Abstract
Hypergammaglobulinemia and defective humoral immunity are hallmarks of HIV-1 infection. Naive B cells have been recently suggested as the major source of hypergammaglobulinemia in chronic viral infections. We recently reported that HIV-1–infected patients carry low levels of memory B cells. Here we studied whether defects in the naive and memory B cells in HIV-1–infected patients translated into hypergammaglobulinemia and defective humoral immunity against specific antigens. Naive B cells from HIV-1–infected patients exhibited abnormal expression of the activation/differentiation markers CD70 and leukocyte-associated Ig-like receptor (LAIR-1). Activated naive B cells from patients showed a significant increase in the intracellular immunoglobulin G (IgG) content ex vivo and this activated phenotype correlated to hypergammaglobulinemia and to the ability of naive B cells from patients to secrete IgG in vitro. We analyzed the levels of antibodies to tetanus toxoid, measles, and HIV-1 in relation to memory B cells and observed a significant reduction of antigen-specific antibodies in patients with low-memory B lymphocytes. Nevertheless, hypergammaglobulinemia and levels of polyspecific self-reactive antibodies were comparable in patients with normal and low memory B cells. We conclude that reduction of memory B lymphocytes in HIV-1 infection correlates with defective humoral immunity and that hyperactivated naive B cells may represent the source of abnormal IgG production in HIV-1 infection. Our results may be relevant to the design of HIV-1 therapeutical vaccines and to the clinical management of HIV-1–infected patients.
Introduction
A profound impairment of immune functions occurs in individuals infected with human immunodeficiency virus type 1 (HIV-1). Both the cellular and the humoral arms of the immune system are unable to control the infection, which ultimately results in severe exhaustion of several lymphocyte functions and increased susceptibility to secondary and opportunistic infections. Major immunologic defects occur in the B-cell compartment.1 Polyclonal B-cell activation is demonstrated by hypergammaglobulinemia and spontaneous antibodies' (Abs) production by cultured peripheral lymphocytes2,3 ; additional signs of B-cell abnormality are the high incidence of B-cell tumors4 and the deregulated expression of several surface molecules like Fas, Fas ligand (FasL), CD5, CD21, and CD27.5-8 B-cell hyperactivity is also accompanied by functional defects since humoral immune responses following immunization are severely impaired in HIV-1–infected subjects and B lymphocytes from patients are poorly responsive to in vitro stimulation.9-11
Several mechanisms may account for the B-cell abnormalities in HIV-1 infection. A direct effect of virus replication or viral proteins on B-cell function has been shown12 and sustained by the observation that polyclonal B-cell activation is strongly reduced following effective antiretroviral treatment.13-15 HIV-driven unbalanced production of several cytokines like tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), IL-10, and IL-15 has also been involved in B-cell dysfunctions.16-18 Defective T-cell help may account for B-cell unresponsiveness to T-cell–dependent antigens.19,20 The defect of B cells in HIV-1 infection appears, however, to be intrinsic since it begins early during infection preceding functional and quantitative defects in T-helper activity and cannot be restored by allogenic normal CD4+ T cells in vitro.3,21
The mechanisms inducing hypergammaglobulinemia in HIV-1 infection are only partially known. Activation driven by CD4+ T cells, monocytes, and natural killer (NK) cells through CD40-CD40 ligand (CD40L) interaction and an inappropriate cytokine supply may have a relevant role in inducing abnormal differentiation of B cells.17,22 In addition, HIV-1 itself may directly affect B-cell activation and dysfunction, inducing the appearance of a subset of CD21– B cells which have been proposed to contribute to increased antibody production.23,24 A recent work by Hunziker et al25 has suggested that naive B cells represent an important source of hypergammaglobulinemia and autoantibody production in chronic viral infections.
Because of the lack of protective humoral immunity, HIV-1–infected individuals receive vaccination against several pathogens. However, many studies have reported an impaired humoral immune response in most of the patients after vaccination.9-11,26,27 Immunologic memory represents an important mechanism of protection against reinfection and the basis for clinically successful vaccines.28,29 We have recently shown that memory B lymphocytes are significantly reduced during HIV-1 infection and are prone to undergo spontaneous apoptosis in vitro.6,30 The loss of memory B cells may be mediated by overexpression of CD70 on activated T lymphocytes6,31 which may induce terminal differentiation to plasma cells and/or by lack of survival factors leading to apoptosis.30
In the present study, we investigated the phenotype and function of naive B cells isolated from HIV-1–infected persons and evaluated the status of antigen-specific humoral immunity in relation to hypergammaglobulinemia and loss of memory B cells. The results of our study indicate that hyperactivated naive B cells may significantly contribute to hypergammaglobulinemia and autoantibody production. Moreover, in the course of HIV-1 infection there is a selective damage to the memory B-cell population that translates into depressed serologic memory.
Patients, materials, and methods
Patients
This study included 72 HIV-1–infected subjects and 23 blood donors of similar age. The ethical committees of the Huddinge Hospital and the South Hospital (Stockholm) approved the study and all individuals gave informed consent to participate. Among the infected subjects, 54 were men and 18 were women. The median CD4+ T-cell count was 400 cells/μL (range, 40 cells/μL-1130 cells/μL). In the patients' cohort, 13 subjects were treatment naive, 5 were treated with reverse transcriptase inhibitors, and 54 were undergoing highly active antiretroviral therapy (HAART), a combination therapy containing inhibitors of the viral protease and reverse transcriptase.
In the population analyzed in the present study, measles represents a naturally acquired infection and more than 95% of the healthy adult Swedish population carry protective antimeasles antibody levels (Kari Johansen, personal written communication, September 2001). Regarding tetanus, 90% of the healthy male Swedish population between 20 and 50 years old is expected to have protective anti–tetanus toxoid (TT) Abs (levels > 0.01 IU/mL) because of the TT boost received during the military service, whereas about 60% of the female population carries protective anti-TT levels.32
Cell preparation and culture
Peripheral blood mononuclear cells (PBMCs) from HIV-1–infected and healthy subjects were purified from 30 mL to 40 mL of EDTA (ethylenediaminetetraacetic acid)–treated whole blood by centrifugation over a Ficoll-Hypaque density gradient. Plasma samples were collected and stored at –70°C until analysis.
Spontaneous IgG secretion by cultured PBMCs was analyzed in 41 HIV-1–infected patients and 20 healthy subjects. PBMCs were cultured in complete RPMI 1640 medium at a concentration of 0.5 × 106 cells/mL for 7 days when culture supernatants were collected and analyzed by IgG enzyme-linked immunosorbent assay (ELISA).
In order to evaluate whether polyclonal B-cell activation in HIV-1–infected subjects was strictly dependent on cell-to-cell contact, we isolated B cells by magnetic sorting from 12 HIV-infected patients. Unfractionated PBMCs were cultured at a cell concentration of 1 × 106 cells/mL. The purified B cells were cultured in Transwell plates (Corning, Corning, NY) with polycarbonate membrane with pore size 5.0 μm as follows. B-cell–depleted PBMCs were layered in the bottom chamber and in the upper chamber purified B cells were added at the same proportion present in unfractionated PBMCs. A similar number of purified B cells present in the total PBMCs were also cultured in parallel. Cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and antibiotics. Supernatants for IgG secretion measurement were collected after 7 days in culture.
Naive (CD19+CD27–) B lymphocytes were obtained from 8 patients and 4 donors by using a 2-step magnetic cell sorting procedure. PBMCs were incubated with CD19 multisort microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) and applied to an MS+ column (Miltonyi Biotech). Positively sorted B cells were eluted from the column, treated with the release reagent to remove magnetic particles, and further applied to a new column to remove remaining magnetically labeled cells. The released B-cell fraction was further incubated with CD27 microbeads and applied to another column to obtain naive B cells from the CD27– fraction. Fluorescence-activated cell-sorting (FACS) analysis by anti-CD27–phycoerythrin (PE) showed that more than 95% of the released naive B cells were CD27–. Naive B cells were cultured in RPMI 1640 medium at 0.5 × 106 cells/mL and cell culture supernatants were collected after 7 days and kept at –20°C for analysis of total IgG content.
Cell death of B lymphocytes
In a group of 26 patients and 8 controls, total B lymphocytes were purified by magnetic cell sorting using CD19 microbeads (Miltenyi Biotech). Purified B cells were cultured overnight in RPMI medium and quantification of cell death was performed by flow cytometry analysis. B cells were stained with anti-CD27–PE monoclonal Ab and annexin-V–fluorescein isothiocyanate (FITC; Pharmingen, San Diego, CA) in order to detect apoptosis of memory B lymphocytes.
Total IgG quantification
The amount of plasma IgG was measured by nephelometric analysis and the secretion of IgG in vitro was measured by sandwich IgG ELISA.33
Specific antibody measurement
Antibody titers against measles, T T, and HIV-1 were measured as follows. A standard in-house indirect quantitative enzyme immunoassay was used to determine measles Ab concentrations while the avidity testing was performed using the Enzygnost Measles virus IgG detection ELISA kit (Dade-Behring, Marburg, Germany) as previously described.34 The cut off for protective levels was set to more than 0.2 IU/mL35 and avidity (%) was calculated as the ratio of the absorbance values with and without urea treatment. Determination of anti–HIV-1 antibody titers was performed by using the Enzygnost HIV ½ Plus ELISA (Dade-Behering) following the manufacturer's instructions. Antibody levels to T T were evaluated by an accredited modified Delfia test previously described.36
Determination of polyspecific self-reactive antibodies
The presence of polyspecific self-reactive antibodies (PSAs) has been described in HIV-1–infected subjects.37,38 Measurement of PSAs was performed as previously described.37 Buffy coat preparations from healthy donors were used to obtain target lymphocytes. The monocyte fraction was removed by plastic adherence incubating the PBMCs in RPMI 1640 medium for 30 minutes at 37°C. The lymphocytes were then washed in phosphate-buffered saline (PBS) and 0.5 × 106 cells were incubated with 100 μL plasma for 1 hour on ice. After 2 washes, the cells were incubated with FITC-conjugated F(Ab′)2 fraction of mouse anti–human IgG for 30 minutes on ice and fixed with 2% paraformaldehyde/PBS. Binding of PSAs to lymphocytes was analyzed by flow cytometry and the reactivity was expressed as mean fluorescence intensity (MFI).
Flow cytometry
Using 3-color flow cytometry, freshly isolated PBMCs were stained with anti-CD19–CyChrome, anti-CD27–FITC and anti-CD70–PE or anti–LAIR-1 (leukocyte-associated Ig-like receptor)–PE-labeled monoclonal Ab (Pharmingen, San Diego, CA) as previously described.6 According to expression of CD27, naive B cells were defined as CD19+CD27– and memory B cells as CD19+CD27+ cells.39 Flow cytometry analysis was performed with a FACScan instrument (Becton Dickinson, Mountain View, CA) using the Cellquest software (Becton Dickinson). Forward/side scatter dot plot was used to gate the live lymphocyte population and 25 000 cells per sample were analyzed.
In order to analyze whether activated naive B cells are primed to produce IgG in vivo, peripheral B lymphocytes were purified from 3 untreated HIV-1–infected and 3 uninfected individuals. The cells were stained for surface expression of CD27 (FITC), CD70 (PE), or LAIR-1 (PE) and intracellular expression of IgG by using an anti–IgG-CyChrome–conjugated Ab (Pharmingen).
Statistical analysis
Differences between HIV-1–infected and uninfected subjects were analyzed by Mann-Whitney test, Wilcoxon signed rank paired test, or Student t test, as appropriate. Correlation analysis between variables was performed by Spearman rank test or regression analysis. Data in the text are expressed as median and range or mean plus or minus SEM.
Results
Spontaneous IgG secretion by PBMCs
We measured in vitro spontaneous IgG secretion by PBMCs from 41 HIV-1–infected and 20 uninfected individuals. As previously reported, PBMCs from HIV-1–infected subjects spontaneously secreted a high amount of IgG as compared with healthy donors (400 ± 47 ng/mL vs 106 ± 8 ng/mL; P < .001). Interestingly, we observed a strong positive correlation between the IgG secretion by PBMCs and the absolute CD4+ T cells count (rho = 0.66, P < .001), indicating that spontaneous IgG secretion by B cells during HIV-1 infection is dependent on CD4+ T cells. This observation is in agreement with the finding that CD4+ T cells are critical in driving hypergammaglobulinemia in chronic viral infections.25
Polyclonal B-cell activation is cell-contact dependent in HIV-1 infection
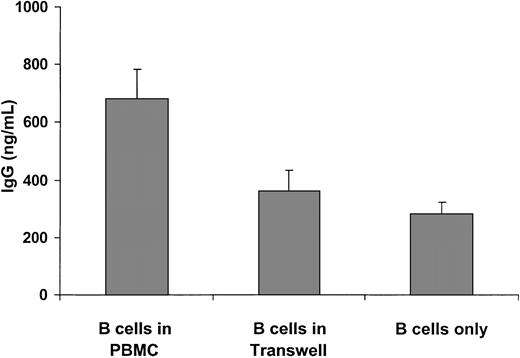
We asked whether direct cell-cell contact or soluble mediators released by PBMCs were involved in the high IgG secretion by B cells. To this end, spontaneous in vitro IgG production was measured from the following 3 culture conditions: (1) unfractionated PBMCs; (2) purified B cells and B-cell–depleted PBMCs separated by Transwell membrane with pore size 5.0 μm in order to inhibit cell-to-cell direct interaction; and (3) purified B cells only. As indicated in Figure 1, B cells from unfractionated PBMCs secreted a significantly higher amount of IgG as compared with B cells separated from PBMCs in the Transwell plates (680 ± 102 ng/mL vs 362 ± 73 ng/mL; P < .001). In line with this observation, B cells alone produced a similar amount of IgG as produced by B cells in the Transwell plate (281 ± 39 ng/mL; P = .81). These findings indicate that polyclonal B-cell activation in HIV-1 infection is dependent on the direct contact between B lymphocytes and other cell types present in the PBMC population, which recently have been suggested to be CD4+ T cells, monocytes, and NK cells.22,40
IgG secretion by cultured B cells from 12 HIV-1–infected subjects. Unfractionated PBMCs were cultured at a concentration of 1 × 106 cells/mL. In the Transwell culture, B-cell–depleted PBMCs were seeded in the lower chamber and purified B cells were seeded in the upper chamber at the same ratio as in the unfractionated PBMCs. The corresponding amount of B cells was cultured with only medium. Supernatants were collected after 7 days. Results are expressed as mean ± SEM.
IgG secretion by cultured B cells from 12 HIV-1–infected subjects. Unfractionated PBMCs were cultured at a concentration of 1 × 106 cells/mL. In the Transwell culture, B-cell–depleted PBMCs were seeded in the lower chamber and purified B cells were seeded in the upper chamber at the same ratio as in the unfractionated PBMCs. The corresponding amount of B cells was cultured with only medium. Supernatants were collected after 7 days. Results are expressed as mean ± SEM.
Activated phenotype of naive B cells in HIV-1 infection
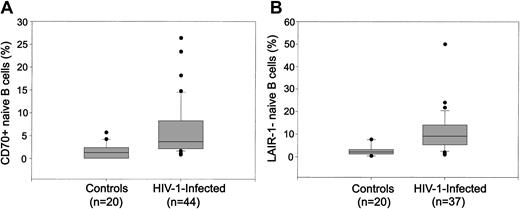
It has recently been described that naive B cells activated in a B-cell receptor (BCR)–independent fashion may be the source of hypergammaglobulinemia in chronic viral infections.25 We analyzed the expression of 2 activation/differentiation markers (CD70 and LAIR-1) on naive B cells from HIV-1–infected and uninfected subjects. In healthy individuals, the expression of CD70 is up-regulated in naive B cells recently stimulated by an antigen41 while the expression of LAIR-1 is down-regulated throughout the process of terminal differentiation into plasma cells.42 In the HIV-1–infected individuals we observed the appearance of a subset of naive B cells with an up-regulated expression of CD70 (4.7% ± 0.8%) that is barely detectable in healthy subjects (1.5% ± 0.4%; P = .01; Figure 2A). The percentage of CD70+ naive B cells was positively correlated with the plasma IgG (rho = 0.34, P = .02). Similarly, we also found that a significant proportion of the naive B cells from patients down-regulated the expression of LAIR-1 as compared with healthy subjects (11.1% ± 1.4% vs 2.7% ± 0.6%; P < .001; Figure 2B), suggesting that these cells are activated and more differentiated. However, the percentage of LAIR-1+ or CD70+ naive B cells was not correlated to CD4+ T-cell count or HIV-1 plasma load (data not shown).
Hyperactivation of naive peripheral B cells in HIV-1 infection. PBMCs were analyzed by 3-color flow cytometry for expression of CD19, CD27, CD70, and LAIR-1. Naive B cells were defined as the CD27– fraction of the gated CD19+ B cells. The expression of CD70 (A) and LAIR-1 (B) on naive (CD19+CD27–) B cells from HIV-1–infected and uninfected subjects are shown.
Hyperactivation of naive peripheral B cells in HIV-1 infection. PBMCs were analyzed by 3-color flow cytometry for expression of CD19, CD27, CD70, and LAIR-1. Naive B cells were defined as the CD27– fraction of the gated CD19+ B cells. The expression of CD70 (A) and LAIR-1 (B) on naive (CD19+CD27–) B cells from HIV-1–infected and uninfected subjects are shown.
The hyperactivation status of naive B cells was also demonstrated by the finding that purified naive B cells from HIV-1–infected patients showed a 2-fold increase in spontaneous IgG secretion in vitro as compared with healthy donors (277 ± 67 ng/mL vs 94 ± 34 ng/mL; P = .06). Interestingly, the spontaneous IgG secretion by naive B cells tended to positively correlate with the CD4+ T-cell count (P = .09), indicating that CD4+ T cells may be involved in priming the activation of naive B cells in HIV-1 infection. Accordingly, the spontaneous IgG secretion by cultured PBMCs from the same patients was significantly correlated with the CD4+ T-cell count (r = 0.77, P = .02).
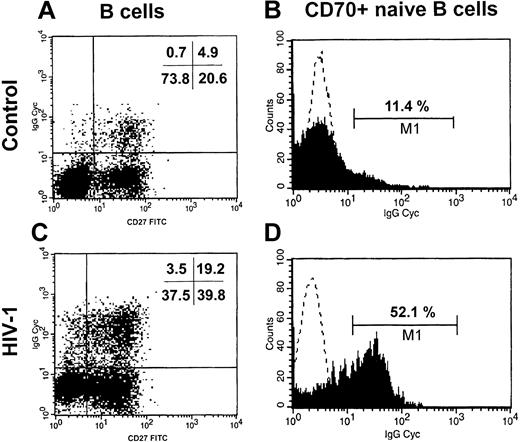
In order to evaluate whether naive B cells are in vivo activated and may be a cellular source of IgG production we purified B cells from 3 untreated patients and 3 donors and analyzed the intracellular content of IgG in naive B cells in relation to the expression of CD70 and LAIR-1. Interestingly, we observed that a significant proportion of naive B cells from HIV-1–infected patients carried intracellular IgG while these cells were low in healthy donors (12.9% ± 0.7% vs 3.4% ± 0.7%; P < .001; Figure 3A,C). Among the population of CD70+ naive B cells (Figure 3B,D), we found that the fraction of IgG+ cells was higher in HIV-1–infected than in uninfected subjects (47.6% ± 2.4% vs 13.1% ± 1.9%; P < .001). HIV-1–infected persons carried a similar proportion of IgG+ cells in the LAIR-1–naive B cells as compared with healthy donors (7.8% ± 3.5% vs 9.2% ± 0.9%; P = NS). These in vivo observations strongly indicate that activated naive B cells from HIV-1–infected patients may have undergone an IgM-IgG class-switch process.
Increased IgG+ naive B cells in HIV-1 infection. B cells were purified from 3 HIV-1–infected and 3 uninfected subjects. The intracellular content of IgG was measured in the total population of naive B cells (CD19+CD27–, panels A,C) and in the activated naive B cells (CD70+CD27–, panels B,D) by 3-color flow cytometry. One representative healthy control (C, D) and one HIV-1–infected patient (A, B) are shown. In panels B and D, the dotted line shows the isotype control Ab and the filled area shows the anti–IgG-CyChrome Ab.
Increased IgG+ naive B cells in HIV-1 infection. B cells were purified from 3 HIV-1–infected and 3 uninfected subjects. The intracellular content of IgG was measured in the total population of naive B cells (CD19+CD27–, panels A,C) and in the activated naive B cells (CD70+CD27–, panels B,D) by 3-color flow cytometry. One representative healthy control (C, D) and one HIV-1–infected patient (A, B) are shown. In panels B and D, the dotted line shows the isotype control Ab and the filled area shows the anti–IgG-CyChrome Ab.
Loss of antigen-specific humoral immunity in HIV-1 infection
The finding that activated naive B cells might contribute to the hypergammaglobulinemia prompted us to evaluate the status of serologic memory in HIV-1 infection. We have recently reported that the reduction of circulating memory B cells observed in HIV-1 infection is associated with a significant increase of spontaneous cell death in vitro of memory B lymphocytes.6,30 In the populations described here we confirm that memory B lymphocytes are reduced in HIV-1 infection as compared with uninfected controls (20% [range, 6.8%-43%] vs 34.3%, [range, 21.1%-60.7%]; P < .001) and that these cells exhibit higher spontaneous apoptosis as compared with the cells from healthy subjects (26.2% ± 2.5% vs 7.9% ± 1.4%; P < .001). Moreover, regression analysis performed on treatment-naive patients showed that the percentage of memory B cells inversely correlates with the HIV-1 plasma viral load (r = 0.45, P = .02). Here, we evaluated whether the reduction and death of memory B lymphocytes were related to decreased levels of antigen-specific Ab. Antibody levels to HIV-1 gp41, tetanus toxoid, and measles were measured. Also, functional affinity (avidity) of anti–measles antibodies (anti-M) was measured. HIV-1–infected subjects were grouped on the basis of the median percentage of memory B cells (20%) into subjects carrying normal (NM, > 20%) or low (LM, < 20%) memory B cells. Age, sex, CD4+ T-cell count, and treatment status were comparable between LM and NM patients (Table 1). Moreover, the absolute number of circulating memory B cells was significantly higher in NM patients (34 cells/μL [range, 13-133 cells/μL]) as compared with LM patients (9.7 cells/μL [range, 3-56 cells/μL]; P < .001).
Characteristics of HIV-1 patients and healthy controls
Subjects . | Female/male . | Age, y (range) . | Naive/treated . | CD4 T cells . | HIV-1 RNA . | Memory B cells . |
|---|---|---|---|---|---|---|
| Healthy donors | 12/8 | 32 (27-46) | NA | ND | NA | 37 ± 3 |
| HIV-1 LM | 7/19 | 37 (24-79) | 5/21 | 442 ± 41 | 3.1 (1.7-5.4) | 13 ± 1 |
| HIV-1 NM | 6/20 | 40 (25-55) | 5/21 | 443 ± 52 | 2.4 (1.7-4.9) | 40 ± 4 |
Subjects . | Female/male . | Age, y (range) . | Naive/treated . | CD4 T cells . | HIV-1 RNA . | Memory B cells . |
|---|---|---|---|---|---|---|
| Healthy donors | 12/8 | 32 (27-46) | NA | ND | NA | 37 ± 3 |
| HIV-1 LM | 7/19 | 37 (24-79) | 5/21 | 442 ± 41 | 3.1 (1.7-5.4) | 13 ± 1 |
| HIV-1 NM | 6/20 | 40 (25-55) | 5/21 | 443 ± 52 | 2.4 (1.7-4.9) | 40 ± 4 |
NA indicates not applicable; ND, not determined.
HIV-1-infected patients with low (LM) or normal (NM) frequency of memory B cells are indicated. The absolute number of CD4+ T cells is indicated in cells/μL, HIV-1 RNA is expressed as log10 (copies/mL) and memory B cells as the percentage of the total B cells. Data are expressed as mean ± SEM or median (5-95 percentiles).
Anti–measles IgG antibodies
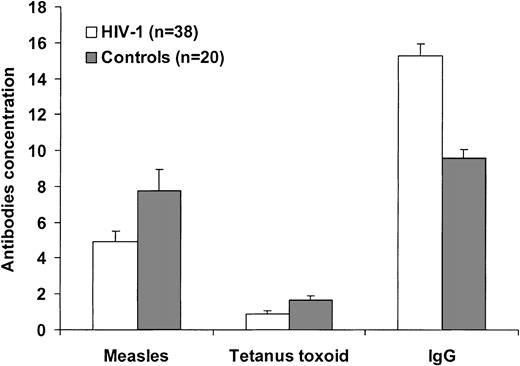
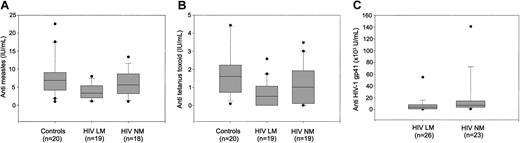
In our cohort, we observed that the levels of anti-M antibodies were lower in HIV-1–infected subjects as compared with healthy individuals (4.92 ± 0.50 IU/mL vs 7.74 ± 1.22 IU/mL; P = .05; Figure 4) and were characterized by a similar avidity (50.8% ± 2.3% vs 54.1% ± 2.6%; P = .37). Interestingly, regression analysis performed on the population of HIV-1–infected subjects showed a correlation between the anti-M antibody levels and the percentage of memory B cells (r = 0.52, P < .001). Among the infected individuals, anti–measles antibodies titers in HIV-1 LM subjects (3.3 IU/mL [range, 1.12-6.68 IU/mL]) were significantly lower as compared with the HIV-1 NM subjects (5.61 IU/mL [range, 1.06-13.36 IU/mL]; P = .04) and to healthy controls (P = .006; Figure 5A).
Hypergammaglobulinemia and antigen-specific antibodies in HIV-1 infection. The plasma concentration of antibodies to measles and tetanus toxoid and of plasma total IgG was analyzed in 52 HIV-1–infected (▪) and 20 healthy individuals (□). Anti-TT and anti–measles antibodies are expressed as IU/mL, plasma IgG are expressed as g/L. Differences were analyzed by Mann-Whitney test, and P values are reported in the sections on specific antibodies.
Hypergammaglobulinemia and antigen-specific antibodies in HIV-1 infection. The plasma concentration of antibodies to measles and tetanus toxoid and of plasma total IgG was analyzed in 52 HIV-1–infected (▪) and 20 healthy individuals (□). Anti-TT and anti–measles antibodies are expressed as IU/mL, plasma IgG are expressed as g/L. Differences were analyzed by Mann-Whitney test, and P values are reported in the sections on specific antibodies.
Memory B cells and specific antibodies. Concentration of antibodies to measles (A), tetanus toxoid (B), and HIV-1 gp41 (C) in healthy controls and HIV-1–infected individuals grouped into patients with normal (NM) or low (LM) memory B lymphocytes.
Memory B cells and specific antibodies. Concentration of antibodies to measles (A), tetanus toxoid (B), and HIV-1 gp41 (C) in healthy controls and HIV-1–infected individuals grouped into patients with normal (NM) or low (LM) memory B lymphocytes.
Anti–tetanus toxoid IgG antibodies
According to the Swedish vaccination program, all subjects in the study had received vaccination with TT. Anti-TT antibody levels were higher in the control group (1.604 IU/mL [range, 0.087-6.002 IU/mL]) as compared with HIV-1–infected subjects (0.624 IU/mL [range, 0-3.483 IU/mL]; P < .01; Figure 4). HIV-1–infected subjects with low memory B lymphocytes had a median anti-TT antibody level of 0.499 IU/mL (range, 0-2.576 IU/mL) that was lower as compared with both healthy controls (P = .001) and HIV-1–infected individuals with normal memory B cells (1.006 IU/mL [range, 0-3.48 IU/mL]; P = .09; Figure 5B).
Anti–HIV-1 antibodies
Subjects with normal frequency of memory B cells harbored a higher concentration of anti–HIV-1 antibodies (6.81 × 103 U/mL [range, 08.38 × 103-149 × 103 U/mL]) as compared with subjects with low memory B cells (3.56 × 103 U/mL [range, 0.19 × 103-71 × 103 U/mL]; P = .01; Figure 5C).
Polyclonal B-cell activation and naive/memory B cells
Despite the consistent reduction of antigen-specific Abs, polyclonal B-cell activation occurred in vivo as shown by the high levels of plasma IgG in HIV-1–infected subjects as compared with healthy controls (15.3 ± 0.7 g/L vs 9.6 ± 0.5 g/L; P < .001; Figure 4). Interestingly, the levels of hypergammaglobulinemia were comparable between HIV-1–infected subjects with normal (15.2 ± 1 g/L) or reduced (15.4 ± 0.9 g/L) memory B cells (Figure 6A). This finding indicates that in HIV-1 infection hypergammaglobulinemia is observed in parallel to selective loss of antigen-specific antibodies. Contrary to hypergammaglobulinemia, the reduction of specific antibodies is associated with decreased memory B lymphocytes.
Memory B cells and hypergammaglobulinemia. Analysis of plasma IgG content (A) and PSA reactivity (B) in healthy controls and HIV-1–infected individuals grouped into patients with normal (NM) or low (LM) memory B lymphocytes.
Memory B cells and hypergammaglobulinemia. Analysis of plasma IgG content (A) and PSA reactivity (B) in healthy controls and HIV-1–infected individuals grouped into patients with normal (NM) or low (LM) memory B lymphocytes.
In order to better define the nature of the IgG detected in HIV-1–infected subjects and since HIV-1 infection is also characterized by increased levels of autoantibodies, we evaluated the presence of polyspecific self-reactive antibodies. High levels of PSA have been detected in HIV-1 infection and these autoantibodies have been shown to bind lymphocytes and mediate T-cell death.38 Human PSA from HIV-1–infected subjects showed a higher reactivity (defined as MFI) as compared with healthy subjects (11.8 [range, 4.6-53.9] vs 7.8 [range, 4.1-12.60; P < .01). In the HIV-1 population, the MFI of PSA was correlated to the plasma IgG (rho = 0.547, P = .001) and PSA reactivity was similar between HIV-1 NM subjects and HIV-1 LM subjects (15.9 [range, 10.3-23.8] vs 7.5 [range, 7-21.6]; P = .226; Figure 6B).
Interestingly, we found that the PSA reactivity was positively correlated with the percentage of CD70+ naive B lymphocytes (rho = 0.36, P < .05) but not with the proportion of LAIR-1+ naive B cells, further supporting the possibility that abnormally activated naive B cells may be responsible for the hypergammaglobulinemia detected in HIV-1–infected persons.
Discussion
In the present study we have evaluated the nature of hypergammaglobulinemia and antigen-specific antibody pattern in relation to naive and memory B lymphocytes in HIV-1–infected subjects.
Hypergammaglobulinemia is commonly observed in autoimmune diseases and chronic infections. Recently, it was proposed that the hypergammaglobulinemia observed in conditions of high and persistent antigen concentrations can be caused by activated naive B cells, which present viral antigens in a BCR-independent manner.25 We observed that in HIV-1–infected subjects naive (CD27–) B lymphocytes have an activated phenotype. In fact, naive B cells from patients showed an increased expression of CD70, indicating recent antigen stimulation.41 The hyperactivation status of naive B cells from HIV-1–infected patients is also confirmed by the down-regulated expression of LAIR-1, a differentiation antigen that is gradually lost during terminal differentiation of B lymphocytes into plasma cells.42 In addition, we found that (1) the proportion of CD70+ naive B lymphocytes is positively correlated to hypergammaglobulinemia and PSA reactivity and that (2) naive B cells from patients are able to spontaneously secrete IgG in vitro and in vivo. We also provide evidence that the naive B cells with up-regulated CD70 expression detected in HIV-1–infected patients carry a higher percentage of IgG+ cells as compared with uninfected donors. These findings support the hypothesis that naive B cells activated by HIV-1 may switch to IgG25 and produce polyclonal IgG and autoantibodies.
We also observed that the capacity of B cells from patients to release immunoglobulins in vitro was dependent on direct cell-cell contact and correlated with CD4+ T-cell count, indicating that CD4+ T cells may play a crucial role in driving abnormal B-cell differentiation in HIV-1 infection, as suggested by Hunziker and colleagues for other chronic viral infections.25
The plasma IgG in HIV-1–infected subjects contains both anti-HIV Abs and other antibodies like PSA and autoantibodies.38,43 In parallel, a loss of specific humoral immunity may occur as indicated by low levels of antibodies against several pathogens26,27,44 and by the rapid loss of antigen-specific antibodies following immunization.9-11,27 We measured antibodies to TT, a common vaccination antigen and to measles, a naturally acquired infection in our cohort, and found that the levels of IgG to both antigens were significantly reduced in HIV-1–infected individuals with low memory B cells. Interestingly, LM and NM patients carried anti–measles IgG with similar avidity indexes, indicating that the defect in anti–measles antibody is not qualitative but quantitative. A similar difference was found in the levels of anti-HIV gp41 antibodies, indicating that the low number of memory B cells was associated with loss of antibodies also against a circulating antigen.
We recently reported that memory B lymphocytes from HIV-1–infected subjects undergo spontaneous apoptosis in vitro,6,30 suggesting that this alteration may account for the dysfunctions in humoral immunity. Many studies have indicated a defect in the humoral immunity toward several antigens9,11,26,27 and such a defect may be due to the deletion of antigen-specific CD4+ T cells or to the loss of memory B cells. Protection against reinfections is based on the immunologic memory carried by neutralizing antibodies. Also, maternally acquired immunity is very important for the protection of the baby against life-threatening infections. Intriguingly, an increased risk of acquiring measles infection early in life was observed in infants of HIV-1–infected mothers,45-47 in association with lower anti–measles antibody titers in the mothers. These findings and the results we report here suggest that impairment in humoral memory immunity may render HIV-1–infected persons more susceptible to infections. In line with our findings, a dysfunction of memory B cells has been suggested to explain the emergence of cytomegalovirus disease in simian AIDS.48
The finding that the VH3 gene family is underrepresented in memory B cells from HIV-1–infected subjects49 also supports this hypothesis. Antibodies of the VH3 family represent an important mechanism of defense against bacterial and fungal infections commonly observed during HIV-1 disease.50 Since the VH3 family encodes for about 50% of all Abs,51 a reduction of this population indicates that the repertoire of antigen-specific Abs is compromised in HIV-1–infected patients.49,52 Such a mechanism could be mediated by viral proteins like gp120 or by virus-induced T-cell activation, as shown by increased CD70 expression on T cells.6,31 In the group of treatment-naive patients we observed that the percentage of circulating memory B lymphocytes is inversely correlated with HIV viremia, indicating that virus-induced immune activation may directly contribute to the loss of memory B cells and to B-cell dysfunctions, as recently suggested.24 On the other hand and perhaps simultaneously, high antigenic pressure might be a bystander driving force to activate B cells to produce polyclonal Abs.25
The B-cell hyperactivity found in our and previous studies can be considered as a paradox since a poor B-cell response occurs in patients after immunization. We found higher levels of antigen-specific Abs in patients with normal memory B cells as compared with patients with low memory B cells. Nevertheless, NM and LM patients had comparable levels of hypergammaglobulinemia and autoantibodies (ie, PSA). Taken together, these observations strongly suggest that antigen-specific Abs do not contribute to hypergammaglobulinemia during HIV-1 infection.
The findings presented in our study may also provide new insights into the mechanisms leading to long-term humoral immunity. The generation of persistent antibody production by plasma cells has been so far explained by 2 models developed in the mouse: (1) plasma cells are long-lived cells resident in the bone marrow and provide life-long antibody production53 ; and (2) plasma cells are continuously generated through the differentiation of memory B cells in the presence of residual antigens.54 A third new intriguing mechanism of maintenance of serologic memory in the human system has been recently proposed by Bernasconi and colleagues.55 These authors provided evidence that the polyclonal activation of human memory B cells by environmental stimuli is responsible for the maintenance of antigen-specific antibodies for a lifetime. Such polyclonal stimuli may be derived from microbial products or from bystander CD4+ T-cell help.55 In the present study we observed that the reduction of memory B cells was related to the reduction of specific humoral immunity to 3 antigens (measles, TT, and HIV-1) to which the immune system was exposed in different manners. The continuous antigenic pressure and immune activation in HIV-1 infection may drive polyclonal activation of memory B cells, eventually leading to cell death and exhaustion of the antigen-specific memory B-cell pool. Although we do not provide direct evidence for the loss of measles- and TT-specific memory B cells, our data support the hypothesis that memory B lymphocytes are needed for the maintenance of serologic memory.55
In conclusion, our study provides evidence that a major impairment occurs in the B-cell compartment during HIV-1 infection: From one side, activated naive B cells contribute to hypergammaglobulinemia, whereas loss of memory B cells is associated with reduced levels of antigen-specific Abs. These findings should be taken in consideration when designing and evaluating therapeutic HIV vaccines. The dysfunctions in the B-cell compartment existing in patients prior to vaccination may hamper effective vaccination and lead to additional immunologic failures.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2375.
Supported by the Swedish Medical Research Council, the Swedish International Development Agency (SIDA-SAREC), the Swedish Physicians Against AIDS Research Foundation, the Swedish Association for Medical Research (SSMF), and Consiglio Nazionale delle Ricerche, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Eva Maria Fenyö of Lund University, Sweden, for helpful discussion and critical reading of the manuscript and to Ann Atlas of Karolinska Hospital, Stockholm, for providing clinical samples.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal