Appel et al1 reported in January that the paradigmatic KIT, PDGFR, ABL tyrosine kinase inhibitor imatinib mesylate (STI571/Gleevec), known to induce potent antitumor effects in chronic myeloid leukemia (CML) and gastrointestinal stromal tumors, acts on nonmalignant hematopoietic cells, inhibiting the differentiation and maturation of CD34+ progenitors into functional dendritic cells (DCs) in vitro. Indeed, DCs can be propagated from mobilized human CD34+ precursors in ex vivo cultures containing granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor α (TNFα) with or without interleukin 4 (IL-4) and with or without Flt3L,2 and display their functional attributes (ie, priming naive T cells), leading to antitumor effects in patients with melanoma.3 The DC growth factor Flt3L (FL) also has been used to propagate DCs from progenitors in vivo in mice,4 healthy volunteers,5 and patients with cancer,6,7 leading to organomegaly and antitumor effects. In the January 15, 2004, issue of Blood, the authors demonstrate that in vitro exposure of CD34+ progenitors to micromolar ranges of STI571 affects the development of DCs and their capacity to induce primary cytotoxic T-lymphocyte (CTL) responses in vitro.1 Extending Appel et al's observation to in vivo settings, we report here that the FL capacity (10 μg/mouse administered intraperitoneally for 10 days) to promote in vivo DC expansion in mice is abrogated when STI571 is concomitantly administered (oral feeding of mice with 150 mg/kg STI571 twice a day from day 5 to day 10 leading to residual plasmatic concentrations of about 1 μM). Indeed, the spleen weight in BL6 mice receiving FL alone reached 215 ± 12 mg at day 12, while reaching 157 ± 8 mg in the combination of FL plus STI571 (P < .001, Mann Whitney 2-sample t test). Accordingly, the number of splenic DCs was dramatically reduced when STI571 was added to FL in both C57BL/6 and SWISSnu/nu mice (35 × 106 ± 6 [BL6] and 45 × 106 ± 6 [nu/nu] with FL alone versus 9.5 × 106 ± 5 [BL6] and 26 × 106 ± 5 [nu/nu] with FL plus STI571, P < .05 in both strains, whereas control BL6 and nu/nu spleens contained 5 × 106 ± 5 and 3.6 × 106 ± 4 DCs, respectively). No significant differences were seen between the groups concerning CD11b, CD11c, IAb, CD80, CD40, and CD86 expression on spleen DCs. Consequently, we demonstrate that the FL-mediated antitumor effects are severely hampered when FL is combined with STI571 in such a setting (Figure 1). In 2 tumor models (the RMA-S lymphoma and the MCA 102 fibrosarcoma), although FL could significantly prevent tumor growth, the coadministration of STI571 at early stages of therapy with FL abrogated tumor regressions (P < .01 in both settings, Kruskall Wallis one-way analysis of variance [ANOVA]). Here again, STI571 inhibits FL bioactivity by preventing DC expansion in vivo, thereby reducing DC-dependent T- and NK-cell–mediated antitumor effects (not shown). Whether STI571 blocks the c-kit pathway involved in the differentiation of the common lymphoid progenitor remains to be investigated.8 Our observation together with Appel et al's report could be taken into account for the management of patients with CML who are treated with DC-based immunotherapy protocols.
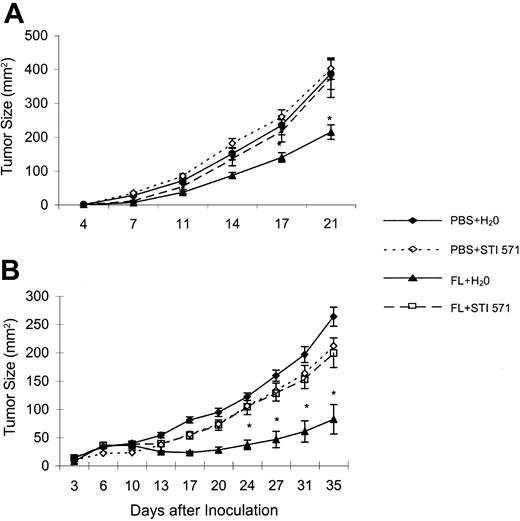
STI571 abrogates FL-mediated antitumor effects. Twice the minimal tumorigenic dose of RMA-S (A) or MCA102 (B) cells was inoculated into the abdominal flank of C57BL/6 mice at day 0. From day –4 to day +5, FL (10 μg/mouse) or phosphate-buffered saline (PBS) only (200 μL) was injected intraperitoneally. From day +1 to day +5, STI571 (150 mg/kg per gavage) or H20 (200 μL) was administered twice a day by oral feeding. Tumor size was monitored with a caliper twice a week, and the product of largest perpendicular diameters is reported for individual time points as mean ± SEM. *Significantly lower than PBS plus H20, PBS plus STI571, and FL plus STI571; P < .05. Each experiment was performed 3 times with similar results and involved 5 to 7 animals per group. A representative experiment is depicted.
STI571 abrogates FL-mediated antitumor effects. Twice the minimal tumorigenic dose of RMA-S (A) or MCA102 (B) cells was inoculated into the abdominal flank of C57BL/6 mice at day 0. From day –4 to day +5, FL (10 μg/mouse) or phosphate-buffered saline (PBS) only (200 μL) was injected intraperitoneally. From day +1 to day +5, STI571 (150 mg/kg per gavage) or H20 (200 μL) was administered twice a day by oral feeding. Tumor size was monitored with a caliper twice a week, and the product of largest perpendicular diameters is reported for individual time points as mean ± SEM. *Significantly lower than PBS plus H20, PBS plus STI571, and FL plus STI571; P < .05. Each experiment was performed 3 times with similar results and involved 5 to 7 animals per group. A representative experiment is depicted.
Response: Is imatinib an immunosuppressive drug?
Imatinib mesylate (STI571/Gleevec, Novartis Pharmaceuticals, Basel, Switzerland) is a competitive inhibitor of the c-Kit, PDGFR, and ABL tyrosine kinases and is currently applied in the treatment of Philadelphia chromosome–positive chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL), as well as gastrointestinal stroma tumors (GISTs) due to its efficiency to induce high rates of disease remissions in these malignancies, with moderate side effects.1 In a recent publication by Bartolovic et al2 it was shown that imatinib mesylate could inhibit the proliferation of human CD34+ progenitor cells that were expanded in serum-free medium and decrease their colony-forming capacity in a dose-dependent manner. However, no such effect on more primitive cobblestone area–forming cells was detectable, suggesting a significant inhibitory effect of imatinib mesylate on normal CD34+ progenitor cells that was largely independent of c-Kit signaling.2 We expanded these observations by demonstrating that this compound can also affect the differentiation of CD34+ progenitor cells into dendritic cells (DCs) characterized by reduced expression of DC-associated cell-surface molecules like CD1a and CD83, as well as HLA class II and costimulatory molecules like CD80 and CD40. In addition to these phenotypic alterations, these cells showed a reduced immune stimulatory capacity and were unable to elicit primary T-cell responses.3 The results by Taïeb et al extend and confirm these observations by analyzing the effects of imatinib mesylate in an animal model. The feeding of mice with imatinib mesylate resulted in the inhibition of FLT3L–induced expansion of DCs in vivo and impaired the induction of a protective antitumor immunity in these animals.
It would be of interest to further analyze the in vivo effects of imatinib mesylate on the function of the effector cells of the immune system (T cells, natural killer cells, B lymphocytes) and their interaction with antigen-presenting cells. These studies have to be accompanied by the analysis of the phenotype and function of DC subpopulations and the induction of immune responses using peripheral blood of patients treated with imatinib mesylate, especially in patients with GISTs. It might be different in patients with CML, as at least at the beginning of the treatment the malignant clone is contributing to the hematopoiesis. Recently, Sato and collegues demonstrated that inhibition of c-Abl activity might even improve the function of antigen-presenting cells.4
The results of these studies would be relevant for the treatment of malignant diseases such as CML, as some of the remissions following interferon α treatment or allogeneic transplantations were shown to be caused by the induction of efficient T-cell–mediated immune responses that might be impaired by coadministration of imatinib mesylate.5
Correspondence: Peter Brossart, University of Tübingen, Department of Hematology, Oncology and Immunology, Otfried-Müller-Str. 10, 72076 Germany.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal