T-cell large granular lymphocytic leukemia (T-LGL) is a clonal lymphoproliferative disorder representing approximately 2% to 3% of all lymphocytic leukemias.1 Clonal lymphocytes express CD3, CD8, CD16, and CD5 and share a common rearranged T-cell receptor (TCR).2 Clinically, the bone marrow, spleen, and liver are extensively infiltrated by LGL, resulting in chronic neutropenia, anemia, and subsequent life-threatening infections. The pathogenesis of these cytopenias is thought to be immune-mediated immunosuppression. Thus, standard therapy utilizes immunosuppressive and/or chemotherapeutic agents.3 However, none have proven to be universally effective in achieving durable disease control.4
Alemtuzumab is a humanized monoclonal antibody directed against the CD52 antigen expressed on all lymphocytes: T cells more than B cells and monocytes.5,6 Alemtuzumab is approved for use in chronic lymphocytic leukemia and is being evaluated in the treatment of other hematologic malignancies as well as autoimmune diseases. The potential efficacy of alemtuzumab in treatment of T-LGL has not been described previously. Herein, we describe a patient with CD52+ T-LGL who, after failing multiple treatment modalities, achieved long-term disease control with alemtuzumab.
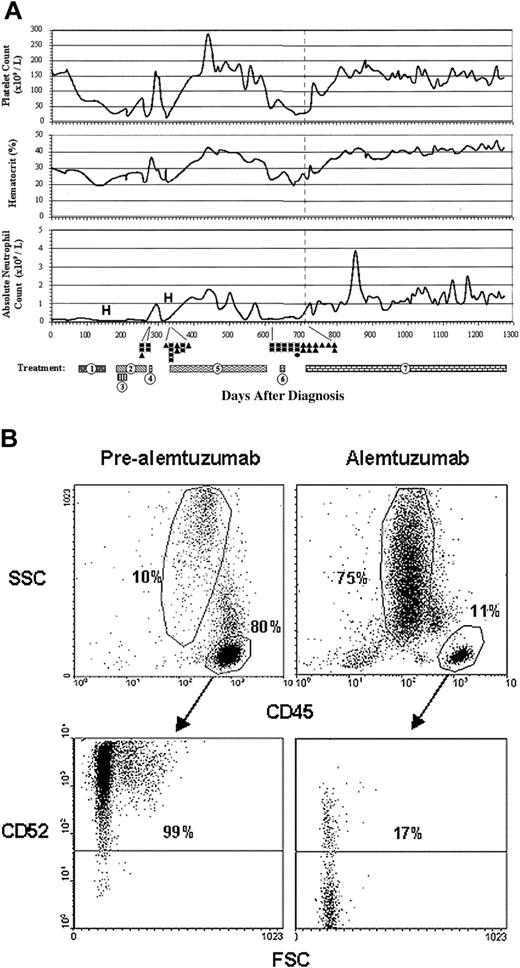
A 53-year-old woman presented in January 2000 with anemia, leukopenia, and neutropenia. Signs or symptoms of an associated rheumatologic disorder were not present. Peripheral blood showed lymphocytosis with 98% of lymphocytes composed of a population of LGL expressing CD2, CD3, CD8, CD16, CD45, and CD57. TCR gene rearrangement studies revealed the presence of a clonal population by polymerase chain reaction (PCR; performed at Mayo Clinic, Rochester, MN). Bone marrow biopsy revealed extensive lymphocytosis with a predominance of large granular T lymphocytes displaying an immunophenotype similar to that observed in the peripheral blood. These findings were consistent with T-LGL. The patient was subsequently treated with a succession of immunosuppressive and/or chemotherapeutic regimens over the course of approximately 20 months as summarized in Figure 1A. She failed to achieve sustained disease control with any of these regimens and remained red blood cell (RBC) and platelet transfusion–dependent throughout her course. In addition, she was hospitalized on 2 separate occasions for neutropenic fevers.
Disease course and immunophenotype of refractory T-LGL before and after alemtuzumab therapy. (A) Data represent all complete blood counts and white blood cell differentials measured from the initial time of diagnosis. Numbered bars represent the mode and duration of treatment modalities as follows: (1) methotrexate 10 mg/m2 twice weekly with prednisone 1 mg/kg (including prednisone taper); (2) cyclosposine 200 mg once a day (days 180-219), 300 mg once a day (days 220-248), and 250 mg once a day (days 249-267); (3) cytoxan 100 mg once a day; (4) ATG 1.5 mg/kg once a day for 4 days; (5) OKT3 20 mg once a day (days 331-339), and 10 mg once a day (days 344-603); (6) pentostatin 4 g/m2 every 2 weeks; (7) alemtuzumab 3 mg to 10 mg to 30 mg within first 3 days of therapy, 30 mg 3 times weekly (days 721-760), weekly (days 766-787), every 2 weeks (days 800-currently/1278). Gray dotted line in each graph represents the beginning of alemtuzumab therapy. • denotes a single packed red blood cell transfusion; ▴, platelet transfusion; and (H) denotes hospitalization for neutropenic fever. (B) Flow cytometry was performed on peripheral blood approximately one week prior to the initiation of alemtuzumab therapy (pre-alemtuzumab) and at approximately 80 weeks into alemtuzumab therapy (alemtuzumab). Cells in all dot plots are gated on mononuclear cells based on forward scatter (FSC) and side scatter (SSC) properties. Cells in lower plots are gated on CD45+ cells as denoted by arrows. Lower quadrants show isotype-matched control antibody staining.
Disease course and immunophenotype of refractory T-LGL before and after alemtuzumab therapy. (A) Data represent all complete blood counts and white blood cell differentials measured from the initial time of diagnosis. Numbered bars represent the mode and duration of treatment modalities as follows: (1) methotrexate 10 mg/m2 twice weekly with prednisone 1 mg/kg (including prednisone taper); (2) cyclosposine 200 mg once a day (days 180-219), 300 mg once a day (days 220-248), and 250 mg once a day (days 249-267); (3) cytoxan 100 mg once a day; (4) ATG 1.5 mg/kg once a day for 4 days; (5) OKT3 20 mg once a day (days 331-339), and 10 mg once a day (days 344-603); (6) pentostatin 4 g/m2 every 2 weeks; (7) alemtuzumab 3 mg to 10 mg to 30 mg within first 3 days of therapy, 30 mg 3 times weekly (days 721-760), weekly (days 766-787), every 2 weeks (days 800-currently/1278). Gray dotted line in each graph represents the beginning of alemtuzumab therapy. • denotes a single packed red blood cell transfusion; ▴, platelet transfusion; and (H) denotes hospitalization for neutropenic fever. (B) Flow cytometry was performed on peripheral blood approximately one week prior to the initiation of alemtuzumab therapy (pre-alemtuzumab) and at approximately 80 weeks into alemtuzumab therapy (alemtuzumab). Cells in all dot plots are gated on mononuclear cells based on forward scatter (FSC) and side scatter (SSC) properties. Cells in lower plots are gated on CD45+ cells as denoted by arrows. Lower quadrants show isotype-matched control antibody staining.
In September 2001, her blood was analyzed for CD52 expression by flow cytometry: over 90% of the lymphocytes expressed CD52 (Figure 1B). Prior to initiating alemtuzumab, the patient had been untreated for approximately 50 days (more than 1.5 years since her last course of prednisone). During this time, her white blood cell, hematocrit, and platelet counts averaged 0.9 × 109/L (0.9 K/μL), 0.25(25%), and 27 × 109/L (27 K/mL), respectively. Peripheral blood mononuclear cells were composed of approximately 80% lymphocytes (normal = 20%-40%) and 10% granulocytes (normal = 40%-60%) (Figure 1B). Alemtuzumab was administered intravenously during the first week as follows: day 1, 3 mg; day 2, 10 mg; day 3, 30 mg. Alemtuzumab was then given in a dose of 30 mg thrice weekly for 6 weeks, once weekly for 3 weeks, and is currently being given in a dose of 30 mg subcutaneously every third week (> 80 weeks). A progression toward normalization of the patient's peripheral blood counts was observed after alemtuzumab treatment (Figure 1A). Platelets, hematocrit, and absolute neutrophil counts increased significantly and remain stable at 150 × 109/L (150 K/mL), 0.4 (40%), and 1.8 × 109/L (1800/μL), respectively. Complete blood count and flow cytometry of peripheral blood 80 weeks after alemtuzumab treatment revealed 75% granulocytes and 11% lymphocytes: 17% of lymphocytes expressing CD52 (Figure 1B). The patient has been treated with alemtuzumab for more than 80 weeks with minimal toxicities. She has not required either RBC or platelet transfusions throughout her alemtuzumab treatment, nor has she had any episodes of neutropenic fevers.
To the best of our knowledge, this is the first report to demonstrate long-term successful treatment of T-LGL with alemtuzumab. The dramatic response of this patient suggests the need for a larger scale clinical trial to explore the potential efficacy of alemtuzumab for first-line therapy of T-LGL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal