 T cells, untreated or pretreated with the specific CAMKII blocker KN62 (10 μM; ▦) and immunoprecipitation with anti-CAMKII–specific mAbs. Results are expressed as cpm × 10–3 and are the mean ± SD from 3 independent experiments with cell lines from 3 different donors. *Student t test; P < .05.
To further confirm these data, CAMKII activity and PI-3K–dependent activation of the downstream serine-kinase Akt/PKB were measured in γδ T cells with the ligation of CXCR3 or CXCR4, in comparison with Vδ1 or Vδ2 TCR, by the use of the specific mAbs. As shown in Figure 6E, triggering CXCR3 on a CXCR3+ Vδ2 T-cell clone (BC25.10) led to the activation of Akt/PKB, which was inhibited by the PI-3K blocker LY294002; of note, CXCR3 oligomerization also elicited CAMKII activation, which was reduced in the presence of the specific blocker KN62 (Figure 6F). In turn, CXCR4 engagement on the CXCR4+ Vδ1 T-cell clone A25.5 could only induce the triggering of Akt/PKB (Figure 6E), but not that of CAMKII (Figure 6F). These data indicate that CXCR3-driven transendothelial migration and signal transduction are dependent on CAMKII in addition to PI-3K.
Although not shown, it should be mentioned that either CAMKII or PI-3K blockers could inhibit spontaneous (in the absence of chemokines) transendothelial migration of Vδ2 (from 15% ± 5% to 5% ± 3% when exposed to KN62) or Vδ1 (from 10% ± 3% to 4% ± 2% in the presence of LY294002 or wortmannin) T cells, respectively. This is because, in the absence of chemokines, during transendothelial migration CAMKII is activated by NKRP1a on Vδ2 T cells, whereas PI-3K is engaged by PECAM-1 on Vδ1 T lymphocytes.7
Interference by HIV-1 Tat amino acid sequence containing CC or CXC motifs on chemokine-driven transmigration of γδ T cells
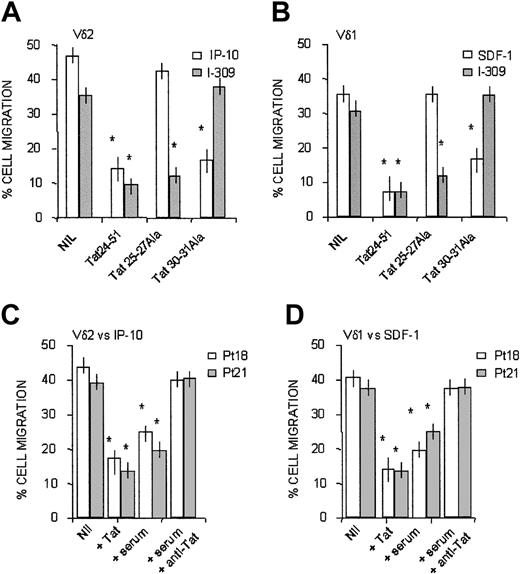
HIV-1 Tat has been reported to function as an antagonist for CXCR4.24 Indeed, HIV-1 Tat contains a CXC chemokine–like sequence (amino acids [aa's] 25-27), in addition to a CC-like sequence (aa's 30-31) residing inside the cysteine-rich domain of the protein.17,24 Furthermore, we reported that exogenous Tat interferes with CAMKII and, to a lesser extent, with PI-3K in dendritic cells and natural killer cells.25,26 Thus, we investigated whether HIV-1 Tat could interfere with the CXC or CC chemokines active on Vδ1 or Vδ2 T lymphocytes by the use of synthetic overlapping peptides.
It is of note that the synthetic peptides Tat24-51 and Tat20-39, both containing the CXC (aa's 25-27) or CC-like (aa's 30, 31) sequences, could exert a dose-dependent inhibition of transendothelial migration induced on Vδ2 T lymphocytes by IP10/CXCL10 and on Vδ1 T cells by SDF-1/CXCL12 (Table 3). As expected, the RGD containing Tat65-80, but not the mutated Tat65-80RGE, peptide also displayed inhibitory activity (Table 3), conceivably because of the block of integrin-mediated cell-matrix adhesion.24 At variance, the basic domain Tat46-60 and the Tat56-70 encompassing the sequence between the basic and the RGD region were not effective (Table 3). The finding that Tat20-39 at low concentrations was less effective than the partially overlapping Tat24-51 in decreasing the migration of the 2 cell subsets might be attributed to the last 4 amino acids (GRKK) of the former peptide, resembling aa's 1987-1990 of fibronectin,27 which is conceivably present in the extracellular matrix of cultured HUVECs. Thus, Tat can downregulate CXCR3- and CXCR4-mediated functions.
Interference of HIV-1 Tat peptides on chemokine-driven Vδ2 and Vδ1 T-cell migration: titration of the inhibitory activity of the peptides Tat24-51 and Tat20-39
Tat peptide (ng/mL) . | Vδ2 vs IP-10 . | Vδ1 vs SDF-1 . |
|---|---|---|
| Nil | 48 ± 3 | 38 ± 3 |
| 24-51 (500) | 10 ± 2 | 12 ± 2 |
| 24-51 (50) | 29 ± 3 | 10 ± 1 |
| 24-51 (5) | 43 ± 1 | 39 ± 2 |
| 20-39 (500) | 14 ± 3 | 11 ± 1 |
| 20-39 (50) | 36 ± 2 | 31 ± 3 |
| 20-39 (5) | 43 ± 2 | 41 ± 4 |
| 46-60 (500) | 42 ± 1 | 32 ± 2 |
| 56-70 (500) | 39 ± 2 | 40 ± 3 |
| 65-80RGD (500) | 19 ± 3 | 18 ± 2 |
| 65-80RGE (500) | 41 ± 4 | 36 ± 3 |
Tat peptide (ng/mL) . | Vδ2 vs IP-10 . | Vδ1 vs SDF-1 . |
|---|---|---|
| Nil | 48 ± 3 | 38 ± 3 |
| 24-51 (500) | 10 ± 2 | 12 ± 2 |
| 24-51 (50) | 29 ± 3 | 10 ± 1 |
| 24-51 (5) | 43 ± 1 | 39 ± 2 |
| 20-39 (500) | 14 ± 3 | 11 ± 1 |
| 20-39 (50) | 36 ± 2 | 31 ± 3 |
| 20-39 (5) | 43 ± 2 | 41 ± 4 |
| 46-60 (500) | 42 ± 1 | 32 ± 2 |
| 56-70 (500) | 39 ± 2 | 40 ± 3 |
| 65-80RGD (500) | 19 ± 3 | 18 ± 2 |
| 65-80RGE (500) | 41 ± 4 | 36 ± 3 |
γδ T-cell lines, cultured in IL-2-containing medium (25 U/mL) for 3 weeks, were radiolabeled with 51Cr and assayed for migration, in response to IP-10 (50 ng/mL) for Vδ2 or SDF-1 (50 ng/mL) for Vδ1, across HUVEC monolayers using a transwell double-chamber system, in the absence (Nil) or presence of the indicated Tat synthetic peptides (500-5 ng/mL, as indicated). After 60 minutes of transmigration, cells were recovered from the lower chamber, lysed, and counted in a γ-counter. Results are expressed as percentage of migrated cells, mean ± SD from 6 independent experiments for each cell population from 6 donors, calculated as described in “Patients, materials, and methods.”
The inhibitory activity of the 2 cysteine-containing, Tat-derived synthetic peptides (Tat24-51 and Tat20-39) on chemokine-driven transmigration of γδ T cells was caused by the CXC- or CC-like sequences of these peptides, as demonstrated by experiments of transendothelial migration performed in the presence of Tat24-51 mutated in the CXC (Tat25-27Ala peptide) or in the CC (Tat 30-31Ala peptide) sequence. Figure 7 shows that transmigration induced by IP-10/CXCL10 on Vδ2 (Figure 7A, white columns) or by SDF-1/CXCL12 on Vδ1 T lymphocytes (Figure 7B, white columns) was strongly inhibited by wild-type Tat 24-51 and by the same peptide that conserves the CXC sequence but is mutated in the CC region (Tat30-31Ala); conversely, no inhibition of the migration induced by the 2 CXC-chemokines was observed when Tat24-51 peptide mutated in the CXC sequence (Tat25-27Ala) was used (Figure 7A-B). Along this line, Tat24-51 mutated in the CC sequence (Tat30-31Ala) did not interfere with the CC chemokine I-309 (Figure 7A for Vδ2 and Figure 7B for Vδ1 T cells, gray columns); on the other hand, Tat24-51 wild-type and Tat25-27Ala, maintaining the CC sequence, were effective in the inhibition of I-309–driven cell migration (Figure 7A-B, gray columns).
Transmigration of γδ T cells in response to CXC or CC chemokines is inhibited by Tat, present in the serum of HIV-1 patients, because of the CXC or CC-like sequences. Vδ2 (A) or Vδ1 (B) cell lines from healthy donors were assayed for transmigration across HUVEC at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 (A, □) or SDF-1/CXCL12 (B, □) or I-309/CCL1 (A-B, ▦), as described in “Patients, materials, and methods.” In some experiments, cells were exposed to 0.1 μg/mL Tat24-51 peptide wild type or mutated in the CXC (Tat25-27 Ala) or in the CC (30-31 Ala) sequence, as indicated. Nil indicates migration in the presence of the indicated chemokine, without Tat peptides. Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). *Student t test; P < .05. (C-D) Vδ2 (C) or Vδ1 (D) T-cell lines obtained from 2 HIV-1–infected patients (patient 18, □; and patient 21, ▨) were assayed for transmigration across HUVEC monolayers at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 (C) or SDF-1/CXCL12 (D), as described in “Patients, materials, and methods.” In some experiments, cells were preincubated with 0.1 μg/mL Tat or with autologous serum (1:2 dilution) alone or with an anti-Tat antiserum (1:400 dilution), as indicated. Nil indicates migration in the presence of the indicated chemokine, without Tat. Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
Transmigration of γδ T cells in response to CXC or CC chemokines is inhibited by Tat, present in the serum of HIV-1 patients, because of the CXC or CC-like sequences. Vδ2 (A) or Vδ1 (B) cell lines from healthy donors were assayed for transmigration across HUVEC at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 (A, □) or SDF-1/CXCL12 (B, □) or I-309/CCL1 (A-B, ▦), as described in “Patients, materials, and methods.” In some experiments, cells were exposed to 0.1 μg/mL Tat24-51 peptide wild type or mutated in the CXC (Tat25-27 Ala) or in the CC (30-31 Ala) sequence, as indicated. Nil indicates migration in the presence of the indicated chemokine, without Tat peptides. Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods” (mean ± SD from 4 independent experiments with cell lines from 4 donors). *Student t test; P < .05. (C-D) Vδ2 (C) or Vδ1 (D) T-cell lines obtained from 2 HIV-1–infected patients (patient 18, □; and patient 21, ▨) were assayed for transmigration across HUVEC monolayers at 60 minutes, in the presence of 50 ng/mL IP-10/CXCL10 (C) or SDF-1/CXCL12 (D), as described in “Patients, materials, and methods.” In some experiments, cells were preincubated with 0.1 μg/mL Tat or with autologous serum (1:2 dilution) alone or with an anti-Tat antiserum (1:400 dilution), as indicated. Nil indicates migration in the presence of the indicated chemokine, without Tat. Results are expressed as percentage of cell migration, calculated as described in “Patients, materials, and methods,” and are the mean ± SD from 3 independent experiments. *Student t test; P < .05.
In keeping with these results, synthetic Tat was able to inhibit transendothelial migration in response to IP-10 or to SDF-1 of Vδ2 or Vδ1 T-cell lines (Figure 7C-D, respectively) obtained from 2 patients (patient 18 and patient 21; superimposable results were obtained with γδ T-cell lines from other 4 patients; not shown). Of note, the autologous serum of these patients could inhibit γδ T-cell migration, and this inhibition was neutralized using a rabbit anti-Tat antiserum (Figure 7C-D), indicating that Tat is present in patient sera, as previously described.28 Indeed, we detected HIV-1 Tat (150-550 ng/mL) in the serum of 24 of 28 HIV-1–infected patients (Table 4), suggesting that the effects of this viral product can conceivably be found in vivo.
Detection of HIV-1 Tat in the serum of HIV-1—infected patients
HIV-1+ patients . | Tat, ng/mL* . |
|---|---|
| 1 | 300 |
| 2 | 400 |
| 3 | 400 |
| 4 | 500 |
| 5 | 550 |
| 6 | 300 |
| 7 | 150 |
| 8 | 400 |
| 9 | 150 |
| 10 | < 50 |
| 11 | 250 |
| 12 | 250 |
| 13 | 250 |
| 14 | < 50 |
| 15 | 400 |
| 16 | 550 |
| 17 | 450 |
| 18 | 450 |
| 19 | 150 |
| 20 | 250 |
| 21 | 300 |
| 22 | 350 |
| 23 | < 50 |
| 24 | 400 |
| 25 | < 50 |
| 26 | 200 |
| 27 | 250 |
| 28 | 550 |
| HIV-1- HBV+/HCV+ patients | |
| 1 | ND |
| 2 | ND |
| 3 | < 10 |
| 4 | < 10 |
| 5 | ND |
| 6 | ND |
| 7 | < 10 |
| 8 | ND |
| 9 | ND |
| 10 | < 10 |
| Healthy donors | |
| H1 | ND |
| H2 | ND |
| H3 | ND |
| H4 | ND |
| H5 | < 10 |
| H6 | < 10 |
| H7 | ND |
| H8 | < 10 |
| H9 | ND |
| H10 | ND |
| H11 | ND |
| H12 | < 10 |
| H13 | ND |
| H14 | ND |
| H15 | ND |
HIV-1+ patients . | Tat, ng/mL* . |
|---|---|
| 1 | 300 |
| 2 | 400 |
| 3 | 400 |
| 4 | 500 |
| 5 | 550 |
| 6 | 300 |
| 7 | 150 |
| 8 | 400 |
| 9 | 150 |
| 10 | < 50 |
| 11 | 250 |
| 12 | 250 |
| 13 | 250 |
| 14 | < 50 |
| 15 | 400 |
| 16 | 550 |
| 17 | 450 |
| 18 | 450 |
| 19 | 150 |
| 20 | 250 |
| 21 | 300 |
| 22 | 350 |
| 23 | < 50 |
| 24 | 400 |
| 25 | < 50 |
| 26 | 200 |
| 27 | 250 |
| 28 | 550 |
| HIV-1- HBV+/HCV+ patients | |
| 1 | ND |
| 2 | ND |
| 3 | < 10 |
| 4 | < 10 |
| 5 | ND |
| 6 | ND |
| 7 | < 10 |
| 8 | ND |
| 9 | ND |
| 10 | < 10 |
| Healthy donors | |
| H1 | ND |
| H2 | ND |
| H3 | ND |
| H4 | ND |
| H5 | < 10 |
| H6 | < 10 |
| H7 | ND |
| H8 | < 10 |
| H9 | ND |
| H10 | ND |
| H11 | ND |
| H12 | < 10 |
| H13 | ND |
| H14 | ND |
| H15 | ND |
Tat measurements in the sera of HIV-1-infected patients, patients with HBV/HCV infection, and healthy donors were performed using ELISA with a rabbit anti-Tat antiserum (10 μg/mL) as capture antibody and a biotinylated rabbit anti-Tat antiserum (1 μg/mL) as detection antibody, followed by Av-HRP (1:2000) and by the specific substrate. Results (ng/mL) referred to synthetic Tat (Tecnogen) used as standard.
ND indicates not detectable.
Discussion
In the present work, we show that (1) Vδ1 T cells are increased in the peripheral blood of HIV-1–infected patients and express CXCR3, CXCR4, or both; (2) transendothelial migration driven by CXC and CC chemokines are inhibited by HIV-1 Tat in the serum of HIV-1–infected patients; (3) transmigration of these cells, obtained from healthy donors, in response to CXCR3-specific chemokines is dependent on CAMKII in addition to PI-3K.
According to their expression of CXCR3 or CXCR4, Vδ1 and Vδ2 T-cell lines and clones from healthy donors transmigrate across endothelial monolayers in response to the specific chemokines, IP-10/CXCL10 for CXCR3+ cells and SDF-1/CXCL12 for CXCR4+ clones. The finding that the homeostatic chemokine 6Ckine/SLC/CCL21 was more effective than IP-10/CXCL10 in driving transendothelial migration of the Vδ1 clones expressing CXCR3 suggests that the resident Vδ1 T-cell population preferentially responds to homeostatic, constitutive chemokines. In this regard, Vδ1 T cells also bear CCR7 (not shown), which is reported to be expressed on bovine γδ T lymphocytes and drives tissue localization of these cells in response to 6Ckine/SLC/CCL21.29 On the other hand, Vδ2 CXCR3+ cells were driven more efficiently by IP-10/CXCL10, supporting that circulating Vδ2 T cells are more sensitive to inflammatory chemokines. However, it is conceivable that the redistribution of Vδ1 and Vδ2 cell subsets during inflammation is regulated by cytokines, such as tumor necrosis factor-α (TNF-α), which increase adhesiveness to vascular endothelium, thus influencing migratory capabilities of lymphocytes. Indeed, when transmigration was performed on endothelial monolayers exposed to TNF-α, the difference in the response of the 2 γδ T-cell subsets to homeostatic and inflammatory chemokines was less evident (not shown).
In addition, we show that CXCR3 can activate CAMKII, which is needed for transendothelial migration mediated by IP-10/CXCL10; conversely, CAMKII is not involved in SDF-1/SLC/CXCL12-driven transmigration. We also found that transmigration of the 2 γδ T-cell subsets induced by IP-10/CXCL10, 6Ckine/SLC/CCL21, or SDF-1/CXCL12 was dependent on PI-3K activation, in agreement with the reported finding that CXCR3 and CXCR4 receptors are coupled to this kinase.20-23
Each of these kinases has been implied in the regulation of cell locomotion—CAMKII controlling calcium-dependent microtubule assembly,30 and PI-3K triggering haptotactic movements driven by extracellular matrix components.31 In agreement with these data, we have reported that CAMKII is engaged by NKRP1a in the Vδ2 γδ T-cell subset during transmigration, whereas the activation of PI-3K through PECAM-1 drives transendothelial migration of Vδ1 T cells.7 Moreover, the chemokine-induced high-affinity state of β2 integrins is controlled by PI-3K–dependent signal transduction, possibly influencing the first steps of tethering and rolling to endothelium.32 Thus, the preferential use of a PI-3K– or a CAMKII-dependent pathway by each subset of γδ T lymphocytes may indicate that they can be recruited by distinct chemokines produced during immune reactions. Indeed, it has been proposed that chemokines selectively expressed by vascular or lymphatic endothelial cells are involved in the regulation of leukocyte extravasation, blood-to-lymph migration, and trafficking to the lymph nodes.33,34 Likewise, chemokine production by intraepithelial γδ T cells and the preferential response of γδ or αβ T cells to defined chemokines may play a key role in the regulation of inflammation and tissue injury or damage.35-37
Of note, Vδ1 and Vδ2 T-cell lines from HIV-1–infected patients coexpress CXCR3 and CXCR4. In healthy donors, who have a lower count of peripheral Vδ1 T cells, CXCR4 was mostly expressed on this subset. According to the receptor expressed, γδ T cells from HIV-1–infected patients migrated in response to the specific chemokines. It is of interest that CXCR3 and CXCR4 are reported markers of T cells associated with certain acute inflammatory and viral diseases, including HIV-1 infection.38,39 Thus, it is conceivable that in the patients analyzed, the up-regulation of CXCR3 and CXCR4 on γδ T cells is a consequence of HIV-1 infection. In addition, it has been proposed that subversion of the immune system through the release of products exerting chemokine mimicry might occur during viral diseases, including the early stages of HIV-1 infection.11,40,41 In particular, specific herpesviruses and lentiviruses can produce chemokine mimics able to block chemokine action and facilitate viral dissemination.41
Our finding that the cysteine-rich domain of HIV-1 Tat protein, which contains chemokine-like sequences, can interfere with the effects of CXC and CC chemokines on γδ T cells is of interest. Indeed, the early production of Tat, which is present in the serum and which inhibits the chemokine-driven transmigration of γδ T cells in these patients, may counteract the effects of chemokines released in response to viral infection, such as I-309/CCL1, and may impair the recruitment of cells with antiviral properties. This mechanism may account, at least in part, for the reported redistribution of the 2 γδ T-cell subsets: the resident one increases in peripheral blood in early AIDS and it decreases from intestine in advanced disease,5,42,43 supporting the importance of γδ T cells as part of the initial defense against HIV-1 infection.5,44-46 In agreement with this, we found that patients with a high percentage of circulating Vδ1 T cells also have considerable amounts of Tat in the serum (200-500 ng/mL); conversely, patients with low or undetectable levels of Tat (50-150 ng/mL) display a number of Vδ1 T lymphocytes in peripheral blood equivalent to that of healthy donors. We are aware that we cannot draw any definitive conclusion from these data because in a few patients, serum Tat levels and Vδ1 T-cell numbers were discordant. Nevertheless, as mentioned, other viral factors may influence the distribution of γδ T-cell subsets in peripheral blood and in mucosal tissues; indeed, the inhibition of chemotaxis in response to SDF-1/CXCL12 has also been reported for at least another HIV-1 product, Nef.44 It is tempting to speculate that evaluating the Vδ1/Vδ2 ratio in peripheral blood might contribute, in the future, to the follow-up of the disease. Finally, studying the viral mimicry of chemokines and chemokine receptors may provide new concepts in the understanding of viral immunopathogenesis and may help in the development of new anti-inflammatory or antiviral drugs and vaccines.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-08-2928.
Supported in part by the Italian Ministero della Salute (A.P., M.R.Z. Coordinated Grant Special Project), the Italian Istituto Superiore di Sanità (IV National Project on AIDS Research), and the Italian Association for Cancer Research (A.P., M.R.Z).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Expression of CXCR3 and CXCR4 on Vδ1 and Vδ2 γδ T cells in healthy donors. Ex vivo analysis of circulating lymphocytes from healthy donors (2 representative phenotypes of 15 donors): double immunofluorescence of lymphocytes in the whole blood, with Alexafluor 488–labeled anti-Vδ1 (A-C) or anti-Vδ2 (D-F) mAbs compared with PE-conjugated anti-CXCR4 mAb (A,C,D,F) or PE-conjugated anti-CXCR3 mAb (B,E). Samples were analyzed using FACS gated to lymphocytes, and results are expressed as log green (x-axis) compared with log red (y-axis) fluorescence intensity (arbitrary units [au]). Numbers in the quadrants of each panel indicate the percentage of double-positive (upper right) or single-positive (upper left and lower right) cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-08-2928/6/m_zh80060458280002.jpeg?Expires=1764984568&Signature=YfQ~rLJ8G-JxS1cfrWNNmVBz09j1UaXnlYmS~7plBu~I6yIJWvqGDApb1R65SMMymaXSTr3F10Cm0AMerqS-y513aG-ttkVCGuhxmCkyUYDGJKd0zbGbeGtjZ~Uj1UNE3SfmSTeDJn96KrKahZTpwaEnWrwpaSxwa2VS17tvZQd2cz98Vxrtef8sjZw5Mb4wb~mL5t6gxqybb75Jthx7LnIPOst59hKdqEieuDngtMJZxZ7OisG6yVtQTVt6QjC-qY4VVyUEJIUH9Q9tg-K1yrDKX8ZGbQVTe45k42zqpfUgvSEzKkWXvNVw2ehx39HR~fxwEpX56YkKNssGxxav5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal