Abstract
This study prospectively evaluated the rate of biologic response to desmopressin (DDAVP) in 66 patients with type 1 or 2 von Willebrand disease (VWD), each of whom had, on the basis of available records, a clinically significant bleeding history and at least one of the following laboratory abnormalities: bleeding time (BT) longer than 15 minutes, ristocetin cofactor activity (VWF:RCo) less than 10 IU/dL, factor VIII coagulant activity (FVIII:C) less than 20 IU/dL (severe VWD). Before the study, responsive patients were defined as those who, 2 hours after infusion of 0.3 μg/kg DDAVP, had increased baseline values of VWF:RCo and FVIII:C by at least 3-fold and achieved levels of at least 30 IU/dL for both and a BT of 12 minutes or less. The rate of biologic response varied according to VWD types and was higher in type 1 (7 of 26, 27%) than in type 2 (7 of 40, 18%) (type 2A [1 of 15, 7%], type 2M [3 of 21, 14%], type 2N [3 of 4, 75%]). Mutations in the VWF gene were previously known or newly identified in most patients with types 2A (n = 15 of 15), 2M (n = 15 of 21), and 2N (n = 4 of 4), but in none of those with type 1 VWD. Genotype provided more information than phenotype in predicting individual responses to DDAVP only in patients with 2A and 2N VWD. This prospective study showed that the rate of biologic response to DDAVP is relatively low not only in type 2 but also in type 1 VWD when uniform and stringent criteria for patient selection and responsiveness are applied.
Introduction
There are 2 treatments of choice for von Willebrand disease (VWD): desmopressin (1-deamino-8-D-arginine vasopressin [DDAVP]) and transfusion of plasma-derived factor VIII/von Willebrand factor (FVIII/VWF) concentrates.1 DDAVP is a synthetic analog of vasopressin that releases and thereby increases 3- to 5-fold the plasma levels of autologous VWF and FVIII.2,3 Although DDAVP has been recommended for the treatment of VWD since 1977,4,5 no prospective studies have been carried out to establish which patients have well-defined biologic responses to the compound and whether there is a relationship between response and phenotypic or genotypic abnormalities of VWF. The goals of this multicenter prospective study were to determine the rate of a predefined biologic response to DDAVP in a relatively large number of patients with types 1 and 2 VWD in Europe, chosen because their clinical histories were severe enough (severe VWD) to predict the need for treatment. The study also tried to establish whether there is a relationship between responsiveness, phenotype, and genotype.
Patients, materials, and methods
Recruitment and inclusion criteria
Patients aged 12 to 65 years—previously classified as having types 1, 2A, 2M, and 2N VWD according to the currently adopted phenotypic criteria of the Subcommittee on VWF of the Scientific and Standardization Committee (SSC) of the International Society of Thrombosis and Haemostasis (ISTH)6 —were enrolled. The institutional review board approved the study, and each patient provided informed, written consent.
The total number of patients with types 1 and 2 VWD who were followed up by the 5 hemophilia centers was 912, but only 150 patients met the inclusion criteria of this study, and only 66 of those gave their informed consent.
Before the study, the participating centers had agreed to define severe VWD as a lifelong history of bleeding (including at least 2 episodes severe enough to require replacement therapy) and at least one of the following laboratory abnormalities according to the records kept at each center: bleeding time (BT) longer than 15 minutes, ristocetin cofactor activity (VWF:RCo) less than 10 IU/dL, and factor VIII coagulant activity (FVIII:C) less than 20 IU/dL. Eleven patients whose historical data fulfilled the enrollment criteria but who subsequently, at the time of DDAVP infusion, were found to have a measurement outside these limits remained in the study based on an intention-to-treat analysis.
Exclusion criteria
Patients with type 3 VWD were excluded because type 3 is known to be unresponsive to DDAVP, and those with type 2B VWD were excluded because none of the participating centers used DDAVP to treat type 2B because of the risk for thrombocytopenia.7 Other exclusion criteria were the presence of acquired von Willebrand syndrome, antibodies to VWF or FVIII:C, a history of severe side effects after DDAVP (such as severe hyponatremia, seizure, or thrombosis), seropositivity for HIV, pregnancy, and concomitant epilepsy or cardiovascular diseases.
Identification of VWF gene mutations
VWF gene screening not previously carried out by participating centers was performed at Laboratoire Français du Fractionnement et des Biotechnologies (C.M., L.H.). The numbering system used for amino acids was in accordance with the recommendations of the VWF Subcommittee of the ISTH.8
DDAVP administration
Intravenous infusions of 0.3 μg/kg body weight DDAVP (diluted in 50-100 mL saline) were given under medical supervision when patients were not bleeding. Hence, this study does not address the clinical response rate. Infusions lasted 30 minutes, and venous blood was withdrawn at baseline and 0.5, 1, 2, and 4 hours after the infusion ended. BT was measured before and 2 hours after DDAVP using a commercially available device that delivers 2 standard 1-mm skin horizontal incisions in the volar part of the forearm (Simplate IIR; BioMérieux, Durham, NC). Aliquots of platelet-poor plasma samples were obtained and frozen at –70°C until tested in each laboratory.
Laboratory methods
FVIII:C, VWF antigen (VWF:Ag), and VWF:RCo assays and multimeric analysis of plasma VWF were performed at each center using locally standardized methods. Commercial standards calibrated against the World Health Organization (WHO) standard were used as reference for FVIII:C and VWF assays. The VWF:RCo/Ag ratio was calculated as a rough index of VWF functional activity. The lower normal limit of each participating laboratory ranged between 0.6 and 0.7; therefore, it was agreed before the study to adopt a lower normal limit of 0.6. This limit is now confirmed in more than 1200 healthy age- and sex-matched subjects collected as a control group by another large, ongoing European study on type 1 VWD (unpublished data). Multimeric analysis was performed by electrophoresis on 1.6% low–gelling temperature agarose gel in patients with type 2A VWD.9
Criteria for biologic response to DDAVP
Before the study was started, responsive patients were defined as those who, 2 hours after the end of DDAVP infusion, had increases of plasma FVIII:C and VWF:RCo of at least 3-fold over baseline and reached levels for both values of at least 30 IU/dL and a BT of 12 minutes or less (12 minutes was the upper BT limit agreed to by all participating centers using the same device).
Statistical analysis
Laboratory data were expressed as means ± SD. The t test was used to compare results of types 2A, 2M, and 2N with those of all type 1 VWD. P values less than .05 were considered statistically significant.
Results
Overall results in patients with types 1 and 2 VWD
Twenty-six type 1 and 40 type 2 patients met the inclusion criteria; none had to be excluded because of severe adverse effects on previous exposures to DDAVP. Table 1 shows the mean laboratory values (mean ± SD) before and 2 hours after DDAVP and the relationship between DDAVP response, phenotypes, and genotypes.
DDAVP response by bleeding time and FVIII/VWF activities before and 2 hours after infusion, in patients with different types of von Willebrand disease divided by phenotypes and gene mutations
. | No. responsive patients of total . | FVIII:C, U/dL . | . | VWF:RCo, U/dL . | . | Bleeding time, min . | . | VWF:RCo/Ag ratio . | . | FVIII:C/VWFAg ratio . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VWD types . | . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | |||||
| All type 1 | 7 of 26 | 39 ± 24 | 130 ± 64 | 19 ± 20 | 59 ± 58 | 24 ± 10 | 11 ± 6 | 0.8 ± 0.4 | 0.9 ± 0.3 | 2.3 ± 0.3 | 2.3 ± 0.2 | |||||
| VWF:RCo/Ag greater than 0.6 | 6 of 18 | 44 ± 28 | 138 ± 72 | 24 ± 22 | 77 ± 62 | 23 ± 9 | 11 ± 7 | 0.9 ± 0.2 | 1.0 ± 0.3 | 2.7 ± 0.3 | 2.6 ± 0.2 | |||||
| VWF:RCo/Ag less than 0.6 | 1 of 8 | 28 ± 5* | 110 ± 38 | 7 ± 1* | 19 ± 10* | 18 ± 12 | 9 ± 5 | 0.5 ± 0.3* | 0.7 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | |||||
| All type 2A | 1 of 15 | 40 ± 14 | 135 ± 56 | 11 ± 9* | 29 ± 20* | 28 ± 5 | 23 ± 7* | 0.3 ± 0.2* | 0.2 ± 0.1* | 1.4 ± 0.8* | 1.4 ± 0.7* | |||||
| G550R | 0 of 1 | 35 | 277* | <6* | <6* | >30* | >30* | 0.1* | 0.1* | 0.6* | 3.2 | |||||
| C1272G | 0 of 1 | 33 | 110 | <6* | 16* | >30* | >30* | 0.3* | 0.2* | 1.6* | 1.3* | |||||
| S1506L | 0 of 5 | 36 ± 12 | 91 ± 18* | 12 ± 13 | 19 ± 9* | >30* | 26 ± 8* | 0.4 ± 0.2* | 0.3 ± 0.2* | 1.7 ± 0.8* | 1.0 ± 0.2* | |||||
| R1597W | 1 of 3 | 45 ± 5 | 144 ± 9 | 10 ± 2 | 39 ± 9* | 24 ± 11 | 18 ± 10* | 0.3 ± 0.1* | 0.3 ± 0.1* | 1.4 ± 0.2* | 1.3 ± 0.1* | |||||
| I1628T | 0 of 3 | 45 ± 8 | 149 ± 9 | 17 ± 5 | 38 ± 12* | 27 ± 4 | 17 ± 6* | 0.4 ± 0.2* | 0.3 ± 0.2* | 0.9 ± 0.3* | 1.1 ± 0.2* | |||||
| G1629R | 0 of 1 | 61 | 203* | 30 | 60 | >30* | 20* | 0.3* | 0.3* | 0.6* | 0-9* | |||||
| V1665E | 0 of 1 | 27 | 102* | <6* | 10* | >30* | >30* | 0.2* | 0.1* | 1.2* | 1.0* | |||||
| All type 2M | 3 of 21 | 37 ± 15 | 113 ± 41* | 12 ± 6 | 39 ± 14* | 17 ± 7 | 12 ± 6 | 0.5 ± 0.2* | 0.7 ± 0.3 | 2.1 ± 1.2 | 1.9 ± 0.9 | |||||
| Vicenza, R1205H | 0 of 1 | 4* | 21* | <6* | 18* | 13 | 7 | 0.5* | 0.6* | 0.3* | 0.7* | |||||
| R1315C | 0 of 3 | 39 ± 9 | 92 ± 11* | 7 ± 2* | 25 ± 3* | 20 ± 0 | 12 ± 6 | 0.3 ± 0.1* | 0.6 ± 0.2 | 2.0 ± 0.7 | 2.1 ± 0.5 | |||||
| R1374C | 1 of 9 | 34 ± 3 | 117 ± 24 | 16 ± 11 | 42 ± 16 | 21 ± 5 | 15 ± 7 | 0.5 ± 0.3* | 0.5 ± 0.3 | 1.9 ± 0.8 | 2.1 ± 0.3 | |||||
| R1374H | 1 of 2 | 31-44 | 100-132 | 13-38 | 12-39 | 16-15 | 11-10 | 0.6-0.5 | 0.4-0.5 | 1.3-1.6 | 0.9*-1.8 | |||||
| undefined | 1 of 6 | 45 ± 12 | 133 ± 18 | 10 ± 5 | 44 ± 6 | 12 ± 4* | 9 ± 3 | 0.5 ± 0.3* | 0.6 ± 0.2 | 2.5 ± 1.2 | 1.9 ± 0.7 | |||||
| Type 2N | 3 of 4 | 16 ± 7* | 106 ± 60* | 40 ± 13* | 153 ± 72* | 7 ± 3* | 6 ± 2* | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.2* | 0.6 ± 0.3* | |||||
| R854Q + R854Q | 1 of 1 | 24 | 165 | 52* | 202* | 5* | 5* | 0.7 | 1.0 | 0.4* | 0.9* | |||||
| R854Q + null | 1 of 1 | 13* | 115 | 26 | 98* | 11* | 8 | 0.8 | 0.8 | 0.3* | 0.8* | |||||
| R854Q + C1060R | 1 of 1 | 18* | 121 | 48* | 227* | 6* | 6* | 0.7 | 0.8 | 0.2* | 0.6* | |||||
| C1060R + null | 0 of 1 | 7* | 23* | 29 | 85* | 5* | 4* | 0.7 | 0.8 | 0.1* | 0.2* | |||||
. | No. responsive patients of total . | FVIII:C, U/dL . | . | VWF:RCo, U/dL . | . | Bleeding time, min . | . | VWF:RCo/Ag ratio . | . | FVIII:C/VWFAg ratio . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VWD types . | . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | Before . | After 2 h . | |||||
| All type 1 | 7 of 26 | 39 ± 24 | 130 ± 64 | 19 ± 20 | 59 ± 58 | 24 ± 10 | 11 ± 6 | 0.8 ± 0.4 | 0.9 ± 0.3 | 2.3 ± 0.3 | 2.3 ± 0.2 | |||||
| VWF:RCo/Ag greater than 0.6 | 6 of 18 | 44 ± 28 | 138 ± 72 | 24 ± 22 | 77 ± 62 | 23 ± 9 | 11 ± 7 | 0.9 ± 0.2 | 1.0 ± 0.3 | 2.7 ± 0.3 | 2.6 ± 0.2 | |||||
| VWF:RCo/Ag less than 0.6 | 1 of 8 | 28 ± 5* | 110 ± 38 | 7 ± 1* | 19 ± 10* | 18 ± 12 | 9 ± 5 | 0.5 ± 0.3* | 0.7 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | |||||
| All type 2A | 1 of 15 | 40 ± 14 | 135 ± 56 | 11 ± 9* | 29 ± 20* | 28 ± 5 | 23 ± 7* | 0.3 ± 0.2* | 0.2 ± 0.1* | 1.4 ± 0.8* | 1.4 ± 0.7* | |||||
| G550R | 0 of 1 | 35 | 277* | <6* | <6* | >30* | >30* | 0.1* | 0.1* | 0.6* | 3.2 | |||||
| C1272G | 0 of 1 | 33 | 110 | <6* | 16* | >30* | >30* | 0.3* | 0.2* | 1.6* | 1.3* | |||||
| S1506L | 0 of 5 | 36 ± 12 | 91 ± 18* | 12 ± 13 | 19 ± 9* | >30* | 26 ± 8* | 0.4 ± 0.2* | 0.3 ± 0.2* | 1.7 ± 0.8* | 1.0 ± 0.2* | |||||
| R1597W | 1 of 3 | 45 ± 5 | 144 ± 9 | 10 ± 2 | 39 ± 9* | 24 ± 11 | 18 ± 10* | 0.3 ± 0.1* | 0.3 ± 0.1* | 1.4 ± 0.2* | 1.3 ± 0.1* | |||||
| I1628T | 0 of 3 | 45 ± 8 | 149 ± 9 | 17 ± 5 | 38 ± 12* | 27 ± 4 | 17 ± 6* | 0.4 ± 0.2* | 0.3 ± 0.2* | 0.9 ± 0.3* | 1.1 ± 0.2* | |||||
| G1629R | 0 of 1 | 61 | 203* | 30 | 60 | >30* | 20* | 0.3* | 0.3* | 0.6* | 0-9* | |||||
| V1665E | 0 of 1 | 27 | 102* | <6* | 10* | >30* | >30* | 0.2* | 0.1* | 1.2* | 1.0* | |||||
| All type 2M | 3 of 21 | 37 ± 15 | 113 ± 41* | 12 ± 6 | 39 ± 14* | 17 ± 7 | 12 ± 6 | 0.5 ± 0.2* | 0.7 ± 0.3 | 2.1 ± 1.2 | 1.9 ± 0.9 | |||||
| Vicenza, R1205H | 0 of 1 | 4* | 21* | <6* | 18* | 13 | 7 | 0.5* | 0.6* | 0.3* | 0.7* | |||||
| R1315C | 0 of 3 | 39 ± 9 | 92 ± 11* | 7 ± 2* | 25 ± 3* | 20 ± 0 | 12 ± 6 | 0.3 ± 0.1* | 0.6 ± 0.2 | 2.0 ± 0.7 | 2.1 ± 0.5 | |||||
| R1374C | 1 of 9 | 34 ± 3 | 117 ± 24 | 16 ± 11 | 42 ± 16 | 21 ± 5 | 15 ± 7 | 0.5 ± 0.3* | 0.5 ± 0.3 | 1.9 ± 0.8 | 2.1 ± 0.3 | |||||
| R1374H | 1 of 2 | 31-44 | 100-132 | 13-38 | 12-39 | 16-15 | 11-10 | 0.6-0.5 | 0.4-0.5 | 1.3-1.6 | 0.9*-1.8 | |||||
| undefined | 1 of 6 | 45 ± 12 | 133 ± 18 | 10 ± 5 | 44 ± 6 | 12 ± 4* | 9 ± 3 | 0.5 ± 0.3* | 0.6 ± 0.2 | 2.5 ± 1.2 | 1.9 ± 0.7 | |||||
| Type 2N | 3 of 4 | 16 ± 7* | 106 ± 60* | 40 ± 13* | 153 ± 72* | 7 ± 3* | 6 ± 2* | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.2* | 0.6 ± 0.3* | |||||
| R854Q + R854Q | 1 of 1 | 24 | 165 | 52* | 202* | 5* | 5* | 0.7 | 1.0 | 0.4* | 0.9* | |||||
| R854Q + null | 1 of 1 | 13* | 115 | 26 | 98* | 11* | 8 | 0.8 | 0.8 | 0.3* | 0.8* | |||||
| R854Q + C1060R | 1 of 1 | 18* | 121 | 48* | 227* | 6* | 6* | 0.7 | 0.8 | 0.2* | 0.6* | |||||
| C1060R + null | 0 of 1 | 7* | 23* | 29 | 85* | 5* | 4* | 0.7 | 0.8 | 0.1* | 0.2* | |||||
Numbers indicate mean ± SD or individual values for single patients.
To calculate the mean values of VWF:RCo and the VWF:RCo/Ag ratios, VWF:RCo levels below the limit of sensitivity of the assay (<6 IU/dL) were taken to 6 IU/dL.
To calculate mean and SD for bleeding times, values >30 min were taken to 30 min.
P<.05 compared with all patients with type 1 VWD.
Criteria for responsiveness to DDAVP were met by 7 of 26 (27%) of type 1 patients and 7 of 40 (18%) of type 2 patients (all subtypes). Details on the changes of the plasma levels of FVIII/VWF measurements and BT after DDAVP, according to VWD phenotypes (types 1, 2A, 2M, and 2N), are given in Figures 1, 2, 4, and 5, respectively.
Biologic responses to DDAVP in 26 patients with type 1 VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see “Criteria for biologic response to DDAVP”) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 26 patients with type 1 VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see “Criteria for biologic response to DDAVP”) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 15 patients with type 2A VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 15 patients with type 2A VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 21 patients with type 2M VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 21 patients with type 2M VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 4 patients with type 2N VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Biologic responses to DDAVP in 4 patients with type 2N VWD. Changes of FVIII:C (U/dL) and VWF:RCo (U/dL) are shown for each patient before and 30 minutes, 2 hours, and 4 hours after DDAVP administration. BT results expressed in minutes are reported only before and 2 hours after DDAVP. Limits of FVIII:C, VWF:RCo, and BT used in this study to define responsiveness (see criteria for DDAVP response) are shown by the zone indicated in gray. Patients responsive (•) and unresponsive (○) to DDAVP.
Responses in patients with type 1 VWD
The VWF gene mutations in patients with type 1 VWD were not identified previously or as part of this study. Figure 1 shows that after DDAVP administration, FVIII:C and VWF:RCo levels increased in most patients, with mean relative increases of 3.6- and 3.7-fold, respectively. However, only 7 of 26 (27%) patients met the criterion of responsiveness because the threshold levels of both measurements were not attained in the remaining 19 patients. BT, prolonged on baseline, was significantly shortened after 2 hours in patients with type 1 VWD and became shorter or normal (less than 12 minutes) in most patients.
The VWF:RCo/Ag ratio was the measurement retrospectively found to better separate responders from nonresponders. Table 1 shows that among 18 patients who had normal VWF:RCo/Ag ratios (greater than 0.6) before and 2 hours after DDAVP, 6 were responsive; among the remaining 8 patients who had VWF: RCo/Ag ratios below 0.6, only one was responsive, even though the mean relative increases of VWF:RCo were similar in the 2 subgroups. Mean FVIII:C/VWF:Ag ratios were similar (2.3) before and 2 hours after DDAVP (Table 1).
Responses in patients with type 2A VWD
Only 1 of 15 (7%) patients with type 2A VWD met the criteria for responsiveness. Figure 2 shows that though all 15 patients had normal FVIII:C levels (more than 50 U/dL) 2 hours after DDAVP administration, VWF:RCo reached the threshold levels of 30 U/dL in 5 patients only. The BT was initially prolonged in most patients and improved or became normal in only 3 patients (Figure 2). Mean FVIII:C/VWF:Ag ratios were similar (1.4) before and 2 hours after DDAVP but were lower than in patients with type 1 VWD (Table 1).
Gene mutations were G550R (1 patient), C1272G (1 patient), S1506L (5 patients), R1597W (3 patients), I1628T (3 patients), G1629R (1 patient), and V1665E (1 patient). In detail, Table 1 shows that the patient with the G550R mutation had a large relative increase in FVIII:C but no change in VWF:RCo or BT. The only patient with the C1272G mutation had increases of both FVIII:C and VWF:RCo, but the BT remained prolonged (Table 1).
All 13 patients with mutations S1506L, R1597W, I1628T, G1629R, V1665E had markedly increased FVIII:C (Table 1) but were ultimately classified as unresponsive, with the exception of 1 of the 3 patients with R1597W.
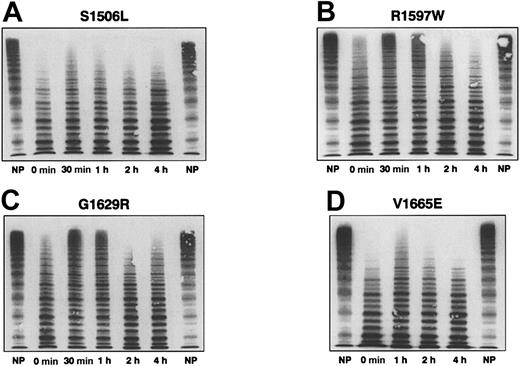
The multimeric pattern of VWF before and after DDAVP was evaluated in 4 patients with mutations most frequently associated with type 2A VWD and correlated with changes in VWF:RCo, VWF:Ag, VWF:RCo/Ag, and BT (Table 2). In patients with mutations S1506L10 and V1665E,11 causing defective intracellular transport and assembly of VWF (so-called group 1 defects), there were few improvements after DDAVP in the defective multimeric structure (Figure 3), persistence of a prolonged BT, and little increase in VWF:RCo (Table 2). In contrast, in patients with mutations R1597W10 and G1629R12 that resulted in VWF multimers more sensitive to proteolysis after secretion in plasma (group 2 defects), there was a transient increase of large multimers (Figure 3) associated with a transient normalization of BT and an improvement of VWF:RCo values (Table 2).
VWF measurements and BT before and after DDAVP in selected patients with type 2A VWD
. | Time after DDAVP, h . | VWF:RCo, U/dL . | VWF:Ag, U/dL . | VWF:RCo/Ag ratio . | Bleeding time, min . |
|---|---|---|---|---|---|
| Normal values (range) | 110 ± 35 (46-202) | 112 ± 36 (50-218) | 0.99 ± 0.23 (0.63-1.75) | 5 ± 2 (3-7) | |
| Patient with S1506L, group 1 | Baseline | <6 | 22 | 0.27 | >30 |
| 0.5 | 14 | 44 | 0.32 | >30 | |
| 1 | 22 | 68 | 0.32 | >30 | |
| 2 | 24 | 77 | 0.29 | >30 | |
| 4 | 14 | 64 | 0.22 | >30 | |
| Patient with R1597W, group 2 | Baseline | 6 | 31 | 0.19 | >30 |
| 0.5 | 65 | 100 | 0.65 | NT | |
| 1 | 60 | 134 | 0.45 | 6.30 | |
| 2 | 53 | 112 | 0.47 | 12 | |
| 4 | 36 | 96 | 0.37 | 15 | |
| Patient with G1629R, group 2 | Baseline | 30 | 100 | 0.30 | >30 |
| 0.5 | 67 | 190 | 0.35 | NT | |
| 1 | 79 | 236 | 0.33 | 10 | |
| 2 | 60 | 232 | 0.26 | 20 | |
| 4 | 62 | 252 | 0.25 | >30 | |
| Patient with V1665E, group 1 | Baseline | <6 | 22 | 0.27 | >30 |
| 1 | 10 | 91 | 0.11 | >30 | |
| 2 | 13 | 95 | 0.14 | >30 | |
| 4 | 10 | 104 | 0.10 | >30 |
. | Time after DDAVP, h . | VWF:RCo, U/dL . | VWF:Ag, U/dL . | VWF:RCo/Ag ratio . | Bleeding time, min . |
|---|---|---|---|---|---|
| Normal values (range) | 110 ± 35 (46-202) | 112 ± 36 (50-218) | 0.99 ± 0.23 (0.63-1.75) | 5 ± 2 (3-7) | |
| Patient with S1506L, group 1 | Baseline | <6 | 22 | 0.27 | >30 |
| 0.5 | 14 | 44 | 0.32 | >30 | |
| 1 | 22 | 68 | 0.32 | >30 | |
| 2 | 24 | 77 | 0.29 | >30 | |
| 4 | 14 | 64 | 0.22 | >30 | |
| Patient with R1597W, group 2 | Baseline | 6 | 31 | 0.19 | >30 |
| 0.5 | 65 | 100 | 0.65 | NT | |
| 1 | 60 | 134 | 0.45 | 6.30 | |
| 2 | 53 | 112 | 0.47 | 12 | |
| 4 | 36 | 96 | 0.37 | 15 | |
| Patient with G1629R, group 2 | Baseline | 30 | 100 | 0.30 | >30 |
| 0.5 | 67 | 190 | 0.35 | NT | |
| 1 | 79 | 236 | 0.33 | 10 | |
| 2 | 60 | 232 | 0.26 | 20 | |
| 4 | 62 | 252 | 0.25 | >30 | |
| Patient with V1665E, group 1 | Baseline | <6 | 22 | 0.27 | >30 |
| 1 | 10 | 91 | 0.11 | >30 | |
| 2 | 13 | 95 | 0.14 | >30 | |
| 4 | 10 | 104 | 0.10 | >30 |
See also Figure 3 for these data.
NT indicates not tested.
VWF multimeric structure before and after DDAVP administration in 4 patients with type 2A VWD. Plasma of healthy controls (NP) and of 4 patients with type 2A VWD with mutations S506L (A), R1597W (B), G1629R (C), and V1665E (D) before and 30 minutes, 1 hour, 2 hours, and 4 hours after DDAVP administration are shown (from left to right). In the 2 patients with the first group of type 2A defects (S1506L and V1665E), there were no changes in the proportion of the high-molecular–weight multimers after DDAVP or in the prolonged BT (more than 30 minutes) and low VWF:RCo/Ag ratios (less than 0.30) observed at baseline (Table 2). Conversely, in the 2 patients with the second group of type 2A defects (R1597W and G1629R), a transient appearance of high-molecular–weight multimers is observed after DDAVP administration and transient normalization (6 and 10 minutes) of the prolonged BT (more than 30 minutes at baseline). The low VWF:RCo/Ag ratios (less than 0.30) observed at baseline did not change appreciably (Table 2).
VWF multimeric structure before and after DDAVP administration in 4 patients with type 2A VWD. Plasma of healthy controls (NP) and of 4 patients with type 2A VWD with mutations S506L (A), R1597W (B), G1629R (C), and V1665E (D) before and 30 minutes, 1 hour, 2 hours, and 4 hours after DDAVP administration are shown (from left to right). In the 2 patients with the first group of type 2A defects (S1506L and V1665E), there were no changes in the proportion of the high-molecular–weight multimers after DDAVP or in the prolonged BT (more than 30 minutes) and low VWF:RCo/Ag ratios (less than 0.30) observed at baseline (Table 2). Conversely, in the 2 patients with the second group of type 2A defects (R1597W and G1629R), a transient appearance of high-molecular–weight multimers is observed after DDAVP administration and transient normalization (6 and 10 minutes) of the prolonged BT (more than 30 minutes at baseline). The low VWF:RCo/Ag ratios (less than 0.30) observed at baseline did not change appreciably (Table 2).
Responses in patients with type 2M VWD
Only 3 of 21 (14%) patients with type 2M VWD met the criteria for responsiveness. Figure 4 shows that all but one patient reached the threshold FVIII:C levels of at least 30 U/dL 2 hours after DDAVP administration, whereas VWF:RCo became higher than 30 U/dL in only 14 patients. The prolonged BT was normal at 2 hours in 13 of 21 (62%) patients. Table 1 shows that in the group as a whole, the relative increase at 2 hours for FVIII:C was similar to that of VWF:RCo, with modest improvements in the mean values of VWF:RCo/Ag ratios and BT.
Gene mutations were known in 15 of 21 (70%) patients: R1205H (1 patient), R1315C (3 patients), R1374C (9 patients), and R1374H (2 patients). The patient heterozygous for the R1205H mutation, Vicenza-type VWD,13 had the following baseline phenotype: VWF:RCo less than 6 U/dL; VWF:Ag, 13 U/dL; FVIII:C, 4 U/dL; and VWF:RCo/Ag ratio, 0.5. Despite the relative 5-fold increase in FVIII:C and the 3-fold increase in VWF:RCo, levels for FVIII:C and VWF:RCo did not reach at least 30 U/dL; therefore, the patient was classified as unresponsive. None of the 3 patients with the R1315 mutation was responsive (Table 1). In 8 of 9 patients from Sweden with the R1374C mutation, there was a consistent lack of response to DDAVP (Table 1). The responses of FVIII:C and VWF:RCo in the 2 patients with the R1374H mutation were similar to those found in patients with R1374C (Table 1); only 1 of these patients was responsive. Five of 6 patients with unknown mutations (2M “undefined”) were classified as unresponsive, despite increases of VWF:RCo and FVIII:C levels (Table 1).
Responses in patients with type 2N VWD
Figure 5 and Table 1 show that in 4 patients with type 2N VWD, FVIII:C was low and VWF:RCo, VWF:RCo/Ag ratio, and BT were normal in all patients at baseline. The 3 patients with the R854Q mutation on one allele were responsive to DDAVP. The fourth patient, with the C1060R mutation, was unresponsive because FVIII:C levels of only 23 U/dL were reached after DDAVP infusion (Table 1). In the 3 patients heterozygous for R854Q, there was a 6.7- to 8.8-fold increase in FVIII:C, and the FVIII:C/VWF:Ag ratios were significantly lower (0.3 ± 0.2) than in patients with types 1, 2A, and 2M and remained lower (0.6 ± 0.3) at 2 hours after DDAVP (Table 1).
Discussion
Although DDAVP has been used for 25 years in the management of VWD, there has been no prospective study in well-characterized patients, with objective criteria to assess biologic or clinical response. In this study the biologic response to DDAVP was evaluated using changes in measurements of FVIII:C, VWF:RCo, and BT, which are commonly used as surrogate markers of clinical efficacy. The patient population included 66 patients with type 1 or 2 VWD who were classified according to preestablished uniform criteria and whose disease was clinically severe enough in the past to require treatment to control bleeding. Inclusion criteria excluded a sizable group of patients with mild and moderately severe VWD, identifying a clinically homogeneous patient population. At the time of infusion no patient was bleeding, so the clinical response rate could not be evaluated. Overall, only approximately one fifth of the patients had a biologic response to DDAVP, defined as the concomitant attainment of at least a 3-fold increase of FVIII:C and VWF:RCo to levels of at least 30 IU/dL and a BT shorter than 12 minutes. These arbitrary criteria for biologic response were the consensus target levels of useful clinical responses based on the experiences of the participating centers.
The rate of biologic response varied in different VWD types. As expected, patients with type 1 VWD responded (27%) better than patients with type 2 taken as a whole (18%), but they responded less frequently than claimed in previous studies.14 The FVIII/VWF ratios of type 1 VWD patients were much higher than those of type 2A VWD patients, as recently reported.15 The values of the ratio between VWF:RCo and VWF:Ag levels, before and 2 hours after DDAVP, provided some understanding of the varied responses. According to the current definition, in type 1 VWD the VWF protein (VWF:Ag) and its functional activity (VWF:RCo) should be similarly low, giving a ratio close to 1, as in healthy persons. However, there were 8 type 1 patients who had ratios below the lower normal limit (0.6), both at baseline and after DDAVP, among whom 6 were unresponsive to DDAVP. According to the current classification criteria, these patients are genuine type 1 because the “distribution of VWF multimers is normal or nearly normal.”16
On the other hand, there is the distinct possibility that these patients with low VWF:RCo/Ag ratio, decreased ristocetin-induced platelet agglutination, and normal or near normal multimeric structure are carriers of a qualitatively abnormal VWF protein in its interaction with platelets and that they should be classified as having type 2M VWD, as recently suggested.17-19 This hypothesis can be only validated by the knowledge of gene defects and by the demonstration that the expressed recombinant proteins have abnormal reactions with platelet membrane glycoproteins. In the absence of these molecular data, the 8 patients with low VWF:RCo/Ag ratios were still kept in the type 1 VWD group, as originally indicated by the participating centers at the time of enrollment.
Only 1 of 17 patients with type 2A VWD responded to DDAVP, because even though FVIII:C levels usually increased and the relative increase of VWF:RCo was on average 3-fold above baseline in most patients, the target levels of 30 U/dL were not reached. It has been reported that some patients with type 2A VWD respond better than others to DDAVP.20,21 However, these reports predated knowledge of gene defects and the description of different mechanisms of type 2A VWD (intracellular processing defects vs increased extracellular proteolysis).10-12 In this study the changes of FVIII/VWF activities, including the multimeric pattern, were documented using 4 patient examples, 2 with the features of group 1 (S1506L and V1665E) and 2 with the features of group 2 (R1597W and G1629R), as established by expression studies.10-12 According to our criteria, all patients with type 2A disease were unresponsive to DDAVP whatever the mutation and the mechanism of VWF dysfunction. However, patients with group 2 mutations behaved differently than did those with group 1 defects, with greater improvements in VWF:RCo and BT values. It remains to be understood whether these observations are associated with a better clinical response.
Most patients with type 2M VWD were poorly responsive to DDAVP. These patients have a full set of multimers, but VWF functions abnormally in the interaction with platelets. This is shown either by finding a low VWF:RCo/Ag ratio in plasma or by demonstrating an abnormal function of the expressed recombinant protein when the mutation is known. In a relatively large group of Swedish patients from different kindreds carrying the same mutation (R1374C), the response to DDAVP was consistently poor. Previous expression studies carried out in these patients have shown that mutant VWF reacts abnormally with platelets.19,22 A transient correction of the VWF:RCo/Ag ratios that correlates with a shortening of the BT can be observed in patients with type 2M VWD (data not shown in detail), as recently reported.23
Patients with Vicenza VWD are characterized by the presence in plasma of an abnormal set of ultralarge multimers, concomitantly low levels of VWF and FVIII:C, and mildly prolonged BT. They are currently classified as having type 2M VWD because multimers are present and VWF is thought to be dysfunctional.13 Only one patient with type 2M Vicenza, carrying the R1205H mutation, was investigated. The patient was unresponsive to DDAVP, with FVIII:C and VWF:RCo levels below 30 IU/dL 2 hours after DDAVP even though the BT was markedly shortened from 13 to 7 minutes. BT was normalized despite the relatively low levels of VWF because the “supranormal” multimers are highly effective in platelet adhesion. FVIII/VWF activities were measured for only 4 hours after DDAVP, so the clearances from plasma could not be determined. Therefore, it is difficult to compare this patient with the recent report of a group of Vicenza patients who exhibited accelerated clearance of FVIII/VWF measurements.23,24 The latter feature does not seem to be specific for Vicenza VWD because other types of VWD have been shown to have a short VWF half-life.25,26
Three of 4 patients of the small group with type 2N VWD, all of whom carried the mutation R854Q, associated with some residual binding of FVIII:C to the abnormal VWF and characterized by low FVIII:C levels and normal VWF levels responded to DDAVP with large increases of FVIII:C.27 On the other hand, the fourth patient, carrying both a null mutation and the C1060R mutation associated with a complete absence of FVIII binding, was unresponsive. Therefore, at least in this group of patients, a relationship appears to exist between the type of gene mutation, the related functional abnormalities, and the response to DDAVP.
From this prospective study designed to understand DDAVP responsiveness through the knowledge of phenotypes and genotypes, we conclude that when preestablished uniform criteria are applied, the rate of biologic response in severe types 1 and 2 VWD is lower than previously described. Genotypic data were more useful than knowledge of phenotype only for patients with types 2A and 2N VWD.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-2072.
Supported by European Community grant BMH4-972256 for a BIOMED 2 Project entitled “Optimizing Orphan Drug Therapy of Severe Forms of von Willebrand Disease.”
A.B.F. and C.M. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof. After this manuscript was accepted for publication, Canadian investigators published a study on responsiveness to desmopressin in 75 patients with type 1 or type 2A von Willebrand disease.28 (S. Revel-Vilk et al, J Pediatr Hematol Oncol 2003;25:874-879).
We thank the technicians at the centers for performing the tests for FVIII/VWF measurements before and after DDAVP. We thank Prof Ulrich Budde for performing densitometric analysis of the gels.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal