Abstract
von Willebrand factor (VWF) released from endothelium is ultralarge (UL) and hyperreactive. If released directly into plasma, it can spontaneously aggregate platelets, resulting in systemic thrombosis. This disastrous consequence is prevented by the ADAMTS13 (ADisintegrin and Metalloprotease with ThromboSpondin motif) cleavage of ULVWF into smaller, less active forms. We previously showed that ULVWF, on release, forms extremely long stringlike structures. ADAMTS13 cleaves these strings under flow significantly faster than it does under static conditions. As ULVWF tethering to endothelium is important for its rapid proteolysis, we investigated 2 molecules for their potential to anchor the ULVWF strings: P-selectin and integrin αvβ3. We demonstrated that P-selectin anchors ULVWF to endothelium by several means. First, Chinese hamster ovary (CHO) cells expressing P-selectin specifically adhered to immobilized ULVWF and ULVWF-coated beads to immobilized P-selectin. Second, an anti-VWF antibody coimmunoprecipitates P-selectin from the histamine-activated endothelial cells. Third, P-selectin antibody or soluble P-selectin, but not a αvβ3 antibody, RGDS peptide, or heparin, blocked the formation of ULVWF strings. Fourth, P-selectin expression was in clusters predominantly along the ULVWF strings. Finally, the strength of the minimal ULVWF–P-selectin bond was measured to be 7.2 pN. We, therefore, conclude that P-selectin may anchor ULVWF strings to endothelial cells and facilitate their cleavage by ADAMTS13.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is characterized by systemic microvascular thrombosis, largely because of the accumulation of ultralarge von Willebrand factor (ULVWF) multimers on the endothelial cell surface and in plasma.1-4 VWF is a large glycoprotein that mediates the initial adhesion of platelets to subendothelium at the sites of vascular injury by binding to the platelet glycoprotein (GP) Ib-IX-V complex.5,6
VWF is synthesized in megakaryocytes/platelets and in endothelial cells, with the latter being the major source of plasma VWF.5,7,8 In endothelial cells, newly synthesized VWF is either secreted constitutively or stored in Weibel-Palade bodies that release their contents on endothelial cell stimulation by a variety of agonists.5,9 VWF multimers released through the inducible pathway are extremely large and form spontaneous high-strength bonds with the platelet GP Ib–IX-V complex,10,11 resulting in platelet adhesion, aggregation, and thrombus formation.9,12-15 Because of this hyperreactivity, the direct release of ULVWF into plasma provokes intravascular thrombosis such as that seen in patients with TTP. Under normal circumstances, thrombosis is prevented by the rapid (although partial) proteolytic processing of ULVWF by a metalloprotease of the ADAMTS family (A Disintegrin and Metalloprotease with ThromboSpondin motif) called ADAMTS13.16-19 This metalloprotease cleaves the Y842/M843 peptide bond in the VWF A2 domain, reducing the average size of VWF multimers and releasing 176-kDa and 140-kDa fragments into the circulation.14,20,21 Not only is the processed form of VWF smaller, but also it is only active in the presence of modulators such as ristocetin or botrocetin, when exposed to high fluid shear stress, or when immobilized onto a solid surface.16,17,19
How ADAMTS13 cleaves ULVWF in vivo remains largely unknown, especially given that under static conditions in vitro, very nonphysiologic conditions are required to affect proteolysis.14,22 Recently, we reported that ULVWF multimers newly released from stimulated endothelial cells form extremely long stringlike structures to which platelets adhere under a wide range of fluid shear stresses. In flowing plasma, ADAMTS13 cleaves these ULVWF strings 1000-fold faster than it does in the static fluid-phase assay, suggesting that ULVWF cleavage in vivo preferentially occurs on the surface of endothelial cells.11 This notion is supported by previous animal studies. Andre et al23 showed that endothelial cell–derived VWF mediates GP Ibα–dependent platelet adhesion to stimulated mouse venular endothelium. The adhesion of platelets begins within 15 seconds of endothelial stimulation and peaks after about 1 minute, with a subsequent decrease in the number of adherent platelets, coinciding with our observations of ADAMTS13 cleavage of ULVWF strings on the surface of stimulated human endothelial cells. Several questions remain regarding this new model of ULVWF cleavage. For example, it is not known how ULVWF, a secreted protein, is anchored to the surface of endothelial cells and how ADAMTS13, which circulates in blood, is captured to the ULVWF to form an enzyme-substrate complex. For the latter, we have recently shown that ADAMTS13 binds to ULVWF under both static and flow conditions, predominantly through the VWF A1 and A3 domains.24
As for what holds the ULVWF multimers to endothelial cells, one interesting observation is that the ULVWF strings appear to be tethered at only a few sites on the endothelial surface, rather than being fully immobilized.11 One obvious unanswered question involves the nature of the anchor for the ULVWF on the endothelial cell surface.
We considered P-selectin as a good candidate to serve as the ULVWF endothelial anchor for several reasons. First, P-selectin colocalizes with VWF in the Weibel-Palade bodies of endothelial cells and the α-granule of platelets.25-28 Second, both are expressed on the endothelial cells or secreted in response to the same agents. In this report, we present evidence that ULVWF interacts with P-selectin and that the interaction may anchor ULVWF strings to the surface of stimulated endothelial cells.
Materials and methods
Endothelial cells
Human umbilical vein endothelial cells (HUVECs) were used to produce ULVWF and to induce VWF strings. The cells were obtained under a protocol approved by the Institutional Review Board of the Baylor College of Medicine, as described previously.11,29 Umbilical cords were washed with phosphate buffer (140 mM NaCl, 0.4 mM KCl, 1.3 mM NaH2PO4, 1.0 mM Na2HPO4, 0.2% glucose, pH 7.4), and then infused with a collagenase solution (0.02%; Invitrogen Life Technologies, Carlsbad, CA). After 30 minutes of incubation at room temperature, the cords were rinsed with 100 mL phosphate buffer. Elutes containing endothelial cells were centrifuged at 250g for 10 minutes. The cell pellets were resuspended in Medium 199 (Invitrogen Life Technologies) containing 20% heat-inactivated fetal calf serum and 0.2 mM l-glutamine and grown to confluence on culture dishes coated with 1% gelatin.
Endothelial cell–derived ULVWF
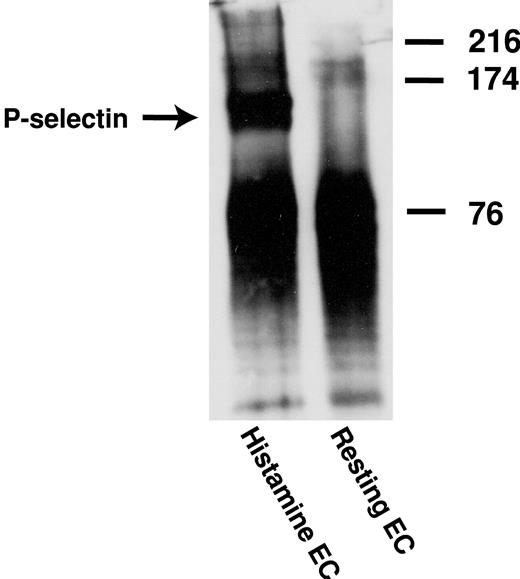
ULVWF was obtained from cultured HUVECs as described previously.13,29,30 Briefly, confluent HUVECs were washed with phosphate-buffered saline (PBS) and incubated with a serum-free M199 medium (Invitrogen Life Technologies) containing 10 μg/mL insulin, 5 μg/mL transferrin, and 1% glutamine for 48 to 72 hours. The cultured cells were then treated with 100 μM histamine (Sigma Chemicals, St Louis, MO) for 30 minutes at 37°C to stimulate the release of ULVWF. After incubation, the serum-free medium was collected and centrifuged at 150g for 10 minutes to remove cell debris. The supernatant was used as the source of ULVWF. The multimeric composition of ULVWF was evaluated by sodium dodecyl sulfate (SDS)–1% agarose gel electrophoresis and chemiluminescence using a polyclonal anti-VWF antibody (DAKO, Carpinteria, CA).
Induction of VWF–platelet strings on endothelial cells
ULVWF–platelet strings were induced by using a method that has previously been described.11 Briefly, confluent HUVECs cultured in 35-mm cell culture dishes were stimulated with 25 μM histamine (Sigma Chemicals). The culture dish was then assembled to form the bottom of a parallel-plate flow chamber (Glycotech, Rockville, MD) connected to a syringe pump, which draws Tyrode buffer containing washed platelets through the chamber at defined flow rates to generate specific wall shear stresses. The assembled chamber was mounted onto an inverted-stage microscope (Eclipse TE300; Nikon, Garden City, NY) equipped with a high-speed digital camera (Model Quantix; Photometrics, Tucson, AZ) and maintained at 37°C by using a thermostatic air bath during the experiments. Acquired images were analyzed off line by using MetaMorph software (Universal Images, West Chester, PA). The ULVWF–platelet strings were quantitated after 2 minutes of perfusion as the number of strings in 20 continuous fields of view.
Potential ULVWF anchors
To identify potential anchor(s) for the ULVWF–platelet strings, HUVECs were stimulated in the presence of either 20 μg/mL polyclonal anti–P-selectin, 20 μg/mL monoclonal antibody LMP609, which binds to the endothelial integrin αvβ331 (kindly provided by Dr David Cheresh of the Scripps Research Institute, La Jolla, CA), 12 μg/mL human P-selectin purified from human platelets, 60 μM RGDS peptide (Sigma Chemicals), or 100 U/mL heparin.
We examined the interaction between P-selectin and ULVWF in a cell-free system to exclude potential roles of other molecules on Chinese hamster ovary (CHO) cell surface. Polystyrene beads coated with ULVWF were perfused over glass-immobilized P-selectin at 2.5 dyn/cm2 wall shear stress. The P-selectin surface was prepared by incubating purified human P-selectin (120 μg/mL) on glass coverslips for 4 hours at room temperature. The number of adherent beads was determined after 5 minutes of perfusion. Negative controls included perfusion of beads coated with bovine serum albumin (BSA) over immobilized P-selectin and perfusion of ULVWF-coated beads over a surface coated with 5% BSA.
ULVWF-coated green fluorescent polystyrene beads (0.5 μm in diameter, fluoresbrite YG microspheres; Polysciences, Warrington, PA) were prepared according to the manufacturer's instructions. Briefly, the beads were incubated with a 96-μg/mL ULVWF solution overnight at room temperature with gentle shaking. Coated beads were washed twice with 0.5 mL borate buffer (pH 8.5) and then incubated with 1 mL 1% BSA for 30 minutes at room temperature. The beads were washed again with borate buffer and resuspended in PBS buffer containing 1% BSA. Control beads were coated with 10% BSA alone.
Fluorescence microscopy
ULVWF and P-selectin distribution on and in endothelial cells was examined by fluorescent microscopy. HUVECs were grown to confluence on small rectangular coverslips coated with 1% gelatin. The cells were then stimulated with 100 μM histamine for 10 minutes at 37°C and immediately rinsed with 10 mL 1% paraformaldehyde in PBS. Each slide was then placed in a parallel-plate flow chamber and slowly perfused (0.1 mL/min) with the same fixative for 20 minutes. After fixation, the cells were stained with fluorescein isothiocyanate (FITC)–conjugated rabbit anti-VWF antibody32 and Texas Red–conjugated monoclonal anti–P-selectin antibody (BD Pharmingen, San Diego, CA). Images were taken with a 100 × objective with appropriate fluorescent filters. For colocalization, images with FITC staining of ULVWF and Texas Red staining of P-selectin were overlaid.
Adhesion of CHO-P cells to immobilized P-selectin
CHO cells expressing human P-selectin (CHO-P cells, kindly provided by Dr C. Wayne Smith at Leukocyte Biology Section, Department of Pediatrics, Baylor College of Medicine) were grown to approximately 90% confluence in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum and penicillin-streptomycin (Invitrogen). They were then detached from culture dishes by 0.025% trypsin, washed with PBS, and resuspended in complete Tyrode buffer (138 mM sodium chloride, 5.5 mM glucose,12 mM sodium bicarbonate, 2.9 mM potassium chloride, and 0.36 mM dibasic sodium phosphate, 0.8 mM calcium chloride, 0.4 mM magnesium chloride, pH 7.4) to a final concentration of 1.5 × 106 cells/mL. Before use, P-selectin expression on the cells was assessed by flow cytometry using a FITC-labeled anti–P-selectin monoclonal antibody (BD Pharmingen). To prepare the immobilized ULVWF matrix, ULVWF (96 μg/mL) was incubated on glass coverslips for 2 hours at room temperature. Unbound protein was removed by washing the coverslips with PBS. Before the cell adhesion assay, CHO-P cells were incubated with 120 μM RGDS peptides (Sigma Chemical) for 20 minutes at room temperature to prevent nonspecific binding of host CHO cell integrins to ULVWF. The RGDS-treated cells were then incubated with the ULVWF matrix for 10 minutes at room temperature, and nonadherent cells were removed by washing the coverslips with complete Tyrode buffer. Cell adhesion was quantitated microscopically by counting the number of adhered cells per view-field (100 ×).
Coimmunoprecipitation of ULVWF and P-selectin
Potential interactions between ULVWF and P-selectin in HUVECs were also examined by coimmunoprecipitation experiments. HUVECs were grown to 90% confluence and washed with PBS. They were first treated with either 100 μM histamine or an equal volume of PBS for 30 minutes at 37°C, and then lysed with a digitonin lysis buffer (20 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.4, 50 mM NaCl, 1% digitonin, 1 mM phenylmethanesulfonylfluoride, 1 μg/mL leupeptin, 1 mg/mL DNase I, 0.1 mg/mL soybean trypsin inhibitor, and 1.6 μg/mL benzamidine) for 20 minutes on ice. Cell lysates were centrifuged at 15 000g for 2 minutes to remove cell debris and precleared with 50 μL Pansorbin beads (Calbiochem, San Diego, CA) for 2 hours at 4°C. At the end of incubation, the cell lysates were centrifuged again at 15 000g for 2 minutes, and the supernatants were incubated with the monoclonal anti-VWF antibody 6G133 overnight at 4°C. For immunoprecipitation, cell lysates were first incubated with 10 μg/mL rabbit antimouse immunoglobulin G (IgG; Zymed, South San Francisco, CA) for 60 minutes and then with 50 μL Pansorbin beads for 60 minutes at 4°C. Immunoprecipitates were resuspended in SDS sample buffer, boiled for 5 minutes to denature proteins, and separated by 7% SDS–polyacrylamide gel electrophoresis (PAGE). The separated proteins were transferred to nitrocellulose membrane.
Coimmunoprecipitated P-selectin was visualized by Western blot. Briefly, the transferred nitrocellulose membrane was incubated with 5% nonfat milk to block nonspecific binding and then with 5 μg/mL polyclonal antihuman P-selectin antibody (BD Pharmingen) in TBS buffer (25 mM Tris, 137 mM NaCl, 0.27 mM KCl, pH 7.4 containing 0.02% Tween-20). Specific antibody binding was recognized by incubating the membrane with a horseradish peroxidase (HRP)–conjugated goat antirabbit IgG for 1 hour at room temperature and developed with Supersignal Westpico Chemiluminescent substrate (Pierce, Rockford, IL) according to the manufacturer's instructions.
Optical tweezer experiments to determine the strength of the ULVWF–P-selectin bond
The method for measuring bond strength by using optical tweezers was previously described.10 Briefly, a titanium-sapphire laser (Model 3900 S; Spectra-Physics, Santa Clara, CA) tuned to a wavelength of 830 nm, which induces minimal or no cellular damage,10,34 was used to set the optical trap. CHO-P cells were incubated on the cell chamber containing PBS buffer for 20 minutes to allow cells to adhere. The cell chamber was then mounted onto a piezoelectrically driven translational stage (Model P-527.3CL; Physik Instrumente, Waldbronn, Germany), placed on the manual stage of an inverted microscope (Axiovert S100TV; Carl Zeiss, Jena, Germany), and illuminated from the top with white light for visualizing the specimens. Images were recorded by a charged-coupled device camera (Model CCD 100; DAGE-MTI, Michigan City, IN).
In each experiment, we optically trapped a 2.0-μm diameter bead coated with ULVWF and moved a CHO-P cell toward the trapped bead by moving the stage. The bead was allowed to remain in contact with the cell for 60 seconds before being pulled away by moving the piezoelectric stage. The minimum laser power required to detach the ULVWF-coated bead from the CHO-P cell was determined and converted to a force value (in pico Newton) that represented the bond strength of the ULVWF–P-selectin interaction by using the calibration curve. The optical trapping force was first calibrated by moving a solution past a trapped bead at a known velocity and calculating the force required to displace the bead from the trap by using the Stokes law,
where η is the solution viscosity (1 cP), v is the solution velocity, r is the bead radius, and h is the distance between the center of the bead and the coverslip.35 For a given laser power, the bead will eventually escape the trap when the drag force exerted by the fluid exceeds the trapping force. The drag force at which the bead escapes from the trap is defined as the escaping force and was determined over a range of laser powers measured past the microscope objective lens. The escaping force is a function of laser power for a 2.0-μm polystyrene bead placed at a height of 10 μm from the coverslip (h = 10 μm). The system was calibrated at a height of 10 μm because the ULVWF-coated bead is in contact with the CHO cell approximately 10 μm from the coverslip during the experiments. There was a linear relationship between the escaping force and laser power with a slope of approximately 0.9 pN/mW.
The forces required to detach the ULVWF-coated bead from CHO-P cells provided distinct groups of data points, whose cluster means were integral multiples of the putative single-bond strength for the ULVWF–P-selectin interaction.
Statistical analysis
All experimental data are presented as mean ± SEM. The unpaired 2-tailed Student t test was used for data analysis, and a P value less than .05 was considered to be statistically significant.
Results
Purified human P-selectin and anti–P-selectin antibody block the formation of ULVWF–platelet strings under flow
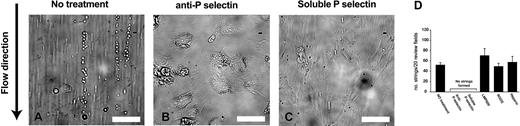
To visualize the formation of ULVWF–platelet strings on the surface of endothelial cells, washed platelets in Ca++, Mg++-free Tyrode buffer were perfused over histamine-stimulated HUVECs at 2.5 dyn/cm2 shear stress. ULVWF–platelet strings initially appeared about 30 seconds after perfusion started (Figure 1A), consistent with our previous report.11 The ULVWF–platelet strings did not appear when cells were stimulated in the presence of either a polyclonal anti–P-selectin antibody (Figure 1B) or soluble human P-selectin (Figure 1C). In contrast, neither RGDS peptide, the anti-αvβ3 antibody LMP609, nor heparin blocked string formation (Figure 1D). In fact, slightly more strings formed in the presence of LMP609 than in its absence. Furthermore, when buffer containing a polyclonal anti–P-selectin antibody was perfused over endothelial cells containing preformed strings, the strings started to detach from endothelial cells after approximately 10 minutes of continuous perfusion (data not shown). These results showed that P-selectin might play a role in the formation or stabilization of ULVWF–platelet strings.
The formation of ULVWF–platelet strings under flow condition was blocked by anti–P-selectin antibody or soluble P-selectin. HUVECs were stimulated with histamine in the absence or presence of a polycloncal anti–P-selectin antibody, purified human P-selectin, the monoclonal anti-αvβ3 antibody LMP609, RGDS peptide, or heparin for 15 minutes at room temperature. Washed platelets suspended in Ca++, Mg++-free Tyrode buffer were then perfused over the stimulated endothelial cells at 2.5 dyn/cm2 for 2 minutes at 37°C. The number of ULVWF–platelets strings was then counted in 20 continuous fields of 400 × (A, bar = 100 μm). Results in (D) are from 12 separate experiments. Data are mean ± SEM.
The formation of ULVWF–platelet strings under flow condition was blocked by anti–P-selectin antibody or soluble P-selectin. HUVECs were stimulated with histamine in the absence or presence of a polycloncal anti–P-selectin antibody, purified human P-selectin, the monoclonal anti-αvβ3 antibody LMP609, RGDS peptide, or heparin for 15 minutes at room temperature. Washed platelets suspended in Ca++, Mg++-free Tyrode buffer were then perfused over the stimulated endothelial cells at 2.5 dyn/cm2 for 2 minutes at 37°C. The number of ULVWF–platelets strings was then counted in 20 continuous fields of 400 × (A, bar = 100 μm). Results in (D) are from 12 separate experiments. Data are mean ± SEM.
P-selectin is clustered and colocalizes with ULVWF strings
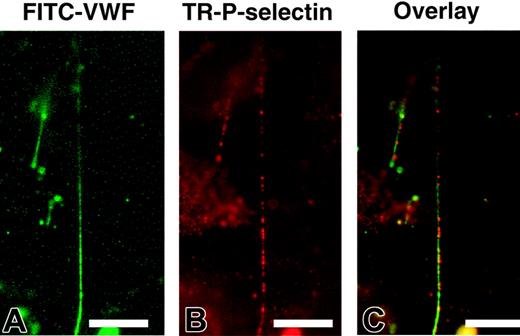
The blocking experiments suggested that P-selectin might serve to anchor ULVWF strings to the endothelial surface. To investigate this possibility, we first examined whether P-selectin on stimulated endothelial cells colocalizes with ULVWF strings by dual staining histamine-stimulated endothelial cells with FITC-conjugated anti-VWF and Texas Red–conjugated anti–P-selectin antibodies. We found that ULVWF strings formed in the absence of platelets (Figure 2A). Compared with the continuous staining with the VWF antibody, P-selectin appeared to be clustered (Figure 2B) at discrete points along the length of the ULVWF strings (Figure 2C).
ULVWF strings colocalized with P-selectin on endothelial cells. HUVECs were stimulated with histamine and perfused with PBS buffer containing 1% paraformaldehyde at a flow rate of 0.1 mL/min for 20 minutes. After perfusion, cells were stained with FITC-conjugated monoclonal anti-VWF and Texas Red–conjugated polyclonal anti–P-selectin antibodies. ULVWF formed stringlike structures in the absence of platelets (A). P-selectin expression was in clusters (B), and most of these P-selectin clusters were located along the ULVWF strings (C). The figure represents 8 independent experiments (bar = 100 μm).
ULVWF strings colocalized with P-selectin on endothelial cells. HUVECs were stimulated with histamine and perfused with PBS buffer containing 1% paraformaldehyde at a flow rate of 0.1 mL/min for 20 minutes. After perfusion, cells were stained with FITC-conjugated monoclonal anti-VWF and Texas Red–conjugated polyclonal anti–P-selectin antibodies. ULVWF formed stringlike structures in the absence of platelets (A). P-selectin expression was in clusters (B), and most of these P-selectin clusters were located along the ULVWF strings (C). The figure represents 8 independent experiments (bar = 100 μm).
CHO cells expressing P-selectin adhere to immobilized ULVWF
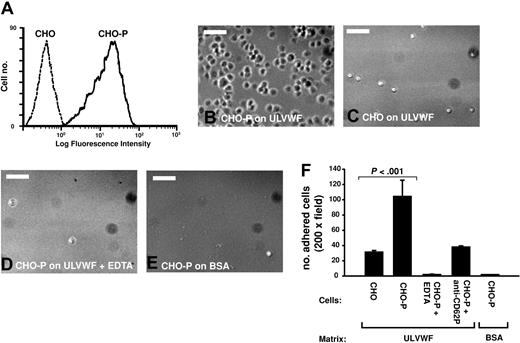
The level of P-selectin expressing in CHO-P cells was first measured by flow cytometry (Figure 3A). CHO-P and parental CHO cells were then incubated with immobilized ULVWF for 10 minutes, the coverslips were then washed, and adherent cells were counted. CHO-P cells, but not parental CHO cells, adhered to immobilized ULVWF (Figure 3B-C). Anti–P-selectin antibody and EDTA (ethylenediaminetetraacetic acid; 5 μM) prevented adhesion (Figure 3D-E, respectively).
CHO cells expressing P-selectin adhered to immobilized ULVWF under static condition. (A) CHO cells stably expressing human P-selectin (CHO-P), as determined by flow cytometry with PBS buffer, and cells that remained adhered were counted (B). Cell adhesion was also measured in the presence of 0.5 μM EDTA (B, bar = 100 μm). CHO-P cells specifically adhered to ULVWF but not to BSA surface, and adhesion was blocked by treating cells with EDTA or a polyclonal anti–P-selectin antibody (C, the Student t test, n = 4).
CHO cells expressing P-selectin adhered to immobilized ULVWF under static condition. (A) CHO cells stably expressing human P-selectin (CHO-P), as determined by flow cytometry with PBS buffer, and cells that remained adhered were counted (B). Cell adhesion was also measured in the presence of 0.5 μM EDTA (B, bar = 100 μm). CHO-P cells specifically adhered to ULVWF but not to BSA surface, and adhesion was blocked by treating cells with EDTA or a polyclonal anti–P-selectin antibody (C, the Student t test, n = 4).
ULVWF-coated beads adhere to immobilized P-selectin under flow
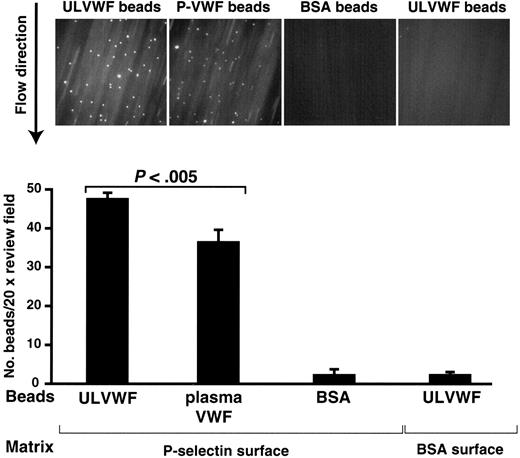
To test whether the ULVWF–P-selectin bond is strong enough to withstand pulling forces applied by fluid shear stress, we evaluated the interaction under flow conditions. ULVWF-coated beads adhered to P-selectin, but not to BSA, under a shear stress of 2.5 dyn/cm.2 Likewise, BSA-coated beads failed to adhere to a P-selectin–coated surface (Figure 4). To determine whether interaction with ADAMTS13 is unique to ULVWF, we also tested adhesion of beads coated with plasma VWF. The plasma VWF-coated beads adhered to immobilized P-selectin, but to a significantly lesser extent (57.4 ± 4.4 beads/viewfield versus 35.6 ± 8.2 beads/viewfield, Student t test, n = 19, P < .005; Figure 4).
ULVWF-coated beads adhered to immobilized P-selectin under flow. Polystyrene beads coated with ULVWF were perfused over immobilized P-selectin under a 2.5-dyn/cm2 shear stress for 5 minutes at room temperature (A). Control experiments were perfusion of ULVWF-coated beads and BSA-coated beads over BSA and immobilized P-selectin, respectively (A). At the end of perfusion, the numbers of beads adhered to P-selectin were counted. Panel B is a summary of 6 separate experiments. Figures are means ± SEM (the studentt test).
ULVWF-coated beads adhered to immobilized P-selectin under flow. Polystyrene beads coated with ULVWF were perfused over immobilized P-selectin under a 2.5-dyn/cm2 shear stress for 5 minutes at room temperature (A). Control experiments were perfusion of ULVWF-coated beads and BSA-coated beads over BSA and immobilized P-selectin, respectively (A). At the end of perfusion, the numbers of beads adhered to P-selectin were counted. Panel B is a summary of 6 separate experiments. Figures are means ± SEM (the studentt test).
P-selectin is coimmunoprecipitated by anti-VWF antibody from stimulated HUVECs
HUVECs with or without prestimulation with 25 μM histamine were lysed with digitonin lysis buffer. VWF was immunoprecipitated by using the monoclonal antibody 6G1; coimmunoprecipitated P-selectin was detected with a polyclonal anti–P-selectin antibody. As shown in Figure 5, P-selectin was coimmunoprecipitated from histamine-stimulated HUVECs but not from unstimulated HUVECs.
Coimmunoprecipitation of P-selectin with anti-VWF antibody from stimulated HUVECs. Histamine-stimulated and unstimulated HUVECs were lysed with a digitonin lysis buffer. ULVWF multimers were precipitated from the cell lysates by the monoclonal anti-VWF antibody 6G1, and coimmunoprecipitation of P-selectin with ULVWF was determined by Western blot using a polyclonal anti–P-selectin antibody. The figure represents 3 independent experiments.
Coimmunoprecipitation of P-selectin with anti-VWF antibody from stimulated HUVECs. Histamine-stimulated and unstimulated HUVECs were lysed with a digitonin lysis buffer. ULVWF multimers were precipitated from the cell lysates by the monoclonal anti-VWF antibody 6G1, and coimmunoprecipitation of P-selectin with ULVWF was determined by Western blot using a polyclonal anti–P-selectin antibody. The figure represents 3 independent experiments.
ULVWF–P-selectin bond strength
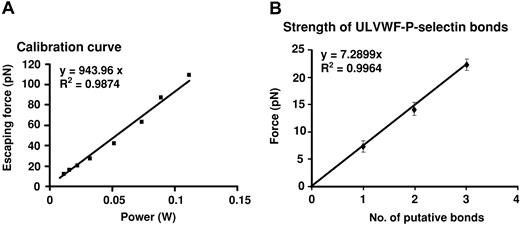
Using the optical trapping system, we measured the strength of the ULVWF–P-selectin bond to be approximately 7.2 pN by detecting the force required to detach ULVWF-coated beads away from CHO cells expressing P-selectin. The lowest detachment forces measured clustered around an average value of 7.2 pN; the higher forces measured clustered at integer multiples of that value, indicating that the most likely value for the strength of the minimal interaction between ULVWF and P-selectin is approximately 7.2 pN (Figure 6). The binding was blocked by either the polyclonal P-selectin antibody or soluble P-selectin but not by RGDS peptide. We were unable to detect any interactions between ULVWF-coated beads and the parental CHO cells.
Measurement of the strength of ULVWF–P-selectin bond by the optical tweezers. (A) Histogram of forces for ULVWF–P-selectin bond detachment. The detachment forces were arranged into bins and aggregate around integer multiples of the lowest cluster average, 7.2 pN. The average force values for the remaining bins were 14.1 and 22.2 pN, designated by the dashed lines for 2 and 3 bonds, respectively. (B) Plot shows the cumulative results of detachment of ULVWF-coated beads from CHO-P cells. The data in the panel represent the mean values of 40 bead-cell detachments. Error bars represent SEM.
Measurement of the strength of ULVWF–P-selectin bond by the optical tweezers. (A) Histogram of forces for ULVWF–P-selectin bond detachment. The detachment forces were arranged into bins and aggregate around integer multiples of the lowest cluster average, 7.2 pN. The average force values for the remaining bins were 14.1 and 22.2 pN, designated by the dashed lines for 2 and 3 bonds, respectively. (B) Plot shows the cumulative results of detachment of ULVWF-coated beads from CHO-P cells. The data in the panel represent the mean values of 40 bead-cell detachments. Error bars represent SEM.
Discussion
We have shown that the formation of ULVWF strings is blocked by either an anti–P-selectin antibody or by soluble P-selectin but not by RGDS peptide, antibody against integrin αvβ3, or heparin (Figure 1). The most parsimonious explanation for these results is that P-selectin tethers the newly formed ULVWF strings to the endothelial surface, thereby possibly facilitating its cleavage by ADAMTS13. This role for P-selectin would require that it interact with VWF, an interaction that we demonstrated by several means. First, we showed that P-selectin clusters along the course of VWF strings (Figure 2), suggesting that a long ULVWF string may be anchored through multiple P-selectin clusters. This unique distribution and colocalization of P-selectin is consistent with our previous observation that ULVWF strings are anchored through multiple anchor points.11 One potential benefit of such a distribution is to increase the ability of the P-selectin anchors to hold on to ULVWF strings made heavy by their extreme length (up to several millimeters) and many adherent platelets. Second, we show that CHO cells that express human P-selectin, but not parental CHO cells, can adhere to immobilized ULVWF under static conditions in a calcium-dependent manner (Figure 3). Third, ULVWF-coated polystyrene beads specifically adhere to immobilized human P-selectin under flow (Figure 4). Fourth, an anti-VWF antibody coimmunoprecipitates P-selectin in histamine-stimulated, but not unstimulated, HUVECs (Figure 5). Finally, the optical tweezer experiments demonstrate a direct interaction between ULVWF and P-selectin with strength of the minimum bond being 7.2 pN (Figure 6). This value is lower than the single bond strength for GP Ibα and ULVWF, which we previously determined to be 11.4 pN, but remarkably similar to the strength of single bonds formed between plasma VWF and GP Ibα in the presence of ristocetin (6.5 pN) or botrocetin (8.8 pN).10
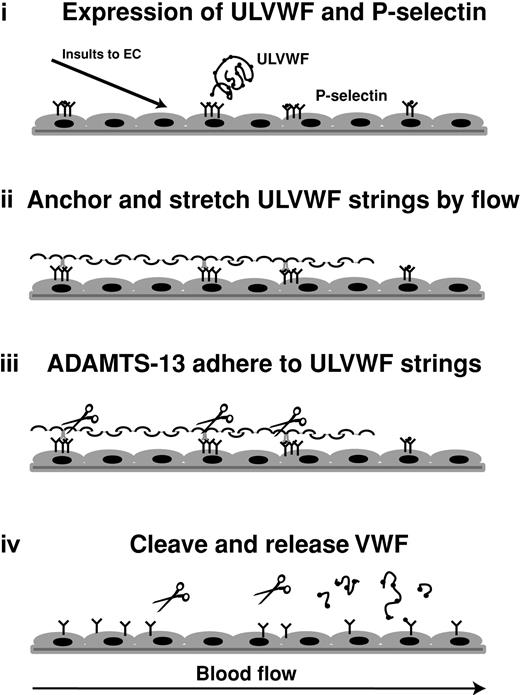
On the basis of these results, we propose a model of how newly released ULVWF multimers are cleaved by ADAMTS13 in vivo (Figure 7). Activated endothelial cells simultaneously release ULVWF and express P-selectin on the surface. The membrane-bound P-selectin anchors ULVWF multimers to the surface of endothelial cells. Because of this surface attachment, ULVWF multimers are stretched in the flowing blood to form elongated stringlike structures. Stretching is enhanced by the adhesion of platelets to the ULVWF strings. The tensile force experienced by the ULVWF–platelet strings may expose the ADAMTS13 cleavage site within the A2 domain, thereby greatly accelerating proteolysis. On cleavage, smaller VWF fragments of various sizes are released into the plasma. The release of the stretching force may once again allow the encryption of the ADAMTS13 cleavage site, thereby limiting further proteolysis. This provides an elegant system to prevent ADAMTS13 from cleaving VWF to the smallest and uniformly sized fragments, even though both ADAMTS13 and VWF both circulate in the blood.
Schematic illustration of a potential mechanism of cleavage of ULVWF string by ADAMTS-13 metalloprotease. On stimulation, endothelial cells release contents from the Weibel-Palade bodies. Membrane-bound P-selectin anchors ULVWF multimers to the surface of endothelial cells to allow long stringlike structures to form under flow. Fluid shear stress stretches these strings to expose sites for ADAMTS-13 to adhere to ULVWF and/or cleavage site in the A2 domain of ULVWF. The cleavage releases ULVWF from endothelial cells (and from wall shear stress) to allow cleaved VWF to adopt different conformation that is no longer available for further cleavage.
Schematic illustration of a potential mechanism of cleavage of ULVWF string by ADAMTS-13 metalloprotease. On stimulation, endothelial cells release contents from the Weibel-Palade bodies. Membrane-bound P-selectin anchors ULVWF multimers to the surface of endothelial cells to allow long stringlike structures to form under flow. Fluid shear stress stretches these strings to expose sites for ADAMTS-13 to adhere to ULVWF and/or cleavage site in the A2 domain of ULVWF. The cleavage releases ULVWF from endothelial cells (and from wall shear stress) to allow cleaved VWF to adopt different conformation that is no longer available for further cleavage.
This model raises the interesting issue of when the interaction between ULVWF and P-selectin first occurs. Our coimmunoprecipitation studies suggest that association may not be constitutive, but rather occurs during endothelial cell activation. This is different from previous studies, suggesting that ULVWF may interact with P-selectin intracellularly. For example, studies have shown that cotransfection of VWF and P-selectin results in the formation of Weibel-Palade bodylike structures in AtT-20 cells, whereas transfection of P-selectin alone does not.36 Furthermore, a defect in the synthesis of VWF leads to the absence of Weibel-Palade bodies and mislocalization of P-selectin,37 suggesting that VWF may target P-selectin to the Weibel-Palade bodies.38,39 One possible explanation for this difference is that in the absence of cell activation the intracellular association between ULVWF and P-selectin is weak but is significantly enhanced during activation of endothelial cells and/or exocytosis of ULVWF and P-selectin from the Weibel-Palade bodies.
Although P-selectin anchorage of ULVWF strings provides an efficient way to cleave ULVWF, it could also render the surface of endothelial cells much more thrombogenic, in the case of ADAMTS13 deficiency, by affixing the hyperreactive ULVWF to the surface of endothelial cells. These membrane-anchored ULVWF would then capture platelets to endothelial cells to build thrombi, which can either occlude vessels locally or, if released through the tensile force applied by the flowing blood, could travel downstream to block small vessels, leading to tissue infarction.
In summary, we have shown that P-selectin interacts with ULVWF multimers on the surface of stimulated endothelial cells. This interaction may anchor the newly released ULVWF multimers to form stringlike structures in flowing blood, in the process facilitating their cleavage by ADAMTS13. This process may be critical for converting the hyperreactive and thrombogenic ULVWF to smaller and adhesively less active plasma forms of VWF.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-08-2956.
Supported by grants from the National Institutes of Health (grants 1-P50-HL65967, HL65229, HL71895), a Grant-in-Aid from the American Heart Association-Texas Affiliate, and the Mary R. Gibson Foundation. J.F.D. is an established investigator of American Heart Association.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal