Abstract
Cardiac T2* (magnetic resonance imaging relaxation parameter) is abnormally low in approximately 40% of adults with thalassemia major (TM), suggesting myocardial iron deposition, but it is unknown at what age this occurs. To address this question, we measured cardiac T2* and function in 19 young patients (aged 7-26 years) with TM as well as 17 patients receiving long-term transfusions for sickle cell anemia (SCA) matched for age, sex, and liver iron content. Cardiac T2* was normal in all of the SCA patients but was low (high iron) in 8 of 19 TM patients. Abnormal T2* was observed only in the TM patients receiving transfusions for 13 years or longer and was correlated with ferritin but not liver iron levels. Cardiac dysfunction was present in 3 of the 8 patients with low T2*. Cardiac T2* changes have a long latency relative to liver iron accumulation. Total transfusional burden is a significant independent risk factor for low cardiac T2* and may partially account for differences observed between patients with SCA and TM.
Introduction
Iron cardiomyopathy is the leading cause of death in patients with thalassemia major (TM).1-4 Once cardiac symptoms become apparent in iron-loaded subjects, decompensation and death occur rapidly unless chelation therapy is dramatically intensified.1,3 The magnetic resonance imaging (MRI) relaxation parameters T2 and T2* have been used to infer cardiac iron loading in patients with TM.5-8 Low T2*, suggesting high myocardial iron content, has been associated with poor ventricular function, myocardial arrhythmias, and need for cardiac medications.5 Although abnormal T2* is common in adults, its prevalence and functional significance in pediatric patients is unknown.5 The purpose of this study was to determine the prevalence of abnormal T2* as well its relationship to hepatic iron content, transfusion burden, and myocardial function in age- and sex-matched pediatric populations with TM and sickle cell anemia (SCA).
Study design
Patients with SCA or TM who received more than 8 transfusions annually for at least 3 years were recruited for this study. All patients had transfusions to keep the pretransfusion hemoglobin level at approximately 95 g/L (9.5 g/dL). Consent was obtained in accordance with the Committee on Clinical Investigation at Childrens Hospital Los Angeles. Clinical information regarding duration of transfusion and chelation therapy, hepatic iron content (Mayo Clinical Laboratories, Rochester, MN), ferritin levels, cardiac medications, and Holter examinations were obtained. Patients were considered to have cardiac disease if they required medication (diuretics, digoxin, afterload reducing agents, or antiarrhythmic drugs), had an ejection fraction less than 60%, or had persistent arrhythmias. Only one patient was receiving a negative inotropic drug, amiodarone, and she had normal function. Cardiac MRI examination was performed using a torso coil on a 1.5 T General Electric CVi scanner. Biventricular volumes and ejection fractions were measured using 12 short-axis segmented cine gradient echo images. Myocardial T2* was measured using a nonsteady-state segmented gradient echo technique with centrally symmetric phase-encoding groups (SCOPEGs), recovery time (TR) 21 ms, and echo time (TE) 2, 3, 4, 6, 9, 12, 15, and 18 ms.5 Signal-decay curves were measured from a region of interest encompassing the interventricular septum and fit to a mono exponential decay.
Hemodynamic data, including pretransfusion heart rate, blood pressure, and hemoglobin levels, were collected from 5 consecutive monthly visits prior to the MRI.
All continuous data were compared using unpaired Student t tests. Prevalence of abnormal T2* in the 2 patient groups was evaluated using the Fischer exact test.
Results and discussion
Demographics, hemodynamic data, and MRI results for all patients are summarized in Table 1. SCA and TM patients included in the study were closely matched for age, sex, and iron burden. Iron burdens were high in both populations with mean hepatic iron content in the presumed “cardiotoxic” range.9 Mean time from liver biopsy to MRI examination was 14 ± 4 months. Duration of transfusion and chelation were not significantly different between the 2 populations.
Demographics and MRI data from patients with SCA and TM.
Parameter . | SCA . | TM . | P . | Normal reference range . |
|---|---|---|---|---|
| Age, y | 17.6 ± 1.3 | 15.7 ± 1.4 | .23 | — |
| Sex, M/F | 9/8 | 10/9 | .999 | — |
| Hepatic iron concentration, mg/g dry weight | 19.3 ± 3.7 | 18.4 ± 3.8 | .997 | — |
| Ferritin concentration | 5422 ± 855 | 3648 ± 1378 | .37 | — |
| Transfusion duration, y | 9.3 ± 1.3 | 12.2 ± 1.5 | .20 | — |
| Chelation duration, y | 6.9 ± 1.2 | 9.8 ± 1.5 | .23 | — |
| BSA, m2 | 1.57 ± 0.09 | 1.25 ± 0.06 | .002* | — |
| Pretransfusion hemoglobin level, g/L | 94 ± 1 | 92 ± 1 | .08 | — |
| Heart rate, bpm | 89.6 ± 3.6 | 84.1 ± 3.5 | .18 | — |
| Systolic blood pressure, mm Hg | 116.9 ± 2.5 | 105 ± 2.3 | .001* | — |
| Diastolic blood pressure, mm Hg | 63.5 ± 1.9 | 60.2 ± 1.8 | .02* | — |
| T2*, ms | 38.1 ± 2.5 | 26.1 ± 4.6 | .005* | 25-46 |
| LV ejection fraction, % | 71 ± 2 | 67 ± 2 | .14 | 61-7714 |
| LV end-diastolic volume index, mL/m2 | 90.0 ± 5.4 | 85.9 ± 3.9 | .53 | 36-8014 |
| LV end-systolic volume index, mL/m2 | 26.1 ± 2.2 | 29.1 ± 2.8 | .41 | 5-3114 |
| LV mass index, g/m2 | 77.0 ± 4.8 | 63.4 ± 3.2 | .008* | 6-9514 |
| Cardiac index, L/min/m2 | 5.9 ± 0.5 | 4.6 ± 0.2 | .03* | 1.7-4.013 |
Parameter . | SCA . | TM . | P . | Normal reference range . |
|---|---|---|---|---|
| Age, y | 17.6 ± 1.3 | 15.7 ± 1.4 | .23 | — |
| Sex, M/F | 9/8 | 10/9 | .999 | — |
| Hepatic iron concentration, mg/g dry weight | 19.3 ± 3.7 | 18.4 ± 3.8 | .997 | — |
| Ferritin concentration | 5422 ± 855 | 3648 ± 1378 | .37 | — |
| Transfusion duration, y | 9.3 ± 1.3 | 12.2 ± 1.5 | .20 | — |
| Chelation duration, y | 6.9 ± 1.2 | 9.8 ± 1.5 | .23 | — |
| BSA, m2 | 1.57 ± 0.09 | 1.25 ± 0.06 | .002* | — |
| Pretransfusion hemoglobin level, g/L | 94 ± 1 | 92 ± 1 | .08 | — |
| Heart rate, bpm | 89.6 ± 3.6 | 84.1 ± 3.5 | .18 | — |
| Systolic blood pressure, mm Hg | 116.9 ± 2.5 | 105 ± 2.3 | .001* | — |
| Diastolic blood pressure, mm Hg | 63.5 ± 1.9 | 60.2 ± 1.8 | .02* | — |
| T2*, ms | 38.1 ± 2.5 | 26.1 ± 4.6 | .005* | 25-46 |
| LV ejection fraction, % | 71 ± 2 | 67 ± 2 | .14 | 61-7714 |
| LV end-diastolic volume index, mL/m2 | 90.0 ± 5.4 | 85.9 ± 3.9 | .53 | 36-8014 |
| LV end-systolic volume index, mL/m2 | 26.1 ± 2.2 | 29.1 ± 2.8 | .41 | 5-3114 |
| LV mass index, g/m2 | 77.0 ± 4.8 | 63.4 ± 3.2 | .008* | 6-9514 |
| Cardiac index, L/min/m2 | 5.9 ± 0.5 | 4.6 ± 0.2 | .03* | 1.7-4.013 |
Data represent the mean value ± SE. Right hand column demonstrates 95% confidence intervals for MRI values; however, all statistical comparisons are between the two patient populations. — indicates not assessed.
Significant P value.
No significant difference was observed in heart rate or pretransfusion hemoglobin level; however, larger body habitus (body surface area [BSA]) was noted in the SCA population, 1.57 ± 0.09 m2 versus 1.25 ± 0.06 m2. The elapsed time between last transfusion and MRI exam was comparable between the 2 groups and was not correlated with any of the MRI measurements. SCA patients had higher systolic and diastolic blood pressures than TM patients, 116 ± 2.5 versus 103 ± 2.3 and 62.2 ± 1.9 versus 57.7 ± 1.2 mm Hg, although all individuals from both groups had blood pressures within 95% confidence intervals for age.
Both patient groups demonstrated dilated ventricles and increased cardiac index, relative to published population norms, consistent with chronic anemia.10-15 Cardiac index was 30% higher in SCA patients (P = .02). Cardiac mass was higher in the SCA group, 77.0 ± 4.8 g versus 63.4 ± 3.2 g, likely reflecting the described differences in cardiac index and blood pressure. Cardiac T2* averaged 38.1 ± 2.5 ms in SCA patients and 26.1 ± 4.6 ms in TM patients (P = .004); the normal range at our institution is 35.7 ± 5 ms (range, 30-43 ms) with a lower limit of 25 ms.
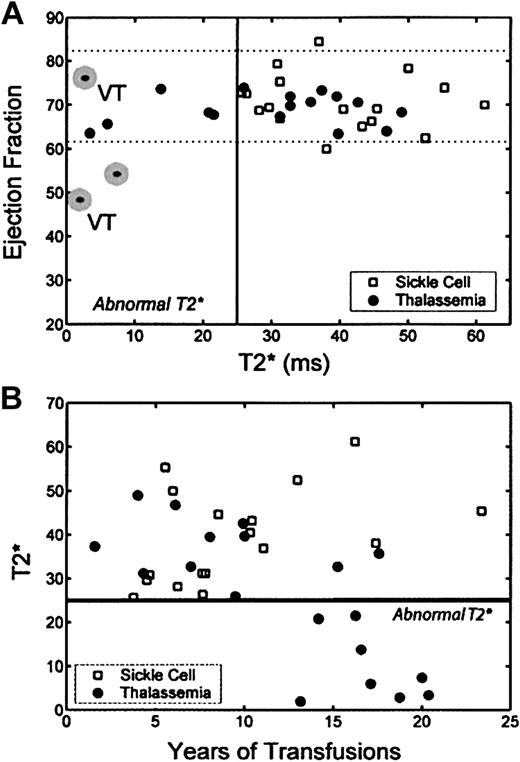
Figure 1A demonstrates the relationship between left ventricular (LV) ejection fraction and myocardial T2* for both patient populations. Patients with SCA had normal T2* and ejection fraction. In contrast, 8 of 19 patients with TM had T2* below the reference range, suggesting myocardial iron loading (P = .003 by Fischer exact test). The 3 patients with T2* between 10 and 25 were asymptomatic, whereas 3 of the 5 patients with T2* less than 10 had ventricular tachycardia, depressed ejection fraction, or both and were receiving cardiac medications.
Relationship among LV ejection fraction, myocardial T2*, and transfusion duration. (A) Plot of ejection fraction by MRI versus cardiac T2* in patients with thalassemia (•) and those with SCD (□). Dotted lines represent reference range for ejection fraction.12-14  indicates need for cardiac medications. Two patients had ventricular tachycardia, indicated by the letters VT. (B) Plot of cardiac T2* as a function of transfusion duration. Patients with SCD were predominantly receiving transfusions for less than 13 years (13 of 17), whereas the majority (10 of 19) of patients with thalassemia had transfusions for longer periods. None of the thalassemia patients receiving transfusions for less than 13 years had abnormal cardiac T2*, whereas 8 of 10 patients receiving transfusions for longer periods had abnormal T2*.
indicates need for cardiac medications. Two patients had ventricular tachycardia, indicated by the letters VT. (B) Plot of cardiac T2* as a function of transfusion duration. Patients with SCD were predominantly receiving transfusions for less than 13 years (13 of 17), whereas the majority (10 of 19) of patients with thalassemia had transfusions for longer periods. None of the thalassemia patients receiving transfusions for less than 13 years had abnormal cardiac T2*, whereas 8 of 10 patients receiving transfusions for longer periods had abnormal T2*.
Relationship among LV ejection fraction, myocardial T2*, and transfusion duration. (A) Plot of ejection fraction by MRI versus cardiac T2* in patients with thalassemia (•) and those with SCD (□). Dotted lines represent reference range for ejection fraction.12-14  indicates need for cardiac medications. Two patients had ventricular tachycardia, indicated by the letters VT. (B) Plot of cardiac T2* as a function of transfusion duration. Patients with SCD were predominantly receiving transfusions for less than 13 years (13 of 17), whereas the majority (10 of 19) of patients with thalassemia had transfusions for longer periods. None of the thalassemia patients receiving transfusions for less than 13 years had abnormal cardiac T2*, whereas 8 of 10 patients receiving transfusions for longer periods had abnormal T2*.
indicates need for cardiac medications. Two patients had ventricular tachycardia, indicated by the letters VT. (B) Plot of cardiac T2* as a function of transfusion duration. Patients with SCD were predominantly receiving transfusions for less than 13 years (13 of 17), whereas the majority (10 of 19) of patients with thalassemia had transfusions for longer periods. None of the thalassemia patients receiving transfusions for less than 13 years had abnormal cardiac T2*, whereas 8 of 10 patients receiving transfusions for longer periods had abnormal T2*.
Cardiac 1/T2* was weakly correlated with ferritin level (r2 = 0.33, P = .01) and uncorrelated with liver iron concentration (r2 = 0.02, P = .54). Cardiac T2* was negatively correlated with transfusion duration for TM but not SCA (Figure 1B; TM, r2 = 0.56, P = .002). No patient receiving transfusions for less than 13 years had an abnormal T2* (P < .001), but 80% of TM patients receiving transfusions for longer periods had abnormal T2* (P = .0006 by Fischer exact test). None of the 4 SCD patients having transfusions for longer than 13 years had abnormal T2* (P = .014 versus TM). Liver iron concentration had no correlation (r2 < 0.1) with transfusion duration for either group (not shown).
Abnormal cardiac T2* and ventricular rhythm or functional abnormalities were common in our pediatric and young adult TM population and absent in age and liver iron burden-matched SCA patients. There are many physiologic, as well as racial and socioeconomic differences, between patients with thalassemia and sickle cell disease (SCD). Shah et al16 also found normal cardiac T2* in their SCA patients and postulated that elevated nontransferrin-bound iron (NTBI) levels and transferrin saturations in the thalassemia population represent a potential mechanism for differential myocardial loading. However, neither their study nor the present study was able to simultaneously match age and transfusion burden. SCA patients often begin chronic transfusion therapy at an older age than patients with thalassemia or receive transfusions intermittently.17 Because of low numbers of SCA patients (4) receiving transfusions for more than 13 years, we cannot yet conclude that patients with SCA are at lower cardiac risk. Anecdotal reports of myocardial iron deposition and iron cardiomyopathy have been described in patients with SCD at autopsy and longer term follow-up is necessary.18,19
Risk factors for cardiac iron accumulation remain unclear. The correlation between myocardial T2* and transfusion duration in our study mirrors the increased risk of cardiac symptoms, over time, observed in longitudinal studies,20,21 supporting the joint role of long-term chelator noncompliance and iron exposure. Although the delay between liver biopsy and MRI examination could contribute to the poor correlation of T2* with liver iron, it is more likely that liver iron indices measured near the time of MRI examination yield little information about long-term chelation compliance and its corresponding cardiac risk. The present study demonstrates a significant latency to T2* changes, relative to liver accumulation, suggesting a long delay between poor iron control and detectable cardiac iron deposition. Other MRI work suggests that a “critical” liver saturation is necessary to achieve positive cardiac iron balance.22 However, careful longitudinal studies of cardiac and liver iron will be necessary to clarify their temporal interdependence. Furthermore, whereas abnormal T2* is associated with cardiac symptoms, further studies are necessary to determine its predictive capability in currently asymptomatic patients.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-1919.
Supported by the General Clinical Research Center (GCRC) (National Institutes of Health no. RR00043-43); the Department of Pediatrics at Childrens Hospital Los Angeles; the University of Southern California School of Medicine; and the Whitaker Foundation.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal