Abstract
We used a morpholino-based gene-targeting screen to identify a novel protein essential for vascular development using the zebrafish, Danio rerio. We show that syndecan-2, a cell-surface heparan sulfate proteoglycan, is essential for angiogenic sprouting during embryogenesis. The vascular function of syndecan-2 is likely conserved, as zebrafish and mouse syndecan-2 show similar expression patterns around major trunk vessels, and human syndecan-2 can restore angiogenic sprouting in syndecan-2 morphants. In contrast, forced expression of a truncated form of syndecan-2 results in embryos with defects in angiogenesis, indicating that the highly conserved cytoplasmic tail is important for the vascular function of syndecan-2. We further show that vascular endothelial growth factor (VEGF) and syndecan-2 genetically interact in vivo using both gain-of-function and loss-of-function studies in zebrafish. VEGF-mediated ectopic signaling is compromised in syndecan-2 morphants, and ectopic syndecan-2 potentiates ectopic VEGF signaling. Syndecan-2 as a novel angiogenic factor is a potential candidate for use in the development of angiogenesis-based therapies.
Introduction
Vascular formation is composed of 2 processes, vasculogenesis and angiogenesis. In vasculogenesis, angioblasts derived from the lateral mesoderm migrate and differentiate into endothelial cells that form the tubular structures of primary vasculature. In angiogenesis, new vessels sprout from pre-existing vasculature and are further remodeled to form mature blood vessels. Angiogenesis is an important process during development, various physiologic phenomena, and pathologic conditions such as tumor growth and diabetic retinopathy.1 Growth factors such as vascular endothelial growth factor (VEGF), angiopoietins, and ephrins play key roles during angiogenesis.1-3 Identification and characterization of novel angiogenic factors during vertebrate development may further the understanding of key molecular players in angiogenesis as well as hold promise for potential application in angiogenesis-based therapies.4-6
Conservation of vascular development in vertebrates7 and the capacity to effectively target this process using morpholinos8 allowed us to embark on a morpholino-based screen for novel angiogenic factors using zebrafish as the model system. Morpholinos were designed based on 5′ leader sequences from a collection of zebrafish expressed sequence tags (ESTs) with high homology to human, Drosophila, or Fugu gene. Bioinformatics methods to enrich for partial sequences of cell-surface or secreted proteins based on full-length reference protein data sets have been determined as previously described.9 As part of this ongoing effort, we have identified syndecan-2 (syn-2), a cell-surface heparan sulfate proteoglycan, as a novel factor essential for angiogenic sprouting during vascular development.
Previous studies have demonstrated the important roles played by heparan sulfate proteoglycans (HSPGs) in a variety of biologic processes such as early embryonic patterning, morphogenesis, and disease.10-12 Regulation of a given biologic process by HSPGs is often achieved through modulation of growth factor–mediated signaling. Genetic studies in Drosophila have shown that loss-of-function mutations in genes encoding membrane-anchored HSPGs, the glypicans dally and dally-like, and in genes encoding enzymes involved in biosynthesis of HSPGs compromise signaling pathways mediated by growth factors such as Wingless (Wg), Decapentaplegic (Dpp), Fibroblast growth factor (FGF), and Hedgehog (Hh).11,13-15 In vitro studies have also demonstrated the requirement of syndecan-4 in FGF-2–induced biologic response16 and dally-like in the activation of a Hedgehog-mediated signaling pathway.17
In this study, we characterize vascular defects in zebrafish embryos resulting from morpholino targeting of syndecan-2. Based on microangiography and molecular analyses, we show that syndecan-2 is essential for angiogenic sprouting during zebrafish vascular development. The vascular function of syndecan-2 during vertebrate development is likely conserved as both zebrafish and mouse syndecan-2 are expressed in cells surrounding the major trunk vessels, and the introduction of human syndecan-2 protein restores angiogenic sprouting in syndecan-2 morphants. Forced expression of a cytoplasmically truncated version of syndecan-2 results in embryos with defects in angiogenic sprouting, highlighting the importance of the cytoplasmic tail for the vascular function of syndecan-2. We further show that syndecan-2 and VEGF-A interact in vivo, as VEGF-165–induced neovascularization is compromised in syndecan-2 morphants, and ectopic syndecan-2 potentiates ectopic VEGF signaling. Our finding that syndecan-2 functions in the angiogenic process of blood vessel formation makes it a potential candidate for anticancer or other angiogenesis-based drug designs.
Materials and methods
Morpholino sequences and injections
The syndecan-2 morpholino phosphorodiamidate oligonucleotides (morpholinos), from Gene Tools (Philomath, OR), have the following sequences (mismatch bases are underlined): syndecan-2 MO no. 1, 5′-GGTTCCTCATAATTCCTCAGTCTTC-3′; 4-base mismatch, d4 MO: 5′-GGTACCTGATAATACCTCACTCTTC-3′; syndecan-2 MO no. 2, 5′-GCTCGTGAAAGCGGAAAATCGC-3′; and syndecan-2 MO no. 3, 5′-CCTCAGTCTTCGCTCGTGAAAGCG-3′.
Raising and staging embryos
Embryos were raised at 29°C. Zebrafish embryos were staged using standard morphologic criteria20 prior to fixation and analysis to compensate for any observed developmental delay.
Syndecan-2 5′ UTR-GFP fusion construct and mRNA synthesis
Polymerase chain reaction (PCR) mutagenesis was used to amplify 5′ syndecan-2 sequences (primers, 5′-GCAGGATCCGCGATTTTCCGCTTTCACGA-3′ and 5′-ACCTGAATTCAGGTTCCTCATAATTCCTCAG-3′) and green fluorescent protein (GFP) sequences (primers, 5′-ACGTGAATTCGAGTAAAGGAGAAGAACTT-3′ and 5′-CAGTCTCGAGTTATTTGTATAGTTCATCCATG-3′) using standard techniques. The resulting EcoRI/XhoI and BamHI/EcoRI digested fragments were subcloned into pCS2+ to generate the plasmid, pCS2+–syndecan-2 5′ untranslated region (UTR)–GFP fusion construct. The syndecan-2 5′ UTR-GFP fusion construct was linearized with NotI. SP6 RNA polymerase was used for in vitro synthesis of capped mRNA (Ambion, Austin, TX).
Manipulation and analysis of embryos
Injections of mRNA21 and quantitative analysis of GFP expression19 were performed as previously described. For microangiography analysis, fluorescein isothiocyanate (FITC)–dextran dye was microinjected into the common cardinal vein of zebrafish embryos as described.6 Hematoxylin and eosin staining was performed using standard protocol.
Cloning of zebrafish, mouse, and human syndecan-2
The full-length zebrafish syndecan-2 coding sequence was determined by standard sequencing of a zebrafish syndecan-2 EST clone obtained from Incyte Genomics (St Louis, MO). The coding sequence of zebrafish syndecan-2 was submitted to GenBank (accession no. AY091914).
cDNA containing the coding sequence of mouse syndecan-2 was isolated from mouse embryonic day-7 cDNA library (Clontech, Palo Alto, CA) using PCR and primers, 5′-TACAGAAGCCAACAAGTGAG-3′ and 5′-ACTGCTAGGCACAGAACACT-3′, which were designed based on the reported sequence (GB accession no. NM009304).
Human syndecan-2 open reading frame was amplified from a human fetal liver cDNA library (Genemed Biotechnologies, San Francisco, CA) using the following primers: 5′-ATGCGGCGCGCGTGGATC-3′ and 5′-TTACGCATAAAACTCCTTAGTAG-3′. The primers were designed based on the human syndecan-2 sequence previously published (GB accession no. XM040582).
Expression constructs and injections
The EcoRI site was introduced at the 5′ and the 3′ ends of the coding sequence of either zebrafish or human syndecan-2 by another round of PCR amplification. The PCR fragment was subsequently subcloned into the EcoRI site of the FRM expression construct.22 The fragment of dS2 was generated by the following primers: 5′-ATGAGGAACCTTTGGATGAT-3′ and 5′-TTACGGTTTCCTCTCTCCCTA-3′. A premature stop codon was introduced by the 3′ primer. The same subcloning procedure as described previously for zebrafish and human syndecan-2 expression constructs was subsequently performed.
The DNA fragment containing VEGF-165 open reading frame was isolated from the CS2+–VEGF-165 plasmid (kindly provided by Dr Ruowen Ge) by EcoRI restriction digest. The purified EcoRI DNA fragment was subsequently subcloned into the EcoRI site of the FRM expression construct.
The mixtures of test DNA construct mixed with FRM–enhanced GFP (EGFP) expression construct was injected into 1-cell embryos at the interface between the yolk and the blastomere to ensure uniform mosaic distribution. Embryos showing GFP expression were selected prior to fixation for subsequent in situ analysis.
In situ hybridization
Zebrafish syndecan-2 in pSport1 plasmid was linearized with SalI, and SP6 polymerase was used for digoxigenin (DIG)–labeled antisense RNA synthesis. The same plasmid was linearized with NotI, and T7 polymerase was used for DIG-labeled sense RNA synthesis. DIG-labeled probes were synthesized using the in vitro DIG labeling kit (Boehringer Mannheim, Mannheim, Germany). The mouse syndecan-2 cDNA fragment was then subcloned into the pCR4-TOPO vector (Invitrogen, Frederick, MD), linearized with NotI for DIG-labeled antisense RNA synthesis (T3 polymerase) or SacI for DIG-labeled sense (control) RNA synthesis (T7 polymerase). Zebrafish and mouse in situ hybridization procedures were performed as previously described.23,24
Radiation hybrid mapping
The radiation hybrid DNA panel LN54,25 a gift from Marc Ekker, was screened using syndecan-2 primers 5′-AGCTGTGATTGCAGGAGGAG-3′ and 5′-TTATGCGTAAAACTCCTTGGTG-3′. “Touchdown” PCR was performed with the starting annealing temperature set at 69°C for 38 cycles, with 0.5°C subtracted from each of the first 29 cycles. The annealing temperature was set at 55°C for the last 9 cycles. The positives were in groups 8, 13, 18, 47, 49, 52, 59, 65, 66, 70, 73, 97, 104, 106, 108, 169, 175, 183, 301, AB9, and Mix. Linkage analysis was performed with Zebrafish Information Network at University of Oregon software (http://zfin.org/cgi-bin/webdriver?MIval=aa-ZDB_home.apg).
Results
Determination of phenotypes from MO injections
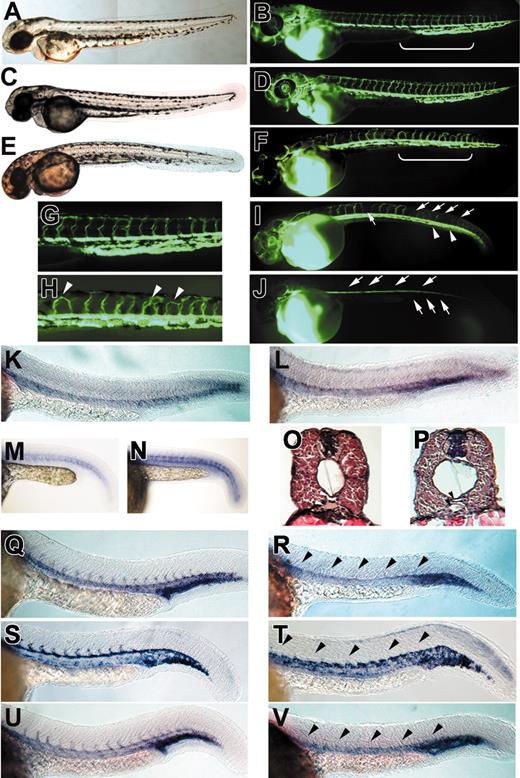
Embryos injected with low doses (1-5 ng) of syndecan-2 morpholino (MO) showed reduced axial or a lack of intersegmental circulation and enlarged pericardium with no other gross morphologic defects (Figure 1E). To assess the functional integrity of the vasculature of each embryo, microangiography analysis was performed on syndecan-2 MO–injected embryos. Embryos injected with syndecan-2 MO showed defective vascular formation in varying extents of severity. In the least severe case, intersegmental vessels either sprouted abnormally (Figure 1F and arrowheads in Figure 1H) or failed to form (Figure 1I, arrows), and the vascular plexus in the tail region was less complex (Figure 1I, arrowheads) compared with that of wild-type embryos (Figure 1B,G). In the more severe case, a complete lack of intersegmental sprouting and defects in formation of the venous plexus in the tail were observed (Figure 1J). In contrast, embryos injected with a 4-base mismatch syndecan-2 MO showed normal morphology and no apparent vascular defects (Figure 1C-D). A greater penetrance of similar vascular phenotypes was observed at higher doses of syndecan-2 MO, with other adverse consequences noted at higher doses (data not shown). We focused our analyses on morpholino doses, which demonstrate clear vascular defects without any additional complicating phenotypes.
syndecan-2 MO–injected embryos showed angiogenic defects. (A) An uninjected wild-type embryo at 48 hpf. (B) Microangiogram of an uninjected wild-type embryo at 48 hpf. Bright field image (C) and microangiogram (D) of an embryo injected with 5 ng of a 4-base mismatch syndecan-2 showing intact vasculature. (E) An embryo injected with 5 ng syndecan-2 MO1, showing no obvious morphologic defects at 48 hpf. (F,I,J) Microangiograms of embryos injected with 5 ng syndecan-2 MO1, showing vascular phenotypes (frequency: 84% ± 4%, n = 55). Magnified images of sections indicated by brackets in panels B and F are shown in panels G and H, respectively. Embryos injected with syndecan-2 MO1 showed either aberrant (F; arrowheads in H compared with wild-type in G) or reduced intersegmental vessels (I, arrows) and reduced tail vessels (I, arrowheads). In the most severe case, embryos showed a complete lack of intersegmental vessels (top arrows in J) and lack of tail vessels (bottom arrows in J). In situ analysis was also performed to assess molecular phenotypes of syndecan-2 MO–injected embryos. (K-L) Expression of ephrin-B2 in a wild-type embryo (K) and a syndecan-2 MO–injected embryo (L) at 24 hpf. Arterial expression of ephrin-B2 was retained in syndecan-2 MO–injected embryos. (M-N) Expression of VEGF-A in wild-type (M) and syndecan-2 MO–injected (N) embryos (n = 20). Somitic expression of VEGF-A was not affected in syndecan-2 MO–injected embryos. Cross sections from the anterior trunk of a wild-type embryo (O) and a syndecan-2 MO–injected embryo (P) at 44 to 48 hpf stained with hematoxylin and eosin. The dorsal aorta (top arrowhead) and the axial vein (bottom arrowhead) were both observed in syndecan-2 MO–injected embryos. (Q) Expression of fli-1 in a wild-type embryo at 24 hpf. (R) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental fli-1 expression (arrowheads). Of the embryos, 58% (n = 35) showed reduced intersegmental fli-1 expression at 5 ng syndecan-2 MO1. (S) Expression of flk-1 in a wild-type embryo at 24 hpf. (T) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental flk-1 expression (arrowheads). Of embryos, 50% (n = 40) showed reduced flk-1 expression at 5 ng syndecan-2 MO1. (U) Expression of tie-1 in a wild-type embryo at 24 hpf. (V) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental expression of tie-1 (arrowheads). At 5 ng syndecan-2 MO1, 63% of embryos (n = 56) showed reduced tie-1 expression. (A,C,E) Bright field images (original magnification, × 5). (B,D,F-J) Microangiography analysis (original magnification, × 5. (K-N,Q-V) In situ hybridization (original magnification, × 10). (O-P) Original magnification, × 40.
syndecan-2 MO–injected embryos showed angiogenic defects. (A) An uninjected wild-type embryo at 48 hpf. (B) Microangiogram of an uninjected wild-type embryo at 48 hpf. Bright field image (C) and microangiogram (D) of an embryo injected with 5 ng of a 4-base mismatch syndecan-2 showing intact vasculature. (E) An embryo injected with 5 ng syndecan-2 MO1, showing no obvious morphologic defects at 48 hpf. (F,I,J) Microangiograms of embryos injected with 5 ng syndecan-2 MO1, showing vascular phenotypes (frequency: 84% ± 4%, n = 55). Magnified images of sections indicated by brackets in panels B and F are shown in panels G and H, respectively. Embryos injected with syndecan-2 MO1 showed either aberrant (F; arrowheads in H compared with wild-type in G) or reduced intersegmental vessels (I, arrows) and reduced tail vessels (I, arrowheads). In the most severe case, embryos showed a complete lack of intersegmental vessels (top arrows in J) and lack of tail vessels (bottom arrows in J). In situ analysis was also performed to assess molecular phenotypes of syndecan-2 MO–injected embryos. (K-L) Expression of ephrin-B2 in a wild-type embryo (K) and a syndecan-2 MO–injected embryo (L) at 24 hpf. Arterial expression of ephrin-B2 was retained in syndecan-2 MO–injected embryos. (M-N) Expression of VEGF-A in wild-type (M) and syndecan-2 MO–injected (N) embryos (n = 20). Somitic expression of VEGF-A was not affected in syndecan-2 MO–injected embryos. Cross sections from the anterior trunk of a wild-type embryo (O) and a syndecan-2 MO–injected embryo (P) at 44 to 48 hpf stained with hematoxylin and eosin. The dorsal aorta (top arrowhead) and the axial vein (bottom arrowhead) were both observed in syndecan-2 MO–injected embryos. (Q) Expression of fli-1 in a wild-type embryo at 24 hpf. (R) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental fli-1 expression (arrowheads). Of the embryos, 58% (n = 35) showed reduced intersegmental fli-1 expression at 5 ng syndecan-2 MO1. (S) Expression of flk-1 in a wild-type embryo at 24 hpf. (T) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental flk-1 expression (arrowheads). Of embryos, 50% (n = 40) showed reduced flk-1 expression at 5 ng syndecan-2 MO1. (U) Expression of tie-1 in a wild-type embryo at 24 hpf. (V) A syndecan-2 MO–injected embryo at 24 hpf, showing a lack of intersegmental expression of tie-1 (arrowheads). At 5 ng syndecan-2 MO1, 63% of embryos (n = 56) showed reduced tie-1 expression. (A,C,E) Bright field images (original magnification, × 5). (B,D,F-J) Microangiography analysis (original magnification, × 5. (K-N,Q-V) In situ hybridization (original magnification, × 10). (O-P) Original magnification, × 40.
Analysis of molecular phenotypes in syndecan-2 morphants
The lack of intersegmental vessels in syndecan-2 MO–injected embryos as revealed by microangiography analysis did not exclude the possibility that the blood vessels might be leaky (for example, due to immature vessel walls) or lack patent lumen. To distinguish the nature of vascular defects in syndecan-2 MO–injected embryos, we analyzed the expression of known vascular markers using in situ hybridization. ephrin-B2 and ephrin-B4 expression was analyzed in syndecan-2 MO–injected embryos to assess the integrity of vasculogenesis. Ephrin-B2 and Ephrin-B4 are a transmembrane ligand-receptor pair of the ephrin family that mark arterial and venous endothelial cells, respectively.26 In embryos injected with 5 ng syndecan-2 MO, arterial expression of ephrin-B2 was not affected (Figure 1L). Expression of ephrin-B4 or rtk-5 was also normal (data not shown), suggesting that primary formation of the axial vessels was normal in syndecan-2 MO–injected embryos. The Hedgehog signaling pathway has been shown to be essential for arterial differentiation and induces the expression of VEGF during the same process.27 Somitic expression of patched-1, a downstream target gene of the Hedgehog signaling pathway, and VEGF-A was not affected in syndecan-2 MO–injected embryos (n = 18 for ptc-1 analysis, data not shown; n = 20 for VEGF-A analysis, Figure 1N), suggesting that syndecan-2 participates in blood vessel patterning downstream of axial vessel differentiation. Histologic analysis using hematoxylin and eosin staining was also performed on syndecan-2 MO–injected embryos. The dorsal aorta and the cardinal vein were both observed in syndecan-2 MO–injected embryos, further indicating that syndecan-2 is not required for axial vessel formation (Figure 1O-P).
We then analyzed expression of flk-1, fli-1, and tie-1, which are expressed in the axial and intersegmental vessels of the trunk as early as 22 hours after fertilization (hpf) for flk-1 and fli-1 and 24 hpf for tie-1.28,29 Axial expression of fli-1, flk-1, and tie-1 was retained at 24 hpf in embryos injected with 5 ng syndecan-2 MO, but intersegmental expression of each marker was absent (Figure 1R,T,V). The same in situ analysis was also performed on embryos injected with higher doses of the syndecan-2 MO, and a higher penetrance of the angiogenic phenotype was observed (data not shown). These results indicate that the process of angiogenic sprouting did not occur in syndecan-2 MO–injected embryos.
As the reduction or the lack of circulation observed in syndecan-2 MO–injected embryos might be attributed to defective blood formation, we examined early blood markers, SCL and gata-1. Expression of SCL (n = 41) and gata-1 (n = 42) was normal in syndecan-2 MO–injected embryos, indicating that early blood formation is normal in syndecan-2 MO–injected embryos (data not shown). However, the lack of intersegmental sprouting in syndecan-2 MO–injected embryos does not solely explain the circulation defects observed. Consistent with this, in some embryos, blood cells were found pooled in caudal regions (data not shown). There could be undetected vasculogenic defects not detected by our microangiography and molecular analyses or another uncharacterized effect from morpholino injections.
A previous study in Xenopus demonstrated the essential role played by syndecan-2 in early left-right asymmetry development.30 Here we show that syndecan-2 MO–injected embryos showed randomized expression of early asymmetric markers, lefty-2 and pitx-2 (Table S1; see the Supplemental Materials link at the top of the online article on the Blood website). In contrast, embryos injected with a 4-base mismatch syndecan-2 MO did not show defects in left-right asymmetry development.
Specificity of morpholino targeting
To assess the efficacy and the specificity of morpholino targeting, several tests were performed (Figure 2). To determine the efficacy of morpholino targeting, we made a syndecan-2 5′untranslated region (UTR)–GFP fusion construct (see “Materials and methods”), with the syndecan-2 5′UTR containing the syndecan-2–MO1 targeting sequence. Embryos were coinjected with the mRNA synthesized from the fusion construct and the syndecan-2 MO1 or the UROD MO31 as the negative control. We showed that the introduction of 5 ng syndecan-2 MO1 in embryos injected with the syndecan-2 5′UTR-GFP RNA resulted in a drastic reduction in the GFP expression (Figure 2B and column 2 in 2E). In contrast, coinjection with 5 ng UROD MO still resulted in strong GFP expression (Figure 2C and column 3 in 2E), comparable with the level determined in embryos injected with the syndecan-2 5′UTR-GFP RNA only (Figure 2A and column 1 in 2E). The same analysis of GFP expression was also performed on embryos coinjected with higher doses of syndecan-2 MO or UROD MO (up to 8 ng). Strong reduction of GFP expression was again observed in embryos coinjected with syndecan-2 MO, but not in those coinjected with UROD MO (data not shown). These results demonstrate the high efficacy of syndecan-2 MO targeting.
Targeting efficacy and specificity of syndecan-2 morpholinos. Messenger RNA synthesized from the syndecan-2 5′UTR-GFP fusion construct was coinjected with syndecan-2 MO1 or UROD MO into zebrafish embryos. GFP expression was assessed at 24 hpf. (A-D) GFP expression was analyzed under the bp-GFP filterset (original magnification, × 2.5). (A) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA. (B) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA and 5 ng syndecan-2 MO1. (C) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA and 5 ng UROD MO. (D) Uninjected wild-type embryos. (E) Quantitation of GFP activity. (F) Embryos were injected with syndecan-2 MO1, syndecan-2 d4 MO1, or syndecan-2 MO3. Injections of syndecan-2 MO1 or MO3 resulted in a high penetrance of vascular phenotypes. In contrast, a strong attenuation of vascular phenotypes was observed in embryos injected with d4 MO1. (G-J) In situ hybridization (original magnification, × 10). (G) In situ analysis of flk-1 expression in a wild-type embryo. (H) flk-1 expression in a syndecan-2 MO–injected embryo, showing a characteristic lack of intersegmental expression. (I) Embryo coinjected with syndecan-2 MO and a mixed solution of zebrafish syndecan-2 and EGFP expression constructs, showing partial sprouts of intersegmental vessels (arrowheads). (J) Embryo coinjected with syndecan-2 MO and EGFP expression construct. No new intersegmental expression was observed. (K) Results from 6 independent rescue experiments. The group coinjected with syndecan-2 MO and syndecan-2 DNA showed a significant increase in the number of embryos showing intersegmental expression of flk-1, compared with the group injected with the syndecan-2 MO only. (L) Results from 4 injection experiments, showing no effect on flk-1 expression by the control DNA (EGFP). Error bars indicate standard errors.
Targeting efficacy and specificity of syndecan-2 morpholinos. Messenger RNA synthesized from the syndecan-2 5′UTR-GFP fusion construct was coinjected with syndecan-2 MO1 or UROD MO into zebrafish embryos. GFP expression was assessed at 24 hpf. (A-D) GFP expression was analyzed under the bp-GFP filterset (original magnification, × 2.5). (A) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA. (B) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA and 5 ng syndecan-2 MO1. (C) Embryos injected with 120 pg syndecan-2 5′UTR-GFP RNA and 5 ng UROD MO. (D) Uninjected wild-type embryos. (E) Quantitation of GFP activity. (F) Embryos were injected with syndecan-2 MO1, syndecan-2 d4 MO1, or syndecan-2 MO3. Injections of syndecan-2 MO1 or MO3 resulted in a high penetrance of vascular phenotypes. In contrast, a strong attenuation of vascular phenotypes was observed in embryos injected with d4 MO1. (G-J) In situ hybridization (original magnification, × 10). (G) In situ analysis of flk-1 expression in a wild-type embryo. (H) flk-1 expression in a syndecan-2 MO–injected embryo, showing a characteristic lack of intersegmental expression. (I) Embryo coinjected with syndecan-2 MO and a mixed solution of zebrafish syndecan-2 and EGFP expression constructs, showing partial sprouts of intersegmental vessels (arrowheads). (J) Embryo coinjected with syndecan-2 MO and EGFP expression construct. No new intersegmental expression was observed. (K) Results from 6 independent rescue experiments. The group coinjected with syndecan-2 MO and syndecan-2 DNA showed a significant increase in the number of embryos showing intersegmental expression of flk-1, compared with the group injected with the syndecan-2 MO only. (L) Results from 4 injection experiments, showing no effect on flk-1 expression by the control DNA (EGFP). Error bars indicate standard errors.
As a primary specificity test, we showed that 2 morpholinos of nonoverlapping sequence targeted against the syndecan-2 gene both generated the same range of vascular phenotypes (Figure 2F), albeit at different penetrance rates. In addition, the introduction of a 4-base modification of the same morpholino achieved a strong attenuation of the observed vascular defects (Figure 2F), indicating a high specificity of the vascular phenotypes observed in syndecan-2 MO–injected embryos.
Analysis of flk-1 expression showed a characteristic loss of intersegmental expression in syndecan-2 MO–injected embryos, indicating defective angiogenic sprouting. We then asked whether the presence of exogenous zebrafish syndecan-2 protein could ameliorate the angiogenic defect observed in syndecan-2 MO–injected embryos. In each experiment, embryos were injected with the syndecan-2 MO designed against the 5′ UTR region of syndecan-2, and a subset of those embryos was also injected with a mixed solution of syndecan-2 and EGFP expression constructs. The EGFP expression construct was used as a lineage tracer to facilitate the identification of successfully injected embryos. Our results from in situ analysis of flk-1 expression indicate a significantly higher fraction of embryos coinjected with syndecan-2 MO and syndecan-2 DNA showing intersegmental vessels (P < .0001 in a student t test; Figure 2I and K, compare left and right columns). In contrast, there was no significant difference in the fraction of embryos showing intersegmental expression of flk-1 in embryos coinjected with syndecan-2 MO and EGFP expression construct compared with those injected with syndecan-2 MO alone (P > .3 in a student t test; Figure 2L). These experiments indicate that the angiogenic defect observed in syndecan-2 MO–injected embryos was specific to a loss of function of the endogenous syndecan-2 gene.
Expression pattern of syndecan-2
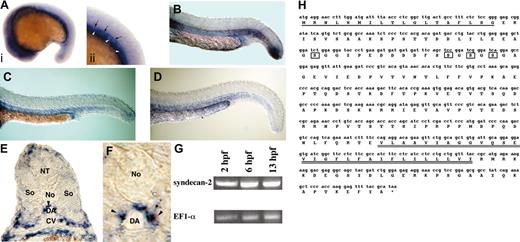
The vascular defects observed in syndecan-2 MO–injected embryos led us to analyze the spatial expression pattern of syndecan-2 at different stages of early vascular development using in situ hybridization. The first localized expression of syndecan-2 was detected in the hypochord (Figure 3A, arrows), a single cell–wide midline structure immediately ventral to the notochord and dorsal to the dorsal aorta, and mesendoderm (Figure 3A, arrowheads) at 17 to 18 hpf, which corresponds to the 15 to 16 somite stage. A cross section in the anterior trunk of a 24-hpf embryo shows expression of syndecan-2 in the hypochord, ventromedial regions of the somites, the mesenchymal cell layer surrounding the axial vessels, and the endoderm (Figure 3E; see syndecan-2–expressing cells surrounding the dorsal aorta in Figure 3F). The expression of syndecan-2 in the mesenchyme surrounding the major trunk vessels and in ventromedial regions of the somites is highly similar to the expression pattern of zebrafish angiopoietin-1 (Ang-1) at a similar stage of development.32 The expression of syndecan-2 in the trunk persists through 33 hpf (Figure 3B-E) and is undetectable by 48 hours. Expression of syndecan-2 is also detected in the head (16-24 hpf; Figure 3A) and the dorsal neural tube (24 hpf; Figure 3E), suggesting possible functions of syndecan-2 in the development of the central nervous system. Nonlocalized RNA expression at earlier stages was confirmed using reverse transcriptase (RT)–PCR (Figure 3G), indicating that syndecan-2 might participate in other early developmental events.
Expression analysis of syndecan-2 mRNA expression in zebrafish. (A) At 17 to 18 hpf (15-16 somite stage), syndecan-2 is expressed in the brain, the hypochord (ii, arrows), and the mesendoderm (ii, arrowheads). Anterior end is to the left. Panel Aii is an expanded image of panel Ai that shows the mid-trunk section of the embryo. This localized syndecan-2 expression pattern persists through 23 hpf (B), 27 hpf (C), and up to 33 hpf (D). (E) A transverse section through the trunk of a 24-hour embryo showing expression of syndecan-2 in the dorsal neural tube, the hypochord (arrowhead), ventromedial somites, the mesenchyme surrounding the dorsal aorta and cardinal vein, and the endoderm. DA indicates dorsal aorta; CV, cardinal vein; NT, neural tube; So, somite; and No, notochord. (F) High-magnification image of the area around dorsal aorta in panel E. Arrowheads indicate syndecan-2–expressing cells surrounding the dorsal aorta (DA). (G) RT-PCR analysis showing expression of syndecan-2 mRNA (top row) and elongation factor 1α (loading control, bottom row) at 2, 6, and 13 hpf. (H) The coding sequence of zebrafish syndecan-2. The single underline indicates the predicted signal sequence. The double underlines denote the predicted hydrophobic transmembrane segment. The rectangles indicate the predicted heparan sulfate attachment sites. The cytoplasmic domain of syndecan-2 consists of 2 conserved (C) regions and one variable (V) region.46 The C1 region consists of amino acids 177 to 188. The variable region (V) consists of amino acids 189 to 199. The C2 region consists of amino acids 200 to 209. (A-F) In situ hybridization; original magnification, × 10 (A-D); × 20 (E); and × 40 (F).
Expression analysis of syndecan-2 mRNA expression in zebrafish. (A) At 17 to 18 hpf (15-16 somite stage), syndecan-2 is expressed in the brain, the hypochord (ii, arrows), and the mesendoderm (ii, arrowheads). Anterior end is to the left. Panel Aii is an expanded image of panel Ai that shows the mid-trunk section of the embryo. This localized syndecan-2 expression pattern persists through 23 hpf (B), 27 hpf (C), and up to 33 hpf (D). (E) A transverse section through the trunk of a 24-hour embryo showing expression of syndecan-2 in the dorsal neural tube, the hypochord (arrowhead), ventromedial somites, the mesenchyme surrounding the dorsal aorta and cardinal vein, and the endoderm. DA indicates dorsal aorta; CV, cardinal vein; NT, neural tube; So, somite; and No, notochord. (F) High-magnification image of the area around dorsal aorta in panel E. Arrowheads indicate syndecan-2–expressing cells surrounding the dorsal aorta (DA). (G) RT-PCR analysis showing expression of syndecan-2 mRNA (top row) and elongation factor 1α (loading control, bottom row) at 2, 6, and 13 hpf. (H) The coding sequence of zebrafish syndecan-2. The single underline indicates the predicted signal sequence. The double underlines denote the predicted hydrophobic transmembrane segment. The rectangles indicate the predicted heparan sulfate attachment sites. The cytoplasmic domain of syndecan-2 consists of 2 conserved (C) regions and one variable (V) region.46 The C1 region consists of amino acids 177 to 188. The variable region (V) consists of amino acids 189 to 199. The C2 region consists of amino acids 200 to 209. (A-F) In situ hybridization; original magnification, × 10 (A-D); × 20 (E); and × 40 (F).
Zebrafish syndecan-2 is orthologous to human and mouse syndecan-2
The complete coding sequence of zebrafish syndecan-2 was identified by sequencing the EST clone (Figure 3H). Based on amino acid sequence comparison, zebrafish syndecan-2 uniquely clusters to the vertebrate syndecan-2 family (Figure 4A). We also performed syntenic analysis between corresponding zebrafish and human chromosome regions. Radiation hybrid mapping placed zebrafish syndecan-2 in the linkage group 19, closely linked to marker Z13292 (distance 16.49 cR, LOD score of 11.3), which is located closest to the TRPS1 gene and the EXT1 gene. This region of linkage group 19 is syntenic to the human chromosome 8 region. Human syndecan-2 maps to 8q23.2,50 human TRPS1 gene maps to 8q24.12,33-35 and human EXT1 maps to 8q24.11-q24.13.36 Thus, human syndecan-2 is likely to be the true orthologue of zebrafish syndecan-2.
Zebrafish syndecan-2 is most similar to vertebrate syndecan-2. (A) A homology tree showing clustering of zebrafish syndecan-2 (gray shading) to the vertebrate syndecan-2 family. (B-G) In situ hybridization. Original magnification, × 5 (B-D); × 10 (E-G). (B) Expression of syndecan-2 mRNA in a wild-type mouse embryo at embryonic day 9.5 showing expression in the head and along the trunk (arrowheads). (C) A mouse embryo at embryonic day 9.5 treated with labeled sense mouse syndecan-2 mRNA probe. (D) A transverse section of a mouse embryo at embryonic day 9.5. The level of the section is indicated by the white line in B. The dark purple staining indicates expression of mouse syndecan-2 RNA. Similar to zebrafish syndecan-2, mouse syndecan-2 is expressed in the dorsal neural tube (arrowheads) and the cells surrounding the right and the left dorsal aorta (arrows). The notochord is indicated by double arrowheads. (E) flk-1 expression in a wild-type embryo. (F) flk-1 expression in a syndecan-2 MO–injected embryo, showing a lack of intersegmental expression. (G) flk-1 expression in an embryo coinjected with syndecan-2 MO and human syndecan-2 expression construct. Sprouts of intersegmental vessels were observed (arrowheads). (H) In situ analysis of flk-1 expression from 5 injection experiments. In each case, a higher fraction of embryos showing intersegmental expression of flk-1 was observed in embryos coinjected with syndecan-2 MO and human syndecan-2 expression construct compared with those injected with syndecan-2 MO only. Error bars indicate SE.
Zebrafish syndecan-2 is most similar to vertebrate syndecan-2. (A) A homology tree showing clustering of zebrafish syndecan-2 (gray shading) to the vertebrate syndecan-2 family. (B-G) In situ hybridization. Original magnification, × 5 (B-D); × 10 (E-G). (B) Expression of syndecan-2 mRNA in a wild-type mouse embryo at embryonic day 9.5 showing expression in the head and along the trunk (arrowheads). (C) A mouse embryo at embryonic day 9.5 treated with labeled sense mouse syndecan-2 mRNA probe. (D) A transverse section of a mouse embryo at embryonic day 9.5. The level of the section is indicated by the white line in B. The dark purple staining indicates expression of mouse syndecan-2 RNA. Similar to zebrafish syndecan-2, mouse syndecan-2 is expressed in the dorsal neural tube (arrowheads) and the cells surrounding the right and the left dorsal aorta (arrows). The notochord is indicated by double arrowheads. (E) flk-1 expression in a wild-type embryo. (F) flk-1 expression in a syndecan-2 MO–injected embryo, showing a lack of intersegmental expression. (G) flk-1 expression in an embryo coinjected with syndecan-2 MO and human syndecan-2 expression construct. Sprouts of intersegmental vessels were observed (arrowheads). (H) In situ analysis of flk-1 expression from 5 injection experiments. In each case, a higher fraction of embryos showing intersegmental expression of flk-1 was observed in embryos coinjected with syndecan-2 MO and human syndecan-2 expression construct compared with those injected with syndecan-2 MO only. Error bars indicate SE.
To address whether syndecan-2 might perform similar vascular functions in other vertebrate organisms, we analyzed the expression of syndecan-2 mRNA in mouse embryos at a similar stage of vascular development. A previous study showed that mouse syndecan-2 is expressed in the mesenchymal tissues surrounding major organs such as kidney, lung, and stomach.37 Here we show that at embryonic day 9.5, mouse syndecan-2 is expressed strongly in the head region and in the cells surrounding axial vessels (Figure 4B,D), similar to the expression pattern of syndecan-2 in zebrafish embryos at 24 hpf (Figure 3E and Figure S1 in the Supplemental Materials).
We then tested the functional conservation of syndecan-2 in vascular development by assessing whether human syndecan-2 protein can ameliorate the angiogenic defect in syndecan-2 morphant embryos. We used intersegmental expression of flk-1 in in situ analysis to assess degrees of angiogenic sprouting in the trunk. New sprouts were observed in embryos coinjected with syndecan-2 MO and human syndecan-2 DNA (arrowheads in Figure 4G compared with F). A significantly higher fraction of embryos coinjected with syndecan-2 MO and human syndecan-2 expression construct showed intersegmental expression of flk-1, compared with those injected with syndecan-2 MO only (P < .0001 in a Student t test; Figure 4H, compare left and right columns). The observation that human syndecan-2 protein alleviated the angiogenic defect observed in syndecan-2 morphant embryos suggests that the vascular function of syndecan-2 is likely conserved.
Forced expression of a cytoplasmically truncated form of syndecan-2 resulted in embryos with vascular defects
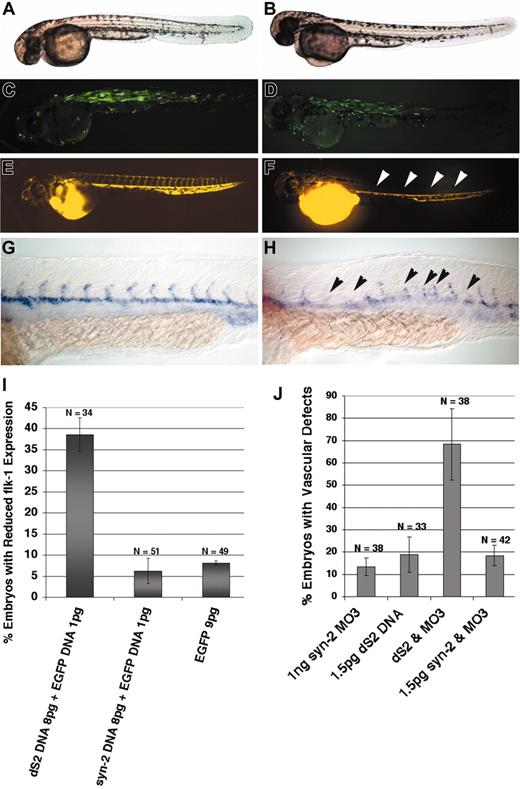
A previous study showed that a cytoplasmically truncated form of syndecan-2 (dS2) functioned as an antimorphic protein in vitro, using an extracellular matrix assembly assay system.38 Another study showed that dS2 acted as a dominant-negative protein and perturbed determination of left-right asymmetry in Xenopus embryos.30 Introduction of dS2 RNA into zebrafish embryos showed normal morphology but randomized expression of lefty-2, an early left-right asymmetry marker (data not shown; 36% ± 2%, n = 28). Overexpression of syndecan-2 RNA at similar doses did not significantly perturb left asymmetry development (10% ± 4%, n = 31). We then asked whether dS2 would have a deleterious effect on vascular development in zebrafish embryos. To deliver efficacious doses of dS2 during organogenesis, we injected embryos using a DNA expression construct for zebrafish dS2 (see “Materials and methods”). Injected embryos were assessed for possible vascular defects by microangiography and molecular analyses. Forced expression of dS2 at lower doses showed grossly normal morphology (Figure 5B), but defective angiogenic sprouting in the trunk upon microangiography analysis (Figure 5F, arrowheads). Analysis of flk-1 expression indicated reduced sproutings in dS2-injected embryos (Figure 5H, arrowheads), mimicking the effect by syndecan-2 MO injections. In contrast, forced expression of syndecan-2 DNA or EGFP DNA alone did not have any significant effect on angiogenic sprouting as revealed by in situ analysis (Figure 5I). We then lowered the dose of dS2 to which only a small percentage of dS2 DNA–injected embryos showed angiogenic defects. The deleterious effect of dS2 on angiogenic sprouting was enhanced by coinjection of a low dose of syndecan-2 MO as indicated by a synergistic increase in the frequency of embryos showing defective angiogenic sprouting (Figure 5J). These results support the antimorphic function of dS2 in angiogenesis and also demonstrate the importance of the cytoplasmic tail in contributing to the vascular function of syndecan-2.
Forced expression of the putative antimorphic form of zebrafish syndecan-2 (dS2) mimicked the weak syndecan-2 morphant phenotype. Wild-type embryos were injected with either 9 pg EGFP expression construct or a mixed solution of 7 pg dS2 and 2 pg EGFP expression constructs. (A-B) Bright field images (A, EGFP-injected embryo; B, dS2-injected embryo) showing normal morphology. (C-D) bp-GFP filter set images. Mosaic expression of EGFP was observed in each case (C, EGFP-injected embryo; D, dS2-injected embryo). (E-F) Microangiography analysis. Microangiography was also performed on embryos at 48 hpf, using TRITC (tetramethylrhodamine-5(and 6)-isothiocyanate)–dextran. EGFP-injected embryos showed normal circulation (E). Embryos injected with dS2 showed reduced or a lack of intersegmental vessels (F, arrowheads) in a subset of injected embryos (65%, n = 22). Analysis of flk-1 expression by in situ hybridization was performed as a separate measurement of blood vessel development. (G-H) In situ hybridization. (G) flk-1 expression in a wild-type embryo. (H) dS2-injected embryo, showing reduced or a lack of flk-1 expression in some intersegmental vessels (arrowheads). (I) Summary of results from in situ analysis of flk-1 expression. A significantly higher fraction of the dS2-injected embryos showed reduced flk-1 expression than was observed in wild-type syndecan-2 DNA or GFP (control) DNA injections. (J) Summary of results from microangiography analysis showing synergy between dS2 DNA at 1.5 pg and syndecan-2 MO at 1 ng in generating embryos with vascular defects (compare column 3 with columns 1 and 2). No synergy was observed between syndecan-2 DNA at 1.5 pg and syndecan-2 MO at 1 ng (column 4). Error bars indicate SE. Original magnification, × 5 (A-B); × 5 (C-F); and × 10 (G-H).
Forced expression of the putative antimorphic form of zebrafish syndecan-2 (dS2) mimicked the weak syndecan-2 morphant phenotype. Wild-type embryos were injected with either 9 pg EGFP expression construct or a mixed solution of 7 pg dS2 and 2 pg EGFP expression constructs. (A-B) Bright field images (A, EGFP-injected embryo; B, dS2-injected embryo) showing normal morphology. (C-D) bp-GFP filter set images. Mosaic expression of EGFP was observed in each case (C, EGFP-injected embryo; D, dS2-injected embryo). (E-F) Microangiography analysis. Microangiography was also performed on embryos at 48 hpf, using TRITC (tetramethylrhodamine-5(and 6)-isothiocyanate)–dextran. EGFP-injected embryos showed normal circulation (E). Embryos injected with dS2 showed reduced or a lack of intersegmental vessels (F, arrowheads) in a subset of injected embryos (65%, n = 22). Analysis of flk-1 expression by in situ hybridization was performed as a separate measurement of blood vessel development. (G-H) In situ hybridization. (G) flk-1 expression in a wild-type embryo. (H) dS2-injected embryo, showing reduced or a lack of flk-1 expression in some intersegmental vessels (arrowheads). (I) Summary of results from in situ analysis of flk-1 expression. A significantly higher fraction of the dS2-injected embryos showed reduced flk-1 expression than was observed in wild-type syndecan-2 DNA or GFP (control) DNA injections. (J) Summary of results from microangiography analysis showing synergy between dS2 DNA at 1.5 pg and syndecan-2 MO at 1 ng in generating embryos with vascular defects (compare column 3 with columns 1 and 2). No synergy was observed between syndecan-2 DNA at 1.5 pg and syndecan-2 MO at 1 ng (column 4). Error bars indicate SE. Original magnification, × 5 (A-B); × 5 (C-F); and × 10 (G-H).
Genetic interaction between VEGF-A and syndecan-2 in vivo
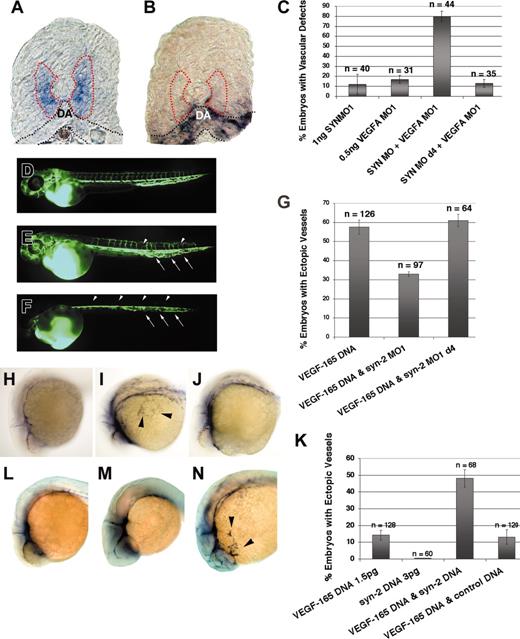
VEGF-A, produced by cells in close vicinity of endothelial cells, plays an essential role in angiogenic sprouting and remodeling during development.39,40 In zebrafish, angiogenic sprouting of intersegmental vessels occurs between 22 hpf and 24 hpf, as indicated by the arterial expression of VEGF receptor, flk-1.28 Prior to the initiation of angiogenic sprouting, zebrafish VEGF is expressed in the medial region of the somite, immediately adjacent to the region where syndecan-2 is expressed41 (Figure 6A-B). VEGF-A morphants also show similar angiogenic defects as observed in syndecan-2 morphants.8 To investigate the effect on angiogenesis resulting from the concomitant loss of endogenous VEGF and syndecan-2, we cotargeted VEGF-A and syndecan-2 by gene-specific morpholinos. Using doses at which each MO alone produced only moderate angiogenic defects in a small fraction of injected embryos, we observed a synergistic inhibition of angiogenesis in embryos coinjected with syndecan-2 MO and VEGF-A MO (Figure 6C). Embryos coinjected with syndecan-2 MO and VEGF-A MO showed aberrant angiogenic sprouting (Figure 6E, arrowheads) and abnormal formation of the venous plexus (Figure 6E, arrows) in the less severe case. In the most severe case, coinjected embryos showed no trunk angiogenesis (Figure 6F, arrowheads).
Genetic interaction between VEGF and syndecan-2 in vivo. (A-B) In situ hybridization. (A) Cross section of a wild-type embryo at 22 hpf, showing VEGF-A expression in medial somitic mesoderm (purple staining within the area as indicated by the red dashed lines). DA indicates dorsal aorta. (B) Cross section of a wild-type embryo at 22 hpf, showing syndecan-2 expression in the mesenchyme (black dashed lines) surrounding the dorsal aorta, ventral to the somitic mesoderm. Note that the region of VEGF-A expression is adjacent to the mesenchyme where syndecan-2 is expressed. (C) Summary of microangiography analysis. Low doses of syndecan-2 MO and VEGF-A MO synergize to generate embryos with vascular defects (compare column 3 with columns 1 and 2). There is no interaction between VEGF-A MO and the 4-base mismatch syndecan-2 MO (d4). The total number of embryos analyzed from 2 independent experiments is indicated. (D-F) Microangiography analysis. (D) Microangiogram of a wild type at 48 hpf. (E) Microangiogram of an embryo coinjected with syndecan-2 MO and VEGF-A MO, showing aberrant sprouting of intersegmental vessels (arrowheads) and abnormal formation of blood vessels in the tail region (arrows). (F) Microangiogram of an embryo coinjected with syndecan-2 MO and VEGF-A MO with no trunk angiogenesis (arrowheads) and reduced tail vessels (arrows). (G) Summary of in situ analysis, using ectopic expression of flk-1 to assess the extent of neovascularization in injected embryos. (H-J) In situ hybridization. (H) flk-1 expression in a wild-type embryo. (I) flk-1 expression in an embryo injected with 5 pg VEGF expression construct, labeling ectopic vessels (arrowheads). (J) flk-1 expression in an embryo coinjected with 5 pg VEGF-165 expression construct and syndecan-2 MO, demonstrating no induction of new vessels. (K) Summary of in situ analysis, assessing ectopic expression of flk-1. (L-N) In situ hybridization. (L) flk-1 expression in a wild-type embryo. (M) flk-1 expression in an embryo injected with a low dose of VEGF-165 DNA, indicating no induced new vessels. (N) flk-1 expression in an embryo coinjected with VEGF-165 DNA and syndecan-2 DNA with ectopic vessels (arrowheads). Original magnification, × 10 (A-B, H-J, L-N); × 5 (D-F). Error bars indicate SE.
Genetic interaction between VEGF and syndecan-2 in vivo. (A-B) In situ hybridization. (A) Cross section of a wild-type embryo at 22 hpf, showing VEGF-A expression in medial somitic mesoderm (purple staining within the area as indicated by the red dashed lines). DA indicates dorsal aorta. (B) Cross section of a wild-type embryo at 22 hpf, showing syndecan-2 expression in the mesenchyme (black dashed lines) surrounding the dorsal aorta, ventral to the somitic mesoderm. Note that the region of VEGF-A expression is adjacent to the mesenchyme where syndecan-2 is expressed. (C) Summary of microangiography analysis. Low doses of syndecan-2 MO and VEGF-A MO synergize to generate embryos with vascular defects (compare column 3 with columns 1 and 2). There is no interaction between VEGF-A MO and the 4-base mismatch syndecan-2 MO (d4). The total number of embryos analyzed from 2 independent experiments is indicated. (D-F) Microangiography analysis. (D) Microangiogram of a wild type at 48 hpf. (E) Microangiogram of an embryo coinjected with syndecan-2 MO and VEGF-A MO, showing aberrant sprouting of intersegmental vessels (arrowheads) and abnormal formation of blood vessels in the tail region (arrows). (F) Microangiogram of an embryo coinjected with syndecan-2 MO and VEGF-A MO with no trunk angiogenesis (arrowheads) and reduced tail vessels (arrows). (G) Summary of in situ analysis, using ectopic expression of flk-1 to assess the extent of neovascularization in injected embryos. (H-J) In situ hybridization. (H) flk-1 expression in a wild-type embryo. (I) flk-1 expression in an embryo injected with 5 pg VEGF expression construct, labeling ectopic vessels (arrowheads). (J) flk-1 expression in an embryo coinjected with 5 pg VEGF-165 expression construct and syndecan-2 MO, demonstrating no induction of new vessels. (K) Summary of in situ analysis, assessing ectopic expression of flk-1. (L-N) In situ hybridization. (L) flk-1 expression in a wild-type embryo. (M) flk-1 expression in an embryo injected with a low dose of VEGF-165 DNA, indicating no induced new vessels. (N) flk-1 expression in an embryo coinjected with VEGF-165 DNA and syndecan-2 DNA with ectopic vessels (arrowheads). Original magnification, × 10 (A-B, H-J, L-N); × 5 (D-F). Error bars indicate SE.
In humans, there are at least 6 splice isoforms of VEGF-A. All except for VEGF-121 contain a heparin-binding domain in the C-terminus.42 VEGF-121 and VEGF-165 are dominant forms expressed in zebrafish and humans.41-43 To investigate whether VEGF and syndecan-2 interact genetically in vivo, we first assessed the ability of VEGF-165 to induce ectopic vessels in syndecan-2 morphants. Overexpression of VEGF-165 expression construct resulted in the formation of ectopic vessels (Figure 6I).44 However, a significant decrease in the frequency of induced ectopic vessels was observed in embryos coinjected with VEGF-165 DNA and syndecan-2 MO, compared with embryos injected with VEGF-165 DNA alone (Figure 6G, compare column 2 with column 1; also compare Figure 6J with I). The introduction of a 4-base mismatch in the syndecan-2 MO abolished the inhibitory effect of syndecan-2 MO on the extent of neovascularization induced by VEGF-165 (Figure 6G, column 3), suggesting that the reduction in VEGF-165–induced ectopic vessel formation in syndecan-2 morphants is due to the specific loss of endogenous syndecan-2 function.
We then asked whether the presence of exogenous syndecan-2 can potentiate ectopic VEGF-165 signaling. At a low dose of VEGF-165 DNA, only a small fraction of injected embryos showed ectopic vessel formation (Figure 6K, column 1). Coinjection of syndecan-2 DNA and VEGF DNA resulted in a synergistic increase in the number of embryos showing ectopic vessels (Figure 6K, column 3). In contrast, coinjection of VEGF-165 and EGFP expression constructs resulted in no increase in ectopic vessel formation compared with those injected with the VEGF-165 DNA alone (Figure 6K, column 4). Our results indicate that ectopic syndecan-2 potentiates ectopic VEGF-165–induced neovascularization.
Discussion
In this report, we characterize the role of the zebrafish syndecan-2 orthologue in vascular development. Specific targeting of syndecan-2 by morpholinos and introduction of a putative dominant-negative form of syndecan-2 result in embryos showing defective sprouting of new blood vessels. The vascular function of syndecan-2 is likely conserved in vertebrates as mouse syndecan-2 is similarly expressed in cells surrounding the developing axial vessels, and human syndecan-2 can restore sprouting of new vessels in syndecan-2 morphants. We also show genetic interaction between syndecan-2 and VEGF-A in loss-of-function and gain-of-function studies in vivo.
The role of the cytoplasmic tail in syndecan-2–mediated angiogenesis
Our characterization of angiogenic defects of syndecan-2 morphants demonstrates the essential role of syndecan-2 in angiogenic sprouting. In contrast, forced expression of a cytoplasmically truncated form of syndecan-2 (dS2) produces angiogenic defects in embryos that phenocopy the loss-of-function morphant phenotypes. This result suggests a role for the cytoplasmic tail in syndecan-2 function during angiogenesis. All vertebrate syndecans contain highly conserved cytoplasmic domains. There are 2 regions, C1 and C2, identical across all syndecans, and a central variable region (V) is unique to each syndecan family member.45 The cytoplasmically truncated form of syndecan-2 (dS2) analyzed in our study lacks the C2 region and the part of the variable region containing the serine phosphorylation site, a substrate for protein kinase C (PKC) and protein kinase A (PKA).46 A previous study has shown that cells transfected with a similar form of truncated human syndecan-2 exhibit defective extracellular matrix assembly as evidenced by the inability of dS2-transfected cells to rearrange laminin or fibronectin substrates into fibrils.38 Other studies have shown that the C2 region of syndecan-2 binds to PDZ domain proteins such as calmodulin-dependent serine protein kinase (CASK) and syntenin, which are implicated in cytoskeletal assembly and organization of signaling complexes at cell-cell junctions.46,47 It is possible that the cytoplasmic tail mediates the function of syndecan-2 in angiogenesis by transducing signals from the extracellular matrix to an assembly of signaling and cytoskeletal components.
In addition to the role in extracellular matrix assembly, the cytoplasmic tail of syndecan-2 has also been shown to mediate the effects of a growth factor on cellular response. A previous study in Xenopus shows that dS2 blocks signaling mediated by Vg-1, a transforming growth factor β (TGF-β) signaling protein, in left-right development.30 dS2 may perturb growth factor–mediated response to inhibit angiogenic sprouting.
A potential role of syndecan-2 in growth factor–mediated signaling
Using both loss-of-function and gain-of-function studies in zebrafish, we show that VEGF-A and syndecan-2 interact in vivo in angiogenesis. Similar genetic approaches were taken by Tsuda et al14 to show the important role played by a Drosophila heparan sulfate proteoglycan, the glypican Dally, in Wingless (Wg) signaling. Loss-of-function mutant phenotypes of dally are similar to those observed in wg mutants. Partial loss-of-function mutations of dally compromises Wg-mediated events, and ectopic expression of dally potentiates Wg signaling.14
There are several possible mechanisms by which syndecan-2 could mediate growth factor signaling during angiogenesis. Many models have been proposed to describe heparan sulfate proteoglycan–growth factor interactions.48,49 Syndecan-2 may directly bind to VEGF, thereby regulating the distribution of VEGF near its receptor. Syndecan-2 could also act as a coreceptor, forming a signaling complex with VEGF and its receptor. Direct physical interaction between VEGF and syndecan-2 has not been shown, however. Consequently, it is also possible that VEGF and syndecan-2 function in distinct pathways during angiogenesis. Syndecan-2 may participate in signaling pathways mediated by other growth factors such as FGFs and thereby indirectly influence the outcome of VEGF-mediated signaling during angiogenesis. Future experiments will help elucidate the precise role of the interactions between syndecan-2 and VEGF or other potential growth factors during angiogenesis.
Clinical significance for the identification of syndecan-2 as a novel angiogenic factor
Our finding that syndecan-2 is essential for the process of angiogenic sprouting has clinical significance as the same process is perturbed in many diseases. In some models of tumor angiogenesis, the process of angiogenic sprouting of new blood vessels into the tumor enhances tumor growth and facilitates metastasis.1-3 Abnormal angiogenesis is also observed in other disease states such as atherosclerosis, inflammatory and infectious diseases, and retinopathy of prematurity.1 Our study shows the important role of syndecan-2 in angiogenesis, supporting syndecan-2 as a protein with potential applications for a variety of angiogenesis-based clinical therapies.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-06-1783.
Supported by National Institutes of Health (NIH) grants to E.C. and S.C.E. (GM55877 and GM63904).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Anna Petryk for providing us with mouse embryos for in situ experiments; Dr Leonard Zon for the tie-1, fli-1, and flk-1 plasmids; Dr Caroline Brennan for the ephrin-B2 plasmid; Dr Jaunian Chen for the RTK-5 plasmid; Dr Ruowen Ge for the VEGF-165 plasmid; Jessica Jandric for helping with probe synthesis; and Ann Davidson, Hyon Kim, Dr Michael Pickart, and Dr Saulius Sumanas for their comments on the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal