Abstract
Primary cutaneous lymphomas constitute a spectrum of diseases characterized by a clonal accumulation of lymphocytes in the skin. Cutaneous T-cell lymphomas of the cytotoxic phenotype, including CD8+ and CD56+ lymphomas, are rare entities that have only been recently recognized and characterized. These lymphomas often show an aggressive clinical course. We investigated the expression of human leukocyte antigen G (HLA-G) and interleukin 10 (IL-10) in conjunction with expression of HLA-G killer-cell inhibitory receptor ligand immunoglobulin-like transcript 2 (ILT2) in 3 CD56+CD4+ and 4 CD8+ cutaneous T-cell lymphomas. HLA-G expression was detected in 2 of 3 lymphomas of the CD56+CD4+ type and in all lymphomas of CD8+ type. It is of note that CD56+CD4+ lymphomas displayed stronger HLA-G reactivity. The expression of IL-10 matched the expression of HLA-G. Together with the expression of IL-10, HLA-G might be one of the factors accounting for the evasion of immunosurveillance, thus contributing to aggressive phenotype of these lymphoma entities.
Introduction
Primary cutaneous lymphomas comprise a spectrum of heterogeneous diseases characterized by clonal accumulation of lymphocytes initially restricted to the skin.1 Clonal T-cell populations of primary cutaneous T-cell lymphomas transcribe and secrete T-helper 2 (Th2) cytokines such as interleukin 10 (IL-10).2 Despite evidence of humoral and cellular antitumor immune response,3-5 cutaneous T-cell lymphomas (CTCLs) eventually progress to systemic disease after a long history of quietness.
Human leukocyte antigen G (HLA-G) represents a nonclassical HLA class Ib molecule whose expression is restricted to immunoprivileged sites, such as the placenta.6,7 HLA-G negatively affects almost every aspect of human immunity, inhibiting allogeneic T-cell and natural killer (NK)–cell cytotoxicity as well as T-cell proliferative response.6,7 Ectopic expression of HLA-G in cancer is thought to enable tumor cells to escape host immunosurveillance.8 Being expressed in advanced stage of the disease, HLA-G and IL-10 are molecules implicated in the progression of CTCL.2,9 One group of primary CTCLs is characterized by initially aggressive clinical behavior and poor outcome. Cytotoxic lymphomas of CD8+ or natural killer (NK)/T-cell phenotype represent rare entities that have only recently been recognized and characterized.10,11 We have recently shown that cutaneous cytotoxic CD8+ and CD56+CD4+ lymphomas express HLA-G ligand, immunoglobulin-like transcript 2 (ILT2).12 We conducted a follow-up study investigating expression of HLA-G and IL-10 in these cytotoxic CD8+ and CD56+CD4+ lymphomas with respect to their killer cell inhibitory receptor (KIR) phenotype.
Study design
Patients
The following criteria were used to select patients from Lymphoma Registry of the Department of Dermatology in Zurich: diagnosis of lymphoproliferative disorder with cytotoxic phenotype with initial presentation in the skin; polymerase chain reaction evidence of T-cell receptor (TCR) rearrangement in biopsied lesion; no iatrogenic or HIV-induced immunosuppression. Patients' characteristics are presented in Table 1.
Expression of ILT2, HLA-G, and IL-10 in primary cutaneous cytotoxic lymphomas
Case no. . | Age, y/sex . | TCR gene rearrangement . | Disease stage . | Immunophenotype . | IL T2*(F278) . | HLA-G*(4H84) . | IL-10* . |
|---|---|---|---|---|---|---|---|
| 1 | 39/F† | Vγ1-8, VJ1/2 | IVb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | + | + |
| 2 | 73/M | Positive‡ | IIb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | - | - |
| 3 | 52/F† | Positive‡ | IIb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | + | + |
| 4 | 69/F | Vγ1-8 | Ib | TIA1+perforin-GrB+CD3+CD8+CD43+ | + | + | + |
| 5 | 39/M | Positive‡ | Ib | TIA1+perforin+GrB-CD2+CD3+CD8+ CD43+ | + | + | + |
| 6 | 45/M | Vγ1-8 | Ib | TIA1+perforin+GrB+CD2+CD3+CD8+ CD43+ | + | + | + |
| 7 | 48/M | Vγ1-8 and JP1 | Ia | TIA1+perforin-GrB+CD3+CD8+CD43+ | + | + | + |
Case no. . | Age, y/sex . | TCR gene rearrangement . | Disease stage . | Immunophenotype . | IL T2*(F278) . | HLA-G*(4H84) . | IL-10* . |
|---|---|---|---|---|---|---|---|
| 1 | 39/F† | Vγ1-8, VJ1/2 | IVb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | + | + |
| 2 | 73/M | Positive‡ | IIb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | - | - |
| 3 | 52/F† | Positive‡ | IIb | TIA1+perforin+GrB+CD4+CD43+CD56+ | + | + | + |
| 4 | 69/F | Vγ1-8 | Ib | TIA1+perforin-GrB+CD3+CD8+CD43+ | + | + | + |
| 5 | 39/M | Positive‡ | Ib | TIA1+perforin+GrB-CD2+CD3+CD8+ CD43+ | + | + | + |
| 6 | 45/M | Vγ1-8 | Ib | TIA1+perforin+GrB+CD2+CD3+CD8+ CD43+ | + | + | + |
| 7 | 48/M | Vγ1-8 and JP1 | Ia | TIA1+perforin-GrB+CD3+CD8+CD43+ | + | + | + |
TIA1 indicates T-cell intracellular antigen 1; and GrB, granzyme B.
Stainings were done on frozen tissue sections.
Deceased.
Positive, but not further specified.
Immunohistochemistry
Both paraffin-embedded as well as frozen material was available from all 7 patients. Patients were extensively phenotyped with the use of a panel of antibodies (CD2, CD3, CD4, CD5, CD7, CD8, CD16, CD30, CD34, CD43, CD45RO, CD56, CD57, CD68, CD79a, and MAC383); only the positive staining is presented in Table 1. A monoclonal antibody recognizing ILT2 (F278) was kindly provided by Maria Christina Mingari (Advanced Biotechnology Center, Genoa, Italy); HLA-G (4H84) immunoglobulin G1 (IgG1) mouse monoclonal antibody was kindly provided by M. McMaster (University of California, San Francisco, CA). Antihuman IL-10 (E-10) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Immunohistochemistry was performed using the alkaline phosphatase–antialkaline phosphatase (APAAP) technique, as previously described.9 HLA-G staining was done according to the HLA-G workshop recommendations.13
Results and discussion
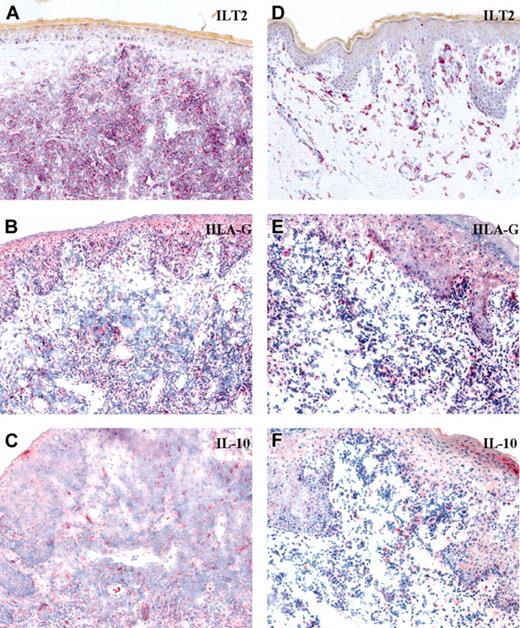
Results of immunostaining for HLA-G and IL-10 are shown in Table 1 and Figure 1. All CD8+ lymphomas expressed HLA-G on a certain proportion of tumor cells. Two of 3 CD56+CD4+ lymphomas showed HLA-G immunoreactivity. It is of note that CD56+CD4+ lymphoma displayed stronger HLA-G immunoreactivity in comparison with CD8+ cases. IL-10 expression matched the expression pattern of HLA-G. IL-10 was either coexpressed or expressed in the vicinity of HLA-G+ cells. All 7 lymphoma cases, both CD56+CD4+ and CD8+, expressed the specific HLA-G ligand, ILT-2 (Table 1). Figure 1 shows that even though infiltrate cells strongly express ILT2 receptor, only a minority of cells actually express HLA-G. Evaluation of serial tissue sections revealed that intraepidermal atypical lymphocytes, a hallmark of CD8+ CTCL, expressed HLA-G as well as ILT2 in all cases (Figure 1D-E). Large blastoid lymphocytes, representing the major infiltrate component in CD56+ CTCL, preferentially expressed ILT2 with occasional HLA-G immunoreactivity (Figure 1A-B). Small-sized reactive lymphocytes displayed sporadic HLA-G positivity.
Expression of ILT2, HLA-G, and IL-10 in primary cutaneous T-cell lymphomas. Original magnification, × 20. (A-C) CD56+CD4+ cutaneous cytotoxic lymphoma. (D-F) CD8+ cutaneous cytotoxic lymphoma.
Expression of ILT2, HLA-G, and IL-10 in primary cutaneous T-cell lymphomas. Original magnification, × 20. (A-C) CD56+CD4+ cutaneous cytotoxic lymphoma. (D-F) CD8+ cutaneous cytotoxic lymphoma.
A recent study by Nikolova et al14 demonstrated increased cell surface expression of ILT2/CD85j in circulating Sézary cells. Sézary syndrome is an aggressive form of cutaneous T-cell lymphoma characterized by erythroderma, lymphadenopathy, and the presence of CD4+CD45RO+ Sézary cells in peripheral blood. ILT2 differs from other KIRs by virtue of its distribution on phagocytic and antigen-presenting cells, such as monocytes, macrophages, dendritic cells, and B lymphocytes.15 ILT2 is also expressed on some of the peripheral NK and T cells, in particular on CD8+ T cells with memory/effector phenotype.16 ILT2 is shown to down-regulate T-cell functions by inhibiting CD3/TCR–mediated activation of both CD4+ and CD8+ clones and by inhibiting antigen recognition by CD8+ cells.17 Nikolova et al14 found that ILT2-expressing Sézary cells, as compared with autologous reactive CD4+ lymphocytes, are resistant to CD3 monoclonal antibody–induced cell death. The expression of ILT2 inhibitory receptor may enable survival of the malignant clone by protecting them from activation-induced cell death, as has already been reported in normal circulating memory/effector CD8+ T cells expressing ILT2 and other KIRs.16 Saverino et al18 showed that the cross-linking of ILT2 receptor on T cells inhibits antigen-specific T-cell proliferation and induces production of immunosuppressive cytokines such as IL-10 and transforming growth factor (TGF)–β. IL-10 exerts a myriad of immunosuppressive effects through inhibition of IL-12 production and Th1 switch, down-regulation of HLA and costimulatory molecules, together with compromised dendritic cell differentiation, maturation, and functionality.8 IL-10 is believed to affect the proliferation and/or cytotoxic phenotype in an autocrine manner in nasal NK-cell lymphomas.19 IL-10 is one of the cytokines implicated in the induction of HLA-G expression.9,20 We have recently analyzed HLA-G and IL-10 expression in various subtypes of CTCL.21 Our study showed that in CD4+ CTCL, the expression of HLA-G associates with IL-10 expression in patients with high-grade histology and advanced disease stage II.1 On the contrary, 2 cases of early stage CD8+ CTCL included in this study expressed HLA-G as well as IL-10.21 Our current results confirm these initial findings, demonstrating HLA-G and IL-10 expression in additional cases of early stage CD8+ CTCL. This early expression of HLA-G and IL-10 might be a parameter of aggressiveness of this lymphoma entity.
Apart from its inhibitory effects on immune response, HLA-G has the capability to modulate cytokine production, that is, to alter the Th1/Th2 balance toward Th2 polarization. The recognition of membrane-bound HLA-G,22 as well as incubation of peripheral blood mononuclear cells with purified HLA-G,23 results in induction of the Th2 shift. By modulating the cytokine microenvironment, HLA-G might be not only attracting immune cells bearing HLA-G–specific KIR ligand but up-regulating a specific KIR ligand on already present infiltrating cells. In contrast to the constitutive and stable KIR expression in mature NK cells,24 the induction of a particular KIR repertoire in T cells is possible under specific conditions (eg, IL-10).25 The expression of ILT2 on tumor cells may render tumor-specific T lymphocytes unable to recognize tumor cells, thus providing an additional immune-escape mechanism. Together with expression of KIRs, IL-10–induced HLA-G up-regulation could account for the growth/survival advantage of the dominant clone and contribute to the aggressive phenotype of these lymphoma entities.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-10-3372.
Supported by the Gottfried and Julia Bangerter-Rhyner Foundation.
M.U. and J.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal