The killer immunoglobulin-like receptor (KIR) family recognizes class I major histocompatibility complex (MHC) molecules and inhibits natural killer (NK) activity. This family is highly polymorphic, and only some of the genes are present in each individual.1 Thus, the frequency of a single inhibitory receptor in a bulk NK population varies between 5% and 50%.2 It was suggested that such diversity of NK receptors enables the killing of infected cells by at least a subset of NK cells.3
A 7-year-old patient from Israel had been hospitalized 12 times due to infections. At the age of 2.5 months, the patient had severe interstitial pneumonitis, hepatosplenomegaly, anemia, and thrombocytopenia. Immunoglobulin M (IgM) antibodies against cytomegalovirus (CMV) were identified, and CMV in the urine was detected. At the age of 2.5 years, the patient had a severe bilateral pneumonia and respiratory failure. Broncho-alveolar lavage recovered CMV from the lungs. In the following years, 8 additional admissions due to lung infections and 1 due to acute gastroenteritis occurred. At the age of 6 years, the child developed chicken pox, with hundreds of skin lesions. Despite acyclovir treatment, a severe pneumonitis with bilateral “white lungs” was evident. All laboratory tests at age 7 years were normal, including blood count, blood chemistry (SMA-18), urinalysis, serum antibodies, complement, and hemolytic complement. IgG antibodies for diphtheria, tetanus, pertussis, poliomyelitis, Epstein-Barr virus, and CMV were positive; tests for HIV were negative. Lymphocyte population was as follows: CD3 (approximately 80%), CD4 (approximately 58%), CD8 (approximately 27%), CD19 (approximately 11%), CD16 (approximately 8%), and CD56 (approximately 9%).
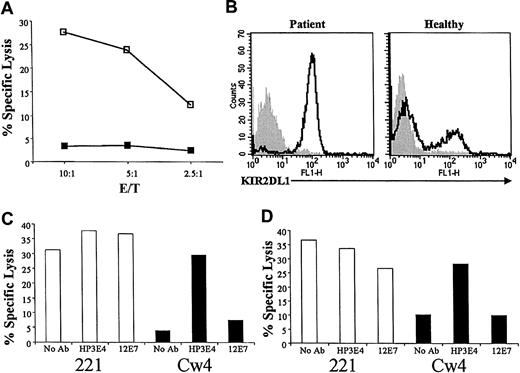
Importantly, the observed symptoms were almost identical to those observed in other patients suffering from NK cell deficiency.4 Surprisingly, the bulk NK cultures of the patient were inhibited by HLA-Cw4 (Figure 1A). Expression of KIR2DL1 receptor was detected on all NK cells in the bulk culture, by the HP3E4 or the EB6 monoclonal antibodies (mAbs) (Figure 1B). Never before had we, or others, observed such an unusual expression of KIR2DL1 on the entire NK cell population. Furthermore, all NK clones derived from the patient expressed KIR2DL1 and were inhibited by HLA-Cw4 (data not shown). Expression of other NK receptors, including KIR2DL2, KIR3DL1, NKG2A, CEACAM1, LIR1, and NKp46, was normal (28%, 21%, 25%, 12%, 12.5%, and 100%, respectively). The inhibition observed was the result of KIR2DL1 interaction with HLA-Cw4, as the HP3E4 mAb abolished the inhibition of either the NK clones or the bulk NK cultures (Figure 1C-D). The MHC haplotype of the patient is A26/A72, B51/B53, Cw06/Cw15, with both HLA-C alleles able to interact with KIR2DL1.
KIR2DL1 is expressed on all NK cells derived from the patient. (A) Bulk NK cultures derived from the patient were incubated with 721.221 targets (221; □), or with 721.221 transfected with HLA-Cw4 (Cw4; ▪), at the indicated effector-target (E/T) ratios. (B) Fluorescence-activated cell sorter (FACS) analysis for KIR2DL1 expression of bulk NK cultures derived from the patient (left) and a representative healthy donor (right) using the EB6 mAb. Staining (open histograms) is shown overlaid on background (shaded histograms). The NK cells were incubated for one hour with or without anti-KIR2DL1 mAb HP3E4 or the anti-CD99 mAb 12E7 and then tested against the various targets as in panel A. Shown is a representative NK clone (C) and NK bulk cultures (D), both derived from the patient.
KIR2DL1 is expressed on all NK cells derived from the patient. (A) Bulk NK cultures derived from the patient were incubated with 721.221 targets (221; □), or with 721.221 transfected with HLA-Cw4 (Cw4; ▪), at the indicated effector-target (E/T) ratios. (B) Fluorescence-activated cell sorter (FACS) analysis for KIR2DL1 expression of bulk NK cultures derived from the patient (left) and a representative healthy donor (right) using the EB6 mAb. Staining (open histograms) is shown overlaid on background (shaded histograms). The NK cells were incubated for one hour with or without anti-KIR2DL1 mAb HP3E4 or the anti-CD99 mAb 12E7 and then tested against the various targets as in panel A. Shown is a representative NK clone (C) and NK bulk cultures (D), both derived from the patient.
Our patient frequently suffers from recurrent infections, mainly CMV. Importantly, the CMV developed many mechanisms to avoid attack by NK cells and by cytotoxic T lymphocytes (CTLs),5 including the down-regulation of HLA-A and -B to avoid CTL attack, while maintaining HLA-C expression to avoid NK attack.6 Since all NK clones in the patient express KIR2DL1, which is able to interact with both HLA-C proteins of the patient, it is possible that in the often observed CMV infections, all NK activity is inhibited. We suggest that overexpression of an inhibitory NK receptor might lead to a novel immune deficiency associated with primary CMV infection.
Supported by grants from the Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum (DKFZ) and Israel's Ministry of Science (MOS), the Israel Cancer Research Foundation, the Israel Science Foundation, the Cancer Research Institute (CRI), the Israel Academy of Sciences, and the European Commission (QLRT-2001-01112).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal