Abstract
Infection of human erythrocytes by the apicomplexan malaria parasite Plasmodium falciparum results in endovacuolar uptake of 4 host proteins that reside in erythrocyte detergent-resistant membranes (DRMs). Whether this vacuolar transport reflects selective uptake of host DRM proteins remains unknown. A further complication is that DRMs of vastly different protein and cholesterol contents have been isolated from erythrocytes. Here we show that isolated DRMs containing the highest cholesterol-to-protein ratio have low protein mass. Liquid chromatography, mass spectrometry, and antibody-based studies reveal that the major DRM proteins are band 3, flotillin-1 and -2, peroxiredoxin-2, and stomatin. Band 3 and stomatin, which reflect the bulk mass of erythrocyte DRM proteins, and all tested non-DRM proteins are excluded from the vacuolar parasite. In contrast, flotillin-1 and -2 and 8 minor DRM proteins are recruited to the vacuole. These data suggest that DRM association is necessary but not sufficient for vacuolar recruitment and there is active, vacuolar uptake of a subset of host DRM proteins. Finally, the 10 internalized DRM proteins show varied lipid and peptidic anchors indicating that, contrary to the prevailing model of apicomplexan vacuole formation, DRM association, rather than lipid anchors, provides the preferred criteria for protein recruitment to the malarial vacuole.
Introduction
Malaria is a major infectious disease and remains an intolerable burden throughout the tropics. The most virulent form of malaria is caused by the protozoan Plasmodium falciparum. The invading “merozoite” parasite invaginates the erythrocyte membrane to reside in an immunologically privileged intracellular parasitophorous vacuolar membrane (PVM). Although the bulk mass of erythrocyte proteins is excluded from the PVM, proteins that reside in cholesterol-rich detergent-resistant membrane (DRM) “rafts” in the host membrane are recruited into the vacuole.1,2 Mild depletion of erythrocyte cholesterol has no detectable effect on major erythrocyte membrane function but disrupts DRM rafts and blocks malarial infection.3 Signaling via erythrocyte β2-adrenergic receptor (β2-AR) and guanine nucleotide-binding protein (Gαs), both of which are raft proteins, regulates entry of P falciparum.4 These data suggest that DRMs and their resident proteins may play a role in the induction and formation of the PVM in the erythrocyte.
Lipid rafts are plasma membrane regions that reflect dynamic, lateral heterogeneities whose lipid and protein content differs from the rest of the membrane.5 Lipid-lipid interactions are thought to underlie the formation of rafts by partitioning into liquid-ordered and -disordered phase domains. Proteins favoring an ordered environment (rich in order-preferring lipids such as cholesterol, sphingomyelin, and glycosphingolipids) partition into raft domains.6 Raft proteins show cholesterol-dependent association with DRMs.3,7 Further, cholesterol renders raft proteins resistant to extraction in detergent8 and thereby provides a method that can be used to isolate DRM proteins from the rest of the membrane.1 At a minimum, DRM proteins represent a consistent subset of the total raft protein population.6,9
Functionally, lipid rafts may increase the duration of interaction of constituent proteins, particularly of signaling complexes.8 Rafts have also been implicated in membrane sorting processes.5,8,10 Hence, the characterization of plasma membrane DRMs and identification of their major and minor resident proteins may provide insights into how DRM rafts may contribute to membrane invagination in cells. However, in most cells, plasma membranes must be purified from membrane-bound organelles. Because erythrocytes lack intracellular organelles, they provide an optimal plasma membrane source uncontaminated by other membranes. During malarial infection, a poorly understood process directs some host raft proteins to the vacuole, while host nonraft proteins remain on the plasma membrane. Thus, study of DRM raft proteins in erythrocytes may lend insight into the role of raft proteins in both malaria infection and other cellular processes.
Plasmodia belong to the phylum Apicomplexa and contain, at their apical end, specialized secretory organelles dedicated for entry into the host cell. Mordue et al proposed that recruitment of host proteins into the related apicomplexan Toxoplasma gondii vacuole is dependent on their membrane anchors and only glycosylphosphatidylinositol (GPI)–anchored proteins are recruited to this vacuole.11 Subsequent studies questioned the suitability of this model for P falciparum because they revealed that multipass transmembrane (Duffy) and integrally associated host proteins (flotillin-2) can be recruited to the plasmodial vacuole.1,3 However, neither provided comprehensive analyses of host membrane protein movement into the apicomplexan vacuole.
The literature suggests that at least 2 distinct DRM preparations with different resident proteins may be isolated from erythrocytes.3,12 The reasons underlying these differences have confused efforts to define resident proteins of erythrocyte DRMs. Here, we show that differences in detergent and protein concentrations in extracts can markedly influence the mass and composition of proteins that associate with DRMs. Further, extraction of DRMs from lysates of low-protein concentrations results in isolation of complexes enriched with high levels of cholesterol. Our studies provide molecular insight on the major and minor protein components of DRM rafts obtained from a model plasma membrane and establish a new model for apicomplexan vacuole formation.
Materials and methods
Parasite culture
Antibodies, immunoblotting, and indirect immunofluorescence assays
Mouse monoclonal antibodies were used against spectrin, actin, and glycophorin A (Sigma, St Louis, MO); flotillin-1 and -2 (BD Transduction Laboratories, Lexington, KY); epidermal growth factor receptor (Ab-12; Lab Vision, Fremont, CA); N- and C-termini of band 3, Rh30, scramblase, and stomatin. Rabbit polyclonal antibodies were directed against aquaporin (Colton antigen, gift from Dr P. Agre15 ); β2-AR (AbCam, Cambridge, MA); Gαs (Santa Cruz Biotechnology, Santa Cruz, CA); glucose transporter 1 (Alpha Diagnostics, San Antonio, TX); ankyrin, band 3, glyceraldehyde-3-phosphate dehydrogenase, C-terminus of scramblase (gift from Dr P. Sims), CD47, Rh, RhAG, and XK (gift from Dr S. Lee). Secondary antibodies were fluorescein isothiocyanate (FITC)–, rhodamine-, or horseradish peroxidase (HRP)–conjugated goat antibodies (Cappel Organon Teknika, West Chester, PA). Western blots and indirect immunofluorescence assays were carried out as previously described3 using primary antibody dilutions of 1:100 to 1:500. Secondary antibodies were diluted according to manufacturer's instructions.
DRM purification
For all experiments, uninfected erythrocyte ghosts were prepared by hypotonic lysis16 and extracted on ice in 0.5%, 1.0%, or 2.0% Triton X-100 (Tx-100) in Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS: 25 mM Tris, 150 mM NaCl pH 7.4) containing Complete miniprotease inhibitor (Roche, Mannheim, Germany) and 1 mM ethylenediaminetetraacetic acid. After 30 minutes at 4°C, the extract was mixed with an equal volume of 80% sucrose, overlaid with 30% and 5% sucrose, and subjected to sucrose flotation step gradient ultracentrifugation, as described.3
For structural studies, microgram quantities of proteins were isolated using a modified version of our previous methods.3 Twenty-fold more packed erythrocytes were used as starting material and, except for proportionally adjusted volumes, the detergent extraction (1% Tx-100) and sucrose flotation gradient steps were unchanged. The sucrose gradient was ultracentrifuged 18 hours rather than the original 4 hours to ensure DRM isolation in larger volumes. Amounts of protein per fraction, refractive index, and protein composition as determined by Western blotting were the same in sucrose fractions obtained from analytic and preparative methods (data not shown). To purify protein from sucrose, sodium dodecyl sulfate (SDS) was added to the raft-containing fraction 2 material to a final concentration of 0.1%, and the sample was dialyzed using presoaked 12- to 14-kDa molecular weight cutoff Sprectra Por dialysis membrane (Baxter, Deerfield, IL) in 10 mM ammonium bicarbonate and 0.02% SDS for 20 hours at 4°C; dialysis was repeated 3 times. The sample was lyophilized and resuspended in water. An equal volume of 20% trichloroacetic acid (TCA) was added, and the sample was incubated on ice for 30 minutes. The precipitated sample was centrifuged at 20800 g (14 000 rpm) at 4°C for 15 minutes, cold acetone was added to the resulting pellet, and the sample was recentrifuged as before. The pellet was dried under nitrogen, resuspended in a small volume of SDS buffer, boiled, and separated by 12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE). Ten percent of the starting material was stained by silver (nonammoniacal), and the rest was stained with colloidal Coomassie overnight at room temperature and destained with acetic acid.
Matrix-assisted laser desorption/ionization mass spectroscopy
To compare extraction conditions, proteins were resolved by SDS-PAGE and stained by colloidal Coomassie. The bands were excised and subjected to mass spectroscopic analysis at the Protein and Nucleic Acid Facility, Medical College of Wisconsin (Milwaukee). In-gel tryptic digests were performed on the excised gel bands, the eluted peptides were desalted with Millipore C18 Zip-Tips (Billerica, MA), and they were analyzed on a Voyager DE PRO matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectrometer (Applied Biosystems, Foster City, CA) operated in reflector mode. A mass list of peptides was obtained for each protein digest. Database searches and protein identification were done with the Protein Prospector software (MS-FIT) and the National Center for Biotechnology Information (NCBI) nonredundant database.
Liquid chromatography MS/MS (LC-MS/MS)
For identification of raft proteins from large-scale preparations, structural analyses were performed on pooled raft proteins at the Wistar Institute (Philadelphia, PA). The protein band of interest was excised from the gel, destained using 200 mM ammonium bicarbonate in 50% acetonitrile for 30 minutes at 37°C, and dried. This was followed by reduction/alkylation of the protein band with 20 mM tris(2-carboxyethyl)phosphine in 25 mM ammonium bicarbonate (pH 8.0) for 15 minutes at 37°C. Supernatant was discarded, and 40 mM iodoacetamide in 25 mM ammonium bicarbonate (pH 8.0) was added to the band for 30 minutes at 37°C. The supernatant was again discarded, and 2 subsequent washes in 25 mM ammonium bicarbonate were performed for 15 minutes each. A final wash was done as described except with the addition of 50% acetonitrile. The band was dried completely and rehydrated with 20 μL of 0.02 μg/μL modified trypsin (Promega, Madison, WI) in 40 mM ammonium bicarbonate overnight with shaking at 37°C. After the overnight incubation, the supernatant was removed, a subsequent 20 μL extraction using 40 mM ammonium bicarbonate was performed, and the 2 extractions were combined and acidified by addition of 4 μL concentrated acetic acid.
Trypsin digests were injected onto a nanocapillary reverse-phase column (self-packed New Objective [Woburn, MA] 75-μm column terminating in a nanospray 15-μm tip) directly coupled to an LCQ Classic quadrupole ion trap mass spectrometer (ThermoFinnigan, San Jose, CA). Data were acquired using triple-play mode to obtain mass spectra (MS) and MS/MS data. The resulting masses and MS/MS data were searched against the nonredundant NCBI database using the TurboSEQUEST browser (ThermoFinnigan) to match MS/MS data to known protein sequences.17,18 Protein identifications were considered high-confidence assignments if the TurboSEQUEST browser report matched at least 3 tryptic peptides with a cross-correlation score of 2.5 or higher to the same protein. Identifications were considered tentative if only 1 to 2 peptides were matched with cross-correlation scores of 2.5 or higher. Where practical, tentative assignments were subsequently confirmed by Western blotting.
Protein and cholesterol estimations
Protein concentrations were estimated using detergent-compatible Bradford assay kits (Bio-Rad, Hercules, CA) or a bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). Cholesterol estimations were carried out using 2 different test kits. The Amplex Red Cholesterol assay kit (A-12216; Molecular Probes, Eugene, OR) was used as a sensitive fluorimetric assay to detect both free cholesterol and cholesteryl esters. A diagnostic cholesterol test kit (139050; Roche) was also used; this kit measures the conversion of cholesterol to cholestenone by cholesterol oxidase using a spectrophotometer.
Results
Isolation of erythrocyte DRM raft proteins: effects of detergent and protein concentration
Salzer and Prohaska reported that DRMs extracted in 1% Tx-100 or 0.5% Tx-100 contained stomatin and flotillin-1 and -2 as well as actin and spectrin (major components of the erythrocyte cytoskeleton) but lacked glycophorin C and band 312 (subsequently, however, minute quantities of band 3 and aquaporin-1 were identified in their preparations19 ). In contrast, Samuel et al extracted DRMs in 1% Tx-100 but at much lower concentrations of protein and found that they were depleted in glycophorin A, contained stomatin and flotillin (as well as Duffy glycoprotein, Gαs, and GPI-anchored CD59), but lacked spectrin and actin.3 To explore the basis for the observed differences, we determined the effects of changing detergent and/or protein concentration during the initial extraction on levels and composition of DRM-associated proteins.
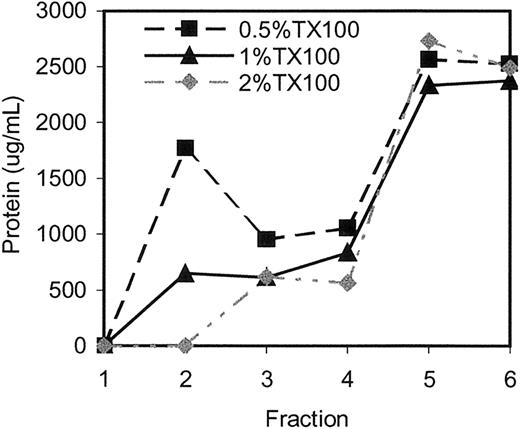
Effects of varying detergent concentration. Erythrocyte membranes were extracted at a high, fixed concentration of protein (7.5 mg/mL) using 0.5%, 1%, and 2% Tx-100. The extracts were subjected to sucrose gradient centrifugation to isolate and analyze DRMs (which float to fraction 2, the interface between 5% and 35% sucrose) as well as the remainder of the fractions (1 and 3-6; 6 being the loading zone). As shown in Figure 1, in 2% Tx-100, no protein was recovered in the DRM fraction, suggesting that the complexes cannot be isolated at elevated detergent concentrations. In contrast, DRMs isolated in 0.5% Triton contained high levels of protein, which reflected approximately 20% of the total protein in the extract. They also contained more than twice the absolute amount of DRM-associated protein obtained after 1% Tx-100 extraction (Figure 1).
Erythrocyte membranes extracted in 0.5% Tx-100 contain twice the amount of protein in DRM fraction 2 compared with membranes extracted in 1% Tx-100, whereas those extracted in 2% Tx-100 do not contain any detectable protein. Protein concentrations in fractions obtained from erythrocyte membranes by Tx-100 extraction and sucrose density gradient centrifugation were measured as described in “Materials and methods.”
Erythrocyte membranes extracted in 0.5% Tx-100 contain twice the amount of protein in DRM fraction 2 compared with membranes extracted in 1% Tx-100, whereas those extracted in 2% Tx-100 do not contain any detectable protein. Protein concentrations in fractions obtained from erythrocyte membranes by Tx-100 extraction and sucrose density gradient centrifugation were measured as described in “Materials and methods.”
To determine whether the increased protein content associated with DRMs prepared in 0.5% Tx-100 was due to unique, major protein species that were absent at 1% Tx-100, we examined the samples by SDS-PAGE. One-dimensional protein profiles showed complex mixtures of proteins in both samples but with relatively similar banding patterns (Figure S1; see the Supplemental Figures link at the top of the online article on the Blood website). This confirmed that increasing the detergent concentration from 0.5% to 1% Tx-100 in lysates of high-starting protein concentration did not lead to the loss of major protein constituents in isolated DRMs. MALDI-TOF analyses of proteins in DRMs isolated in 0.5% Tx-100 revealed the presence of flotillin-2 and stomatin and major erythrocyte membrane components (spectrin, ankyrin, band 3, protein 4.1) (Figure S2; see Supplemental Figures), reflecting the presence of a sizeable portion of erythrocyte components in these DRM preparations.
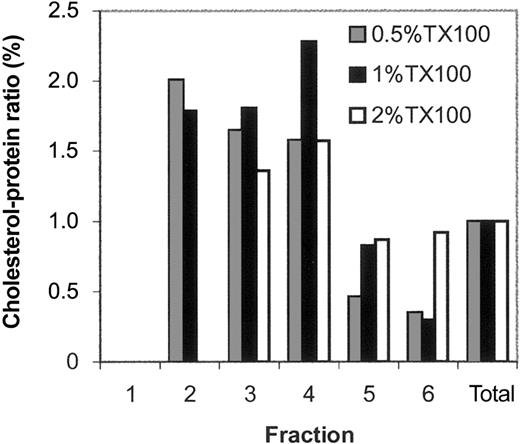
Because DRMs are cholesterol rich, we quantitated the cholesterol-to-protein ratio for each individual gradient fraction. This was done by dividing the amount of cholesterol in an individual fraction by the total cholesterol in fractions 1 to 6 and further dividing by the same calculation for relative protein content in each fraction (Figure 2). As shown, a 2-fold increase was seen in the cholesterol-to-protein ratio in DRMs isolated in 0.5% and 1% Tx-100 relative to the loading zone (and, as expected in 2% Tx-100, no cholesterol was detected in the fraction 2). Taken together, these findings suggest that DRMs prepared in either 0.5% or 1% Tx-100 contain complex mixtures of protein, have similar cholesterol-to-protein ratios, and may share major protein determinants. Further, because the higher DRM protein mass extracted in 0.5% Tx-100 showed the same cholesterol-to-protein ratio as a lower DRM protein mass extracted at 1% Tx-100, this suggests that membrane lipid interactions other than those mediated by cholesterol are refractory to disruption at 0.5% Tx-100.
The cholesterol-to-protein ratio of the DRM-containing fraction is high for samples extracted in 0.5% and 1% Tx-100. As expected, no cholesterol was detected in fraction 2 of samples extracted in 2% Tx-100. The cholesterol-to-protein ratio equals the percentage of cholesterol in a given fraction relative to the total cholesterol obtained for fractions 1 to 6 divided by the percentage of protein in a given fraction relative to the total protein obtained for fractions 1 to 6. A high cholesterol-to-protein ratio reflects enrichment in cholesterol or loss of protein relative to the membrane profile as a whole.
The cholesterol-to-protein ratio of the DRM-containing fraction is high for samples extracted in 0.5% and 1% Tx-100. As expected, no cholesterol was detected in fraction 2 of samples extracted in 2% Tx-100. The cholesterol-to-protein ratio equals the percentage of cholesterol in a given fraction relative to the total cholesterol obtained for fractions 1 to 6 divided by the percentage of protein in a given fraction relative to the total protein obtained for fractions 1 to 6. A high cholesterol-to-protein ratio reflects enrichment in cholesterol or loss of protein relative to the membrane profile as a whole.
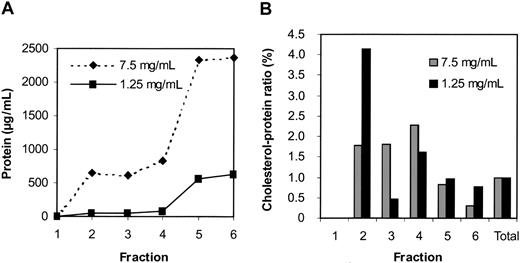
Effects of varying protein concentration. In another series of experiments, we compared DRMs prepared in 1% Tx-100 at protein concentrations of 7.5 mg/mL and 1.25 mg/mL. Extraction at the higher concentration resulted in substantially more protein detected in the DRM fraction (Figure 3A). However, DRMs extracted at the lower protein concentration of 1.25 mg/mL protein showed a 4-fold increase in the cholesterol-to-protein ratio, whereas this ratio was only 2-fold for DRMs extracted at 7.5 mg/mL protein (Figure 3B). As we have previously reported,3 Western blots confirmed that skeletal components were excluded from DRMs extracted at 1.25 mg protein per milliliter but were present in DRMs extracted at 7.5 mg protein per milliliter (data not shown). These data suggest that at 1% Tx-100 the initial protein concentration in the extract has a profound influence on the composition of proteins detected in the DRM fraction. At relatively high (but not low) protein concentrations, cytoskeletal proteins associate with erythrocyte DRMs. Proteins such as glycophorin A were not detected in DRMs prepared at either 7.5 or 1.25 mg/mL protein, while raft markers (flotillin-2, stomatin, Gαs) were found in DRMs prepared at either concentration (data not shown). Thus, some proteins were consistently excluded from DRMs while others were retained over a wide (5-fold) range of protein concentrations.
The cholesterol-to-protein ratio in DRM and non-DRM fractions is sensitive to a 6-fold difference in starting protein levels. DRMs were cholesterol rich and protein poor when prepared from a low starting protein concentration. Membranes were extracted using 1% Tx-100 from material containing either a high (7.5 mg/mL protein) or low (1.25 mg/mL protein) starting protein concentration and subjected to sucrose gradient centrifugation as described. For each fraction, the level of protein (A) and the cholesterol-to-protein ratio (B) were measured and calculated as described for Figures 1 and 2.
The cholesterol-to-protein ratio in DRM and non-DRM fractions is sensitive to a 6-fold difference in starting protein levels. DRMs were cholesterol rich and protein poor when prepared from a low starting protein concentration. Membranes were extracted using 1% Tx-100 from material containing either a high (7.5 mg/mL protein) or low (1.25 mg/mL protein) starting protein concentration and subjected to sucrose gradient centrifugation as described. For each fraction, the level of protein (A) and the cholesterol-to-protein ratio (B) were measured and calculated as described for Figures 1 and 2.
Our results in Figure 3B also suggest that reducing the protein concentration in the initial extraction preserves cholesterol association with DRMs to a much greater degree than protein association with DRMs, resulting in DRMs that are enriched in cholesterol even though they are protein poor. Earlier studies have shown that depletion of about 23% of erythrocyte membrane cholesterol abolishes isolation of these protein-poor DRM complexes (with little effect on the protein-to-cholesterol ratio in non-DRM fractions of the sucrose gradient).3 These cholesterol-rich DRM complexes, whose formation is also dependent on cholesterol, were of particular interest because the presence of cholesterol has been shown to be critical to raft formation in biologic membranes. Hence, they were further characterized by structural methods and with antibodies to erythrocyte proteins.
Identification of major erythrocyte raft proteins by structural and antibody analyses
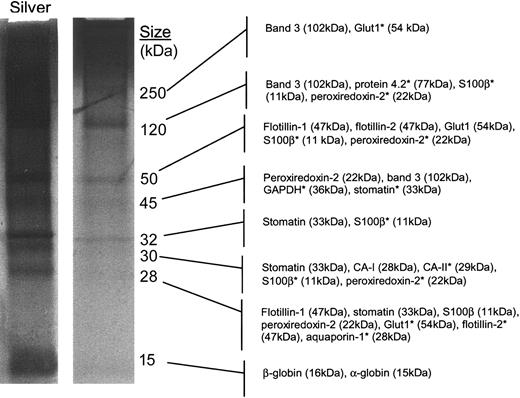
Structural studies. We initiated our structural studies by purifying large quantities of DRM proteins using a scaled-up isolation procedure. Compared with previous methods,1,3 the ultracentrifugation step was extended from 4 to 18 hours, and dialysis was added to maximize yield and remove sucrose from the DRM fraction. The resulting DRM fraction contained 4 readily detectable bands in the colloidal Coomassie-stained lane (90% of purified material) and an additional 3 bands in the silver-stained lane (remaining 10% of purified material; Figure 4); the protein banding pattern was similar to that seen with previous silver-stained gels published by Samuel and colleagues.3 Using albumin standards and SDS-PAGE, we estimate that about 1 to 5 μg DRM protein was obtained per 1 × 1010 erythrocytes.
Major erythrocyte raft components include band 3, the flotillins, and stomatin. Detergent-resistant membrane fractions were prepared from normal human erythrocytes. Membranes were purified, extracted in 1% cold Tx-100, and subjected to sucrose density gradient centrifugation for 18 hours as described in “Materials and methods.” The raft-containing fraction was dialyzed, dried, TCA precipitated, dried, and resuspended. The purified fraction was separated by reducing SDS-PAGE and stained with collodial Coomassie or silver. Structural analyses were performed on excised Coomassie-stained protein bands using a nanocapillary reverse-phase column coupled to a ThermoFinnigan LCQ Classic quadrupole ion trap mass spectrometer. Resulting masses and MS/MS data were searched against the nonredundant NCBI database to make the listed protein predictions. *Tentative predictions.
Major erythrocyte raft components include band 3, the flotillins, and stomatin. Detergent-resistant membrane fractions were prepared from normal human erythrocytes. Membranes were purified, extracted in 1% cold Tx-100, and subjected to sucrose density gradient centrifugation for 18 hours as described in “Materials and methods.” The raft-containing fraction was dialyzed, dried, TCA precipitated, dried, and resuspended. The purified fraction was separated by reducing SDS-PAGE and stained with collodial Coomassie or silver. Structural analyses were performed on excised Coomassie-stained protein bands using a nanocapillary reverse-phase column coupled to a ThermoFinnigan LCQ Classic quadrupole ion trap mass spectrometer. Resulting masses and MS/MS data were searched against the nonredundant NCBI database to make the listed protein predictions. *Tentative predictions.
Structural studies were initially performed on 7 Coomassie-stained protein bands (Figure 4). Silver staining of an identical fraction confirmed that these indeed represented the major protein bands in the gel. As shown in Table 1, most of the major proteins that were detected in this study were actually observed at multiple positions in the gel in addition to the band that corresponded to the expected protein molecular weight. These multiple identifications of proteins are most likely due to the detection by the highly sensitive liquid chromatography MS/MS (LC-MS/MS) method used in this study of minor amounts of proteolytic fragments and cross-linked homo- and possibly hetero-oligomers. This phenomenon is also typically observed when using this method to analyze other types of samples (data not shown). Alternatively, the proteins detected at anomalous molecular weights could have been caused by altered SDS binding caused by the low protein, high cholesterol, and high sucrose content of these DRM preparations.
Erythrocyte raft proteins identified by LC-MS/MS
Gel band . | Protein . | No. of peptides . |
|---|---|---|
| High-confidence assignments* | ||
| 250 | Band 3 | 14 |
| 120 | Band 3 | 21 |
| 50 | Flotillin-1 | 16 |
| 50 | Flotillin-2 | 11 |
| 50 | Glucose transporter 1 | 3 |
| 45 | Peroxiredoxin-2 | 7 |
| 45 | Band 3 | 6 |
| 32 | Stomatin | 13 |
| 30 | Stomatin | 5 |
| 30 | Carbonic anhydrase I | 4 |
| 28 | Flotillin-1 | 4 |
| 28 | Stomatin | 4 |
| 28 | S100β | 3 |
| 28 | Peroxiredoxin-2 | 3 |
| 15 | α-globin | 6 |
| 15 | β-globin | 14 |
| Tentative assignments* | ||
| 250 | Glucose transporter 1 | 2 |
| 120 | Protein 4.2 (pallidin) | 1 |
| 120 | S100β | 1 |
| 120 | Peroxiredoxin-2 | 1 |
| 50 | S100β | 1 |
| 50 | Peroxiredoxin-2 | 1 |
| 45 | GAPDH | 2 |
| 45 | Stomatin | 1 |
| 32 | S100β | 2 |
| 30 | Carbonic anhydrase II | 2 |
| 30 | S100β | 1 |
| 30 | Peroxiredoxin-2 | 1 |
| 28 | Glucose transporter 1 | 2 |
| 28 | Flotillin-2 | 2 |
| 28 | Aquaporin-1 | 1 |
Gel band . | Protein . | No. of peptides . |
|---|---|---|
| High-confidence assignments* | ||
| 250 | Band 3 | 14 |
| 120 | Band 3 | 21 |
| 50 | Flotillin-1 | 16 |
| 50 | Flotillin-2 | 11 |
| 50 | Glucose transporter 1 | 3 |
| 45 | Peroxiredoxin-2 | 7 |
| 45 | Band 3 | 6 |
| 32 | Stomatin | 13 |
| 30 | Stomatin | 5 |
| 30 | Carbonic anhydrase I | 4 |
| 28 | Flotillin-1 | 4 |
| 28 | Stomatin | 4 |
| 28 | S100β | 3 |
| 28 | Peroxiredoxin-2 | 3 |
| 15 | α-globin | 6 |
| 15 | β-globin | 14 |
| Tentative assignments* | ||
| 250 | Glucose transporter 1 | 2 |
| 120 | Protein 4.2 (pallidin) | 1 |
| 120 | S100β | 1 |
| 120 | Peroxiredoxin-2 | 1 |
| 50 | S100β | 1 |
| 50 | Peroxiredoxin-2 | 1 |
| 45 | GAPDH | 2 |
| 45 | Stomatin | 1 |
| 32 | S100β | 2 |
| 30 | Carbonic anhydrase II | 2 |
| 30 | S100β | 1 |
| 30 | Peroxiredoxin-2 | 1 |
| 28 | Glucose transporter 1 | 2 |
| 28 | Flotillin-2 | 2 |
| 28 | Aquaporin-1 | 1 |
In addition to the reported protein identifications, β-globin and sometimes α-globin were detected in all bands analyzed. For simplicity, these results were omitted from the table.
Protein identifications were considered high-confidence assignments if the TurboSEQUEST browser report matched 3 or more tryptic peptides with a cross-correlation score of 2.5 or higher. Identifications were considered tentative if only 1 or 2 peptides were matched with cross-correlation scores of 2.5 or higher. The number of peptides meeting these criteria is shown.
The DRM sample contained a relatively simple mixture of 10 confidently and 4 tentatively identified proteins. Although mass spectrometry is not a quantitative method unless stable isotope-labeled internal standards are used, the peptide patterns obtained strongly suggested that the major protein in the 250- and 120-kDa bands was band 3 dimer and monomer, respectively. Band 3 is the most abundant protein in the erythrocyte plasma membrane, present at about 1 × 106 copies per cell (reviewed by Poole20 ). Its presence in DRMs raised a question of whether band 3 was merely a contaminant from other fractions. However, glycophorin A, a protein of almost comparable abundance to band 3, is not detected in DRMs,1 supporting the idea that band 3 is not a contaminant in DRM fraction 2. Judging from the intensity of protein bands in fraction 2 shown in Figure 4 and the LC-MS/MS results, band 3 appears to be one of the major resident proteins of erythrocyte DRMs. Likewise, flotillin-1 and -2 are most probably the major components in the 50-kDa band; peroxiredoxin and band 3 proteolytic fragments are the major components in the 45-kDa band; stomatin is the major component in the 32-kDa band; and globins are the major components in the 15-kDa band. The 30- and 28-kDa bands were much more complex mixtures. By comparing the staining intensities and MS/MS peptide yields, the most abundant proteins in this fraction are probably band 3, flotillin-1 and -2, peroxiredoxin-2, stomatin, and hemoglobin. S100β may also be a major protein because it was observed in multiple positions on the gel, and usually the detection of multiple oligomers of a protein is only observed if that protein is fairly abundant in the sample. Previously, S100β and peroxiredoxin-2 had not been identified in erythrocyte lipid rafts. S100β, a neurotrophic signaling molecule, is part of a complex that regulates calcium homeostasis and influences cytoskeletal morphology.21 Peroxiredoxin-2, the second most abundant cytoplasmic erythrocyte protein,22 reduces peroxides and prevents membrane lipid peroxidation.23
In addition to the 6 major proteins described above, glucose transporter (Glut1), carbonic anhydrase I (CA-I), and several hemoglobin monomers were also confidently identified in the DRM sample. Protein 4.2, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), carbonic anhydrase II (CA-II), and aquaporin (AQP1) were identified with less confidence. The glucose transporter 1 (Glut1) is a major integral erythrocyte protein capable of importing, sequestering, or exporting sugars.24,25 We were not surprised to find carbonic anhydrase I, because other members of the carbonic anhydrase family bind to band 3.26 Hemoglobin, particularly the β-globin subunit, was found in all excised bands, most prominently at 15 kDa as expected. The detection of hemoglobin at many positions in 1-dimensional SDS gels has also been observed using this LC-MS/MS method on proteome studies of other types of blood-derived samples (D.W.S. et al, manuscript in preparation). The presence of globins in band 3–containing bands was not surprising, because up to 4 hemoglobin tetramers can bind to the band 3 tetramer.27
In summary, these data show that 5 major protein bands besides hemoglobin represent the major protein components of erythrocyte DRMs. Importantly, the overall erythrocyte DRM protein profile (at least 14 proteins) appears less complex than that reported for neutrophil DRMs (23 proteins)28 and does not contain actin, spectrin, or band 4.1, which were all reported in erythrocyte DRMs prepared in 0.5% Tx-100.12 However, similar to these previous studies,12,28 major raft markers flotillin-1 and -2 and stomatin were indeed present in our DRMs. Proteins of quantitative importance in erythrocyte DRMs may also prove important in malarial infection.
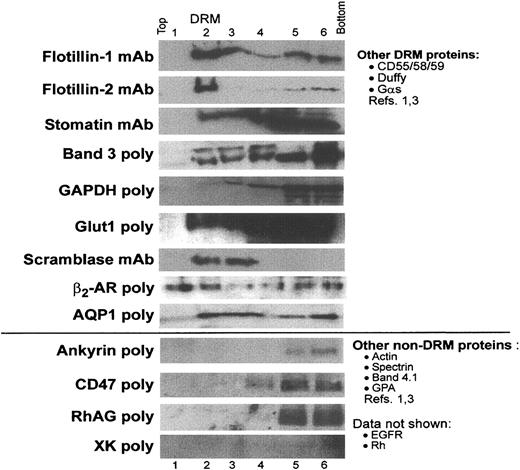
Western blots. Because many biologically important proteins may not be abundant enough to be detected by our structural approaches and to corroborate LC-MS/MS studies, DRM and non-DRM fractions isolated from sucrose gradients were examined for the distribution of a range of known erythrocyte proteins using SDS-PAGE and Western blots. As expected, DRM proteins flotillin-1 and -2, stomatin, and band 3 were detected in the DRM-containing fraction by immunoblotting (Figure 5). Flotillin-1 and especially flotillin-2 appear to float in a quantitative manner, suggesting that these proteins are uniquely suited for the cholesterol-rich microenvironment of DRMs. In contrast, only a small percentage of stomatin partitions into DRMs; this result contrasts with findings by Salzer and Prohaska, which showed that stomatin was strongly DRM associated in erythrocytes.12 A small portion of band 3 was DRM associated and, because band 3 is a major erythrocyte component, it is likely that DRM-associated band 3 is present at levels at least comparable to flotillin-1 and -2. The copy numbers of the flotillins and stomatin in erythrocytes are unknown. In addition, less abundant DRM proteins Glut1 and GAPDH identified in Figure 4 and Table 1 were also detected in DRM fraction 2 in Western blots, although, here again, most of the total Glut1 and GAPDH protein was present in the non-DRM fraction. These data confirm that both major and minor DRM proteins detected by structural methods could also be detected by a second, independent method of immunblotting.
Major and minor erythrocyte membrane proteins partition into DRM rafts to varying degrees. Uninfected erythrocyte membranes were extracted in 1% Tx-100 and subjected to sucrose density gradient centrifugation as described in “Materials and methods.” Fractions 1 to 6 were acetone precipitated and separated by SDS-PAGE. Blots were probed with appropriate primary and horseradish peroxidase–conjugated secondary antibodies and imaged as described. Results are shown for selected antibodies, oriented with the lightest, low-sucrose fraction on the left and the heaviest, sucrose-rich loading zone on the right. Fraction 2 contains DRMs. Adobe Photoshop (Adobe, San Jose, CA) was used to adjust brightness and contrast. EGFR indicates epidermal growth factor receptor; GPA, glycophorin A.
Major and minor erythrocyte membrane proteins partition into DRM rafts to varying degrees. Uninfected erythrocyte membranes were extracted in 1% Tx-100 and subjected to sucrose density gradient centrifugation as described in “Materials and methods.” Fractions 1 to 6 were acetone precipitated and separated by SDS-PAGE. Blots were probed with appropriate primary and horseradish peroxidase–conjugated secondary antibodies and imaged as described. Results are shown for selected antibodies, oriented with the lightest, low-sucrose fraction on the left and the heaviest, sucrose-rich loading zone on the right. Fraction 2 contains DRMs. Adobe Photoshop (Adobe, San Jose, CA) was used to adjust brightness and contrast. EGFR indicates epidermal growth factor receptor; GPA, glycophorin A.
In addition, 3 proteins not identified by structural methods were detected in our DRM samples by Western blotting. As shown in Figure 5, scramblase, the β2-AR, and the water channel AQP1 were enriched in DRMs. Interestingly, quantitative amounts of these minor components are detected in DRMs. In this regard, they behave more like the major DRM-associated flotillin-1 and -2 rather than stomatin or band 3 (Figures 4 and 5). Thus, both major and minor DRM components may float in a quantitative manner. Finally, the erythrocyte proteins ankyrin, CD47, RhAG, and XK were not detected in DRM fractions (Figure 5), although CD47 can be detected in DRM rafts in other cells.29 These immunoblotting experiments add several proteins of potential functional importance to the list of proteins (Table 2) assessed for internalization to the malarial vacuole.
Summary of erythrocyte lipid raft proteins
Protein . | Molecular weight, kDa . | Membrane association* . | Remarks . | Internalized to the PVM? . |
|---|---|---|---|---|
| α-globin | 15 | Cytoplasmic | Hemoglobin complex | N/D |
| β-globin | 16 | Cytoplasmic | Hemoglobin complex | N/D |
| GAPDH | 36 | Cytoplasmic | Conversion of G3P to 1,3-BPG | Not internalized |
| Peroxiredoxin-2 | 21.9 | Cytoplasmic | Eliminates peroxides; signaling? | N/D |
| S-100β | 10.5 | Cytoplasmic | Dimer with α chain; binds p53, tubulin, and Ca2+; (dis)assembly of microtubules and intermediate filaments | N/D |
| CA-I | 28.8 | Cytoplasmic | Reversible hydration of CO2 | N/D |
| Flotillin-1 | 47 | Endofacing hairpin loop | Organization of caveolae and/or lipid rafts | Internalized |
| Flotillin-2 | 45 | Endofacing hairpin loop | High-order flotillin oligomers; raft scaffolding component | Internalized |
| Stomatin | 31 | Endofacing hairpin loop | Associates with Glut1; cation transport | Not internalized |
| Gαs | 44 | Endofacing | GPCR activation of adenylate cyclase | Internalized |
| CD55 | 55-70 | GPI-linked | Decay accelerating factor | Internalized |
| CD58 | 64-73 | GPI-linked | Unknown function in erythrocytes | Internalized |
| CD59 | 20-40 | GPI-linked | Membrane inhibitor of reactive complement lysis | Internalized |
| Glut1 | 54 | Multipass (12) | May bind stomatin; passive glucose transport | Not internalized |
| Band 3 | 101 | Multipass (14) | Binds protein 4.2 and ankyrin; “Cl shift” | Not internalized |
| AQP1 | 28 | Multipass (6) | Water channel protein for erythrocytes and renal PCT | Internalized |
| β2-AR | 65 | Multipass (7) | Gαs-coupled receptor | Internalized |
| Duffy | 35-43 | Multipass (7) | Chemokine and P vivax receptor; GPCR-like | Internalized |
| Scramblase | 35 | Single-pass | Movement of membrane phospholipids | Internalized |
Protein . | Molecular weight, kDa . | Membrane association* . | Remarks . | Internalized to the PVM? . |
|---|---|---|---|---|
| α-globin | 15 | Cytoplasmic | Hemoglobin complex | N/D |
| β-globin | 16 | Cytoplasmic | Hemoglobin complex | N/D |
| GAPDH | 36 | Cytoplasmic | Conversion of G3P to 1,3-BPG | Not internalized |
| Peroxiredoxin-2 | 21.9 | Cytoplasmic | Eliminates peroxides; signaling? | N/D |
| S-100β | 10.5 | Cytoplasmic | Dimer with α chain; binds p53, tubulin, and Ca2+; (dis)assembly of microtubules and intermediate filaments | N/D |
| CA-I | 28.8 | Cytoplasmic | Reversible hydration of CO2 | N/D |
| Flotillin-1 | 47 | Endofacing hairpin loop | Organization of caveolae and/or lipid rafts | Internalized |
| Flotillin-2 | 45 | Endofacing hairpin loop | High-order flotillin oligomers; raft scaffolding component | Internalized |
| Stomatin | 31 | Endofacing hairpin loop | Associates with Glut1; cation transport | Not internalized |
| Gαs | 44 | Endofacing | GPCR activation of adenylate cyclase | Internalized |
| CD55 | 55-70 | GPI-linked | Decay accelerating factor | Internalized |
| CD58 | 64-73 | GPI-linked | Unknown function in erythrocytes | Internalized |
| CD59 | 20-40 | GPI-linked | Membrane inhibitor of reactive complement lysis | Internalized |
| Glut1 | 54 | Multipass (12) | May bind stomatin; passive glucose transport | Not internalized |
| Band 3 | 101 | Multipass (14) | Binds protein 4.2 and ankyrin; “Cl shift” | Not internalized |
| AQP1 | 28 | Multipass (6) | Water channel protein for erythrocytes and renal PCT | Internalized |
| β2-AR | 65 | Multipass (7) | Gαs-coupled receptor | Internalized |
| Duffy | 35-43 | Multipass (7) | Chemokine and P vivax receptor; GPCR-like | Internalized |
| Scramblase | 35 | Single-pass | Movement of membrane phospholipids | Internalized |
Nineteen proteins were reliably identified in the floating fraction of Tx-100-resistant erythrocyte membranes in this and other studies,1,3,12 but only a subset of these proteins enters the malarial vacuole.
N/D indicates no data; G3P, glyceraldehyde-3-phosphate; 1,3-BPG, 1-3,bisphosphoglycerate; GPCR, G protein—coupled receptor; PCT, proximal convoluted tubule.
Numbers in parentheses represent the number of transmembrane domains in multipass proteins.
Movement of DRM raft and non-DRM raft markers in P falciparum–infected erythrocytes
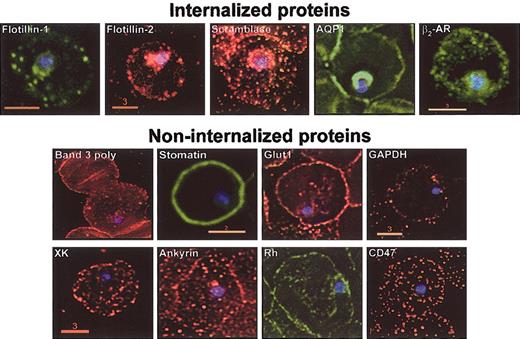
To date, DRM raft-associated proteins are the only host proteins known to enter the malarial vacuole.1-3 Therefore, we investigated whether both major and minor host DRM proteins are internalized and the distribution of host non-DRM proteins in infected erythrocytes. Erythrocytes infected with P falciparum were subjected to indirect immunofluorescence assays (Figure 6). As previously reported,2,3 major raft proteins flotillin-1 and -2 were internalized to the plasmodial vacuole. However, we failed to detect vacuolar uptake of major proteins band 3 or stomatin (although low levels of stomatin may be seen in association with the earliest stages of infection). Additional minor transmembrane proteins internalized into the malarial vacuole include the β2-AR, AQP1, and scramblase. All are DRM proteins; however, the property of detergent resistance alone is not sufficient for extensive vacuolar entry, because not all major (band 3, stomatin) or minor (Glut1, GAPDH) DRM proteins are internalized into infected erythrocytes (Figure 6). Finally, nonraft proteins fail to be quantitatively recruited to the vacuole (ankyrin, Rh, CD47, XK in Figure 6) compared with multiple raft counterparts.
DRM association is necessary but not sufficient to target host proteins to the malarial vacuolar membrane. Normal human erythrocytes were infected with asexual stage P falciparum. Infected cultures were sampled and prepared for indirect immunofluorescence assays. Cells were adhered to 0.1% poly-l-lysine–coated glass coverslips, fixed, washed, and permeabilized before treatments with appropriate primary and fluorescein- or rhodamine-conjugated secondary antibodies. Hoechst stain was used to visualize parasite nuclei. Fluorescence microscopy images were obtained using DeltaVision deconvolution microscopy (Applied Precision, Seattle, WA) as previously described.1 Images are single optical sections of cells infected with ring-stage parasites. Scale bars present size (in μm). All images were obtained with a 100 × objective.
DRM association is necessary but not sufficient to target host proteins to the malarial vacuolar membrane. Normal human erythrocytes were infected with asexual stage P falciparum. Infected cultures were sampled and prepared for indirect immunofluorescence assays. Cells were adhered to 0.1% poly-l-lysine–coated glass coverslips, fixed, washed, and permeabilized before treatments with appropriate primary and fluorescein- or rhodamine-conjugated secondary antibodies. Hoechst stain was used to visualize parasite nuclei. Fluorescence microscopy images were obtained using DeltaVision deconvolution microscopy (Applied Precision, Seattle, WA) as previously described.1 Images are single optical sections of cells infected with ring-stage parasites. Scale bars present size (in μm). All images were obtained with a 100 × objective.
A compilation of the results from this and previous studies (from our laboratory1,3 and others12 ) that identifies 19 host DRM proteins (of which 10 of 14 tested proteins are internalized during P falciparum infection) is shown in Table 2. Thus, DRM association is necessary but not sufficient for vacuolar recruitment. Of the internalized proteins, 3 are GPI anchored, 3 have multipass membrane-spanning domains, 1 has a single-pass transmembrane domain, and 2 are integrally associated but cytoplasmic-facing hairpin loop proteins. This uptake of multiple DRM raft proteins with different types of membrane anchors has implications for mechanisms of host protein trafficking and the function of internalized host proteins in the P falciparum–infected erythrocyte.
Discussion
In this study, we have utilized the erythrocyte membrane as a model to characterize plasma membrane DRMs. Our results suggest that protein association with DRMs is indeed sensitive to protein concentrations of the initial extracts. Extraction at low protein concentrations produces complexes with the highest cholesterol content. Because the latter is a hallmark for plasma membrane rafts, these complexes may be specifically enriched in raft proteins.
In previous studies, we estimated that DRMs constitute as much as 3% to 4% of the total erythrocyte membrane protein, based on Bradford assays.1 However, estimates based on colloidal Coomassie staining of proteins recovered in DRM fractions suggest that less than 0.1% of the total erythrocyte membrane protein is isolated in cholesterol-rich, protein-poor raft complexes. Together, these results suggest that cholesterolrich DRMs isolated from the erythrocyte plasma membrane represent a minor fraction of erythrocyte membrane proteins. Further, they are relatively simple with 5 major constituents but show a higher level of complexity of their minor resident proteins. Importantly, these DRMs lack cytoskeletal components (implying that rafts are not anchored to the membrane skeleton) but contain integral proteins like band 3. Thus, DRMs may contain a portion of band 3 that has increased lateral mobility by virtue of being spectrin- and/or ankyrin-free.30,31
Despite the controversy about the association of band 3 with erythrocyte DRMs,3,12,19 we find that band 3 is indeed associated with DRMs and provides a major component of DRM rafts even under the most stringent conditions of erythrocyte DRM extraction reported in the literature. Previous studies have reported that cholesterol can be tightly associated with band 3,32 and this may promote association with DRMs. Whether band 3 also influences raft structure has yet to be determined. Association with band 3 may account for DRM association of globins, GAPDH, protein 4.2, and possibly also CA-I.26 However, proteins such as Rh, RhAG, and CD47 that also complex with band 3 26 do not associate with DRMs. This may be due to their preferred association with band 3 anchored to the cytoskeleton. On the basis of these data and published models of the band 3 complex,26 we would further expect that untested proteins p55, LW, and glycophorin B would be found in non-DRM complexes.
An understanding of the relationship between internalization of host raft proteins and their subsequent function in the malaria-infected cell is slowly emerging from a variety of studies in our laboratory and others. Minor components such as the β2-AR and the heterotrimeric protein Gαs regulate entry of P falciparum into erythrocytes.4 The heptahelical Duffy receptor (the receptor for a second species of human malaria, Plasmodium vivax, which is not G protein coupled) is also internalized in infected cells.1 The internalized water channel AQP1 is vital for diffusional and osmotic water permeability in normal erythrocytes33 and, in theory, could play a role in homeostasis of the malarial vacuole as well. Internalization of the GPI-anchored proteins CD55 and CD59, which are the major inhibitors of membrane complement, may influence lysis of infected erythrocytes upon their depletion from the plasma membrane. Salzer and Prohaska have suggested that flotillin oligomers may organize raft components from the cytoplasmic side of the erythrocyte plasma membrane.12 Hence, the internalization of flotillin could be important for the formation of the raft-enriched malarial vacuolar membrane.
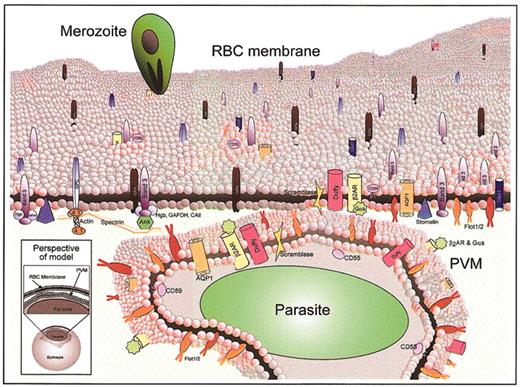
The internalization of multiple erythrocyte DRM proteins with transmembrane as well as GPI anchors strongly suggests that, contrary to the prevailing model of apicomplexan vacuole formation developed in T gondii, a peptidic anchor per se does not exclude host proteins from the apicomplexan, P falciparum vacuole. Rather, we propose a model whereby vacuolar formation involves interactions among a select group of host DRM raft proteins, possibly through a yet-undefined active pathogenic mechanism (Figure 7). In this model, the uninfected erythrocyte membrane possesses a dynamic mix of cholesterol-rich microdomains; some proteins partition wholly into these raft microdomains (ie, flotillin-2 and others), while others are only partial or temporary residents in rafts (ie, band 3, GAPDH, and others). Upon malarial invasion, the vacuolar membrane becomes highly cholesterol-enriched and a subset of the major and minor raft proteins enters the PVM while other proteins are excluded. Because subsets of both major and minor proteins are internalized, the model suggests heterogeneity in the interaction of erythrocyte plasma membrane rafts with the forming parasitic vacuole.
Model of erythrocyte DRM rafts and their enrichment in the malarial vacuolar membrane. The uninfected erythrocyte membrane contains a dynamic milieu of generalized lipid domains (gray spheres) and raft microdomains (pink spheres) containing various proteins. Some proteins partition heavily into raft domains (ie, flotillins), while others are only minimally present in rafts (ie, band 3, Glut1). During malaria infection, merozoite-stage parasites invade erythrocytes to reside in a membrane-bound parasitophorous vacuole. The PVM becomes selectively cholesterol-enriched, and 10 of the known raft proteins are internalized to the PVM (flotillin-1 and -2, Gαs, β2-AR, AQP1, Duffy, CD55, CD58, CD59, scramblase). Most of the abundant erythrocyte membrane proteins are not internalized to the PVM (ie, glycophorins A and C, cytoskeleton-associated band 3, and others). The lower left inset shows the perspective of the model, which depicts a whole infected erythrocyte and a magnified view through the plasma membrane and PVM of a malaria-infected erythrocyte. Because the PVM is formed by invagination of the plasma membrane, proteins that are cytoplasmically oriented in uninfected cells remain so upon infection; protein structures exposed to the extracellular space face the vacuolar space upon infection. 4.1 indicates band 4.1.
Model of erythrocyte DRM rafts and their enrichment in the malarial vacuolar membrane. The uninfected erythrocyte membrane contains a dynamic milieu of generalized lipid domains (gray spheres) and raft microdomains (pink spheres) containing various proteins. Some proteins partition heavily into raft domains (ie, flotillins), while others are only minimally present in rafts (ie, band 3, Glut1). During malaria infection, merozoite-stage parasites invade erythrocytes to reside in a membrane-bound parasitophorous vacuole. The PVM becomes selectively cholesterol-enriched, and 10 of the known raft proteins are internalized to the PVM (flotillin-1 and -2, Gαs, β2-AR, AQP1, Duffy, CD55, CD58, CD59, scramblase). Most of the abundant erythrocyte membrane proteins are not internalized to the PVM (ie, glycophorins A and C, cytoskeleton-associated band 3, and others). The lower left inset shows the perspective of the model, which depicts a whole infected erythrocyte and a magnified view through the plasma membrane and PVM of a malaria-infected erythrocyte. Because the PVM is formed by invagination of the plasma membrane, proteins that are cytoplasmically oriented in uninfected cells remain so upon infection; protein structures exposed to the extracellular space face the vacuolar space upon infection. 4.1 indicates band 4.1.
Interestingly, internalized proteins are also those that quantitatively float to fraction 2 in DRM assays, suggesting that the extent of flotation may provide a measure of endovacuolar protein mobility in this model host cell. One explanation may be that distinct raft populations exist, each with a different propensity toward detergent resistance. In fact, previous studies proposed that stomatin and the flotillins reside in distinct rafts, because stomatin was released into exovesicles induced by high cytosolic calcium conditions but the flotillins were not.34 A third class of calcium-dependent rafts was also recently described and found to contain the proteins synexin and sorcin.34 Our studies found differential internalization of DRM proteins and thus provide further support for the existence of multiple raft populations. In theory, the sorting of host raft proteins into the nascent vacuole could occur on the basis of individual protein parameters or by sorting of flotillin- or stomatin-type rafts described by Salzer and Prohaska.12 Further, the contribution of parasite ligands to PVM formation should not be overlooked. In P falciparum–infected cells, selective recruitment of host raft components may be induced by proteinaceous parasite ligands resident in the invasion-associated apical organelles. Recent studies suggest that a plasmodial ortholog of stomatin (Pfstomatin)35 may provide a leading candidate to investigate the molecular basis of parasite and host cell raft interactions during parasite entry.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-09-3165.
Supported by grants from the National Institutes of Health (NIH) (AI39071 [K.H.], DK32094 [N.M.], HL38794 [D.W.S.], HL54459 [M.E.R.], GM24417 [P.S.L.]), Austrian Science Fund (PI5486 [R.P.]), and American Heart Association (0315210Z [S.C.M.]).
S.C.M. and B.U.S. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs Peter Sims (The Scripps Research Institute, La Jolla, CA), Soohee Lee (New York Blood Center, New York, NY), and Peter Agre (Johns Hopkins University, Baltimore, MD) for their donations of antibodies. We also thank Thomas Beer (Wistar Institute) for his efforts on the LC-MS/MS studies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal