Abstract
Chronic immune thrombocytopenic purpura (ITP) is manifested by autoantibody-induced platelet destruction. Platelet turnover studies suggest that autoantibody may also affect platelet production. To evaluate this, we studied the effect of plasma from adult patients with chronic ITP on in vitro megakaryocyte production. CD34+ cells, obtained from healthy donors, were cultured in medium containing PEG-rHuMGDF and 10% plasma from either ITP patients or healthy subjects. Cultures containing plasma from 12 of 18 ITP patients showed a significant decrease (26%-95%) in megakaryocyte production when compared with control cultures. Positive ITP plasmas not only reduced the total number of megakaryocytes produced during the culture period but also inhibited megakaryocyte maturation, resulting in fewer 4N, 8N, and 16N cells. The role of antibody in this suppression is supported by 2 factors: (1) immunoglobulin G (IgG) from ITP patients inhibited megakaryocyte production when compared with control IgG; and (2) adsorption of autoantibody, using immobilized antigen, resulted in significantly less inhibition of megakaryocyte production when compared with unadsorbed plasma. These results show that plasma autoantibody from some adult patients with ITP inhibits in vitro megakaryocyte production, suggesting that a similar effect may occur in vivo.
Introduction
In 1950, Dr William Harrington was infused with blood from a patient with chronic immune thrombocytopenic purpura (ITP), resulting in the rapid onset of severe thrombocytopenia. He recovered within the next few days. Further studies by Harrington et al1 and Shulman et al2 showed clearly that patients with chronic ITP have a circulating factor capable of destroying homologous and autologous platelets. The severity of the thrombocytopenia was dose dependent, and the plasma factor could be adsorbed by platelets and was present in the immunoglobulin G (IgG)–rich fraction after ion-exchange chromatography. For the next several years, it was concluded that thrombocytopenia in patients with chronic ITP was caused solely by autoantibody-induced platelet destruction.
However, in the early 1980s, autologous platelet survival studies from several laboratories showed that in approximately two thirds of ITP patients, platelet turnover is either reduced or normal, not increased, as would be expected if platelet destruction were the only mechanism causing thrombocytpenia.3-6 Because megakaryocytes express GPIIb-IIIa and GPIb-IX on their surfaces during maturation7 and because most ITP autoantibodies react with one or both of these glycoprotein complexes,8,9 it follows that autoantibody binding to megakaryocytes could interfere with platelet production and release from the bone marrow either by causing intramedullary megakaryocyte or platelet destruction or by interfering with megakaryocyte maturation.
Recently, Chang et al10 evaluated the effect of plasma, from patients with childhood ITP (44 with acute ITP and 9 with chronic ITP), on thrombopoietin-induced production of megakaryocytes from cord blood cells in liquid culture. They noted that plasma from ITP patients with detectable antiplatelet antibodies suppressed the in vitro production of megakaryocytes from cord blood cells, whereas plasma from control subjects or ITP patients without demonstrable antibodies did not.
We have developed a similar culture system except that we use CD34+ cells, obtained by leukapheresis, which allows us to study individual patient antibodies because of the high percentage of megakaryocytes (65%-85%) resulting from this source of CD34+ cells. We describe our results on the study of plasma autoantibodies from patients with adult chronic ITP.
Patients, materials, and methods
These studies were approved by the Human Subjects Committee of the Scripps Clinic, Scripps Green Hospital, and The Scripps Research Institute.
Plasma preparation
Platelet-rich plasma was obtained from EDTA (ethylenediaminetetraacetic acid)–anticoagulated blood by centrifugation for 10 minutes at 250g, and plasma was obtained by centrifugation of the platelet-rich plasma for 15 minutes at 1100g. The plasma was stored at –70°C until used.
Patients
We studied plasma from 18 patients with chronic ITP, 1 patient with high-titer HLA antibodies that bound to the CD34+ cell donor platelets, and 28 control subjects. Clinical details of the ITP patients are shown in Table 1.
Patients with chronic ITP
. | Splenectomy Response . | Anti-GPIIb/IIIa* . | . | Anti-GPIb/IX* . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patient . | . | Plt-assoc . | Plasma . | Plt-assoc . | Plasma . | Medications . | ||
| ITP-1 | NR | 313.9 | 52 | 134.5 | 22.0 | IgG | ||
| ITP-2† | NR | ND | ND | ND | ND | SM | ||
| ITP-3 | NR | 194.8 | 28.0 | Neg | ND | Pred | ||
| ITP-4 | NR | 1210.8 | 287.0 | Neg | ND | Pred | ||
| ITP-5† | NR | 1109.0 | 53.0 | Neg | ND | Az, Dn, Pred | ||
| ITP-6† | NR | Neg | ND | 226.7 | 128.0 | None | ||
| ITP-7 | NR | 10.8 | Neg | 518.5 | 112.0 | Pred | ||
| ITP-8† | NR | 35.9 | 10.0 | 40.4 | 13.0 | None | ||
| ITP-9 | NR | 163.0 | 152.0 | Neg | ND | Pred | ||
| ITP-10 | NR | 104.1 | 24.0 | Neg | ND | None | ||
| ITP-11 | NR | 28.8 | Neg | 135.6 | 113.0 | Pred | ||
| ITP-12 | NR | 228.0 | 8.0 | 5.6 | ND | CSA, Pred | ||
| ITP-13 | CR | 347.1 | 53.0 | 10.4 | ND | Pred | ||
| ITP-14 | NR | 1310.0 | 130.0 | 13.0 | 13.0 | IgG | ||
| ITP-15† | NR | 24.3 | Neg | 415.5 | 18.0 | Vinc | ||
| ITP-16 | PR | 21.0 | Neg | 29.3 | 120.0 | Pred | ||
| ITP-17† | NR | 182.9 | 41.0 | 21.1 | Neg | SM | ||
| ITP-18 | NR | 80.8 | 79.0 | Neg | Neg | Az, Pred | ||
. | Splenectomy Response . | Anti-GPIIb/IIIa* . | . | Anti-GPIb/IX* . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patient . | . | Plt-assoc . | Plasma . | Plt-assoc . | Plasma . | Medications . | ||
| ITP-1 | NR | 313.9 | 52 | 134.5 | 22.0 | IgG | ||
| ITP-2† | NR | ND | ND | ND | ND | SM | ||
| ITP-3 | NR | 194.8 | 28.0 | Neg | ND | Pred | ||
| ITP-4 | NR | 1210.8 | 287.0 | Neg | ND | Pred | ||
| ITP-5† | NR | 1109.0 | 53.0 | Neg | ND | Az, Dn, Pred | ||
| ITP-6† | NR | Neg | ND | 226.7 | 128.0 | None | ||
| ITP-7 | NR | 10.8 | Neg | 518.5 | 112.0 | Pred | ||
| ITP-8† | NR | 35.9 | 10.0 | 40.4 | 13.0 | None | ||
| ITP-9 | NR | 163.0 | 152.0 | Neg | ND | Pred | ||
| ITP-10 | NR | 104.1 | 24.0 | Neg | ND | None | ||
| ITP-11 | NR | 28.8 | Neg | 135.6 | 113.0 | Pred | ||
| ITP-12 | NR | 228.0 | 8.0 | 5.6 | ND | CSA, Pred | ||
| ITP-13 | CR | 347.1 | 53.0 | 10.4 | ND | Pred | ||
| ITP-14 | NR | 1310.0 | 130.0 | 13.0 | 13.0 | IgG | ||
| ITP-15† | NR | 24.3 | Neg | 415.5 | 18.0 | Vinc | ||
| ITP-16 | PR | 21.0 | Neg | 29.3 | 120.0 | Pred | ||
| ITP-17† | NR | 182.9 | 41.0 | 21.1 | Neg | SM | ||
| ITP-18 | NR | 80.8 | 79.0 | Neg | Neg | Az, Pred | ||
Plasma of patients ITP-1 through ITP-12 suppressed in vitro megakaryocyte production; plasma of patients ITP-13 through ITP-18 did not. Plt-assoc, platelet-associated; NR, no response; ND, not done; CR, complete remission; SM, methylprednisolone (Solu-Medrol); Neg, negative; Pred, prednisone; Az, azathioprine; Dn, danazol; IgG, intravenous gamma globulin; CSA, cyclosporine; and Vinc, vincristine.
Polystyrene beads, coated with monoclonal anti-GPIIb or anti-GPIb and blocked with BSA, were incubated with washed, solubilized patient platelets (108) or with normal platelets (108) that had been incubated with 1.0 mL patient plasma, followed by washing and solubilization. After incubation and washing, bound autoantibody-GP immune complexes were detected with radiolabeled monoclonal antihuman IgG. Results are expressed as a ratio of cpm patient sample/mean cpm of 3 control samples. A ratio of more than 3.0 is considered positive.
Patient died of disease.
All ITP patients had severe disease, with platelet counts lower than 25 000/μL before therapy. All patients underwent splenectomy; 16 did not respond to surgery and required additional therapy. Six patients subsequently died of the disease. Seventeen patients had high levels of platelet-associated antibody and plasma antibody to GPIIb-IIIa or GPIb-IX, or both complexes. In 1 patient (ITP-2), antibodies were not measured. All but 3 patients received some form of therapy within the 4 weeks before the study: methylprednisolone (Solu-Medrol; Pharmacia & Upjohn, Bridgewater, NJ) or prednisone, 12 patients; IgG, 2 patients; azathioprine, 2 patients; danazol, 1 patient; cyclosporine, 1 patient, and vincristine, 1 patient.
IgG purification
IgG was purified from patient and control plasmas by affinity chromatography using the ImmunoPure IgG Purification Kit (Pierce, Rockford, IL) according to the manufacturer's instructions, and the IgG concentration was adjusted to 1.2 mg/mL. The final IgG preparations were dialyzed overnight with culture medium.
Adsorption of anti-GPIIb/IIIa plasma autoantibodies
Plasma was adsorbed twice with immobilized GPIIb-IIIa. For each adsorption, biotinylated murine monoclonal anti-GPIIb (2A9, 24 μg in 600 μL of 0.1 M sodium bicarbonate) was added to 250 μL avidin-Sepharose beads (Pierce) and incubated overnight at 4°C. After centrifugation for 5 minutes at 1000g, the beads were washed 3 times with phosphate-buffered saline (PBS) and were mixed with 1 mL normal platelet lysate (109 platelets per milliliter solubilized in 1% Triton X-100), as a source of GPIIb/IIIa. After overnight incubation at 4°C and washing 3 times with PBS and once with normal plasma, the immobilized GPIIb-IIIa was added to 500 μL patient or control plasma and was incubated overnight at 4°C followed by centrifugation at 1000g.
Liquid suspension cultures
CD34+-rich cells were obtained from 2 unstimulated, healthy donors of O Rh-positive blood by leukapheresis, followed by CD34+ cell selection (CellPro Inc.). The final CD34+ cell concentration was 30% to 35% of the total cells. Cultures were prepared in 24-well plates (Costar, Cambridge, MA). Each well contained 105 CD34+-rich cells, 100 μL heparinized patient or control plasma (2 U heparin/mL ultracentrifuged plasma at 20 000 rpm; Beckman L8-80), 5 ng megakaryocyte growth and development factor (PEG-rHuMGDF; Amgen, Thousand Oaks, CA), and 900 μL Iscove modified Dulbecco medium (Invitrogen, Grand Island, NY) supplemented as described by Mazur et al.11 Cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 for the desired time (usually 10 days). At harvesting, 111 μL prewarmed 10 × CATCH/prostaglandin E1 (PGE1) buffer (calcium/magnesium-free Hanks solution containing 3.8% sodium citrate, 10 mM adenosine, 20 mM theophylline, and 18 μg/mL PGE1) was added to each culture. After gentle mixing with a plastic pipette, cells were counted and flow cytometric analysis was performed.
Flow cytometric analysis
Each cell suspension was transferred to a separate polystyrene tube, and 20 μL murine fluorescein isothiocyanate (FITC)–labeled antihuman CD41 (Biodesign, Saco, ME) was added; FITC-labeled MOPC-21 (BD PharMingen, San Diego, CA) was used as an isotype control. Preliminary studies demonstrated that the plasma autoantibodies did not interfere with binding of the FITC-labeled anti-CD41. After incubation for 60 minutes on ice, the cells were centrifuged at 175g for 5 minutes and resuspended in 1 mL 1 × CATCH/PGE1 buffer.
Cells were analyzed with a Becton Dickinson FACScalibur cytometer equipped with a 15 mW argon ion laser emitting at 488 nM. For each experiment, 3000 to 10 000 cells were analyzed; electronic compensation was used to eliminate spectral overlap between the fluorochromes. Megakaryocytes were selected on the basis of the specific immunofluorescence at levels above that of cells labeled with the isotype control antibody.
To determine megakaryocyte ploidy distribution, cellular DNA was stained by modification of a previously described method.11 Briefly, 500 μL of each FITC-conjugated anti-CD41–labeled cell suspension was diluted 1:2 with hypotonic citrate buffer (0.37 g sodium citrate/L, 0.15 g sodium chloride/L, and 0.63 g dextrose/L; pH adjusted to 6.2), containing 20 μg/mL propidium iodide and 0.1% Triton-X100). After 15-minute incubation at 4°C, ploidy distribution was assessed by the intensity of the propidium iodide red fluorescence, as previously described.12
Statistics
Results from each patient's plasma were compared with the results of at least 3 (range, 3-10, depending on the experiment) duplicate control plasmas using the Student t test.
Results
Megakaryocyte production
Megakaryocytes were defined by fluorescence-activated cell sorter (FACS) analysis as CD41+ cells. The possibility that platelet-monocyte rosettes might be present and influence the results was ruled out by preliminary studies in which cultured cells were labeled with FITC–anti-CD41 and PE–anti-CD14 and then were examined for double-labeled cells. There was an absence of CD41+/CD14+ cells, indicating that no platelet-monocyte rosettes were present (data not shown).
Control plasma. The reproducibility of the culture system was evaluated by determining the variation in results among cultures containing stem cells from the same donor cultured for 10 days in 28 different plasmas from healthy subjects. The average percentage variations (± SD) from the mean control results were as follows: total cell count per well, 9.0% ± 6.8%; percentage megakaryocytes, 14.7% ± 11.1%; total megakaryocytes per well, 21.3% ± 15.9%.
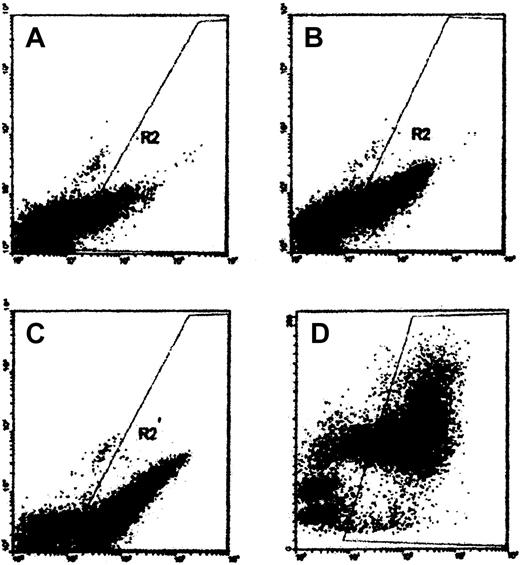
Figure 1 shows a representative series of cultures containing control plasma. Megakaryocytes were easily noted by day 5 and their numbers increased progressively on days 7 and 10. Most CD41+ cells, noted on day 10, were large, as determined by forward light scatter (right panel), consistent with megakaryocytes. FACS results were confirmed by direct observation of stained smears.
In vitro megakaryocyte production in normal plasma. CD34+-rich cells were cultured in media containing rHuMGDF and control plasma. Cultures were harvested on day 5 (A), day 7 (B), or day 10 (C-D). The abscissa shows data from FL-1H (FITC–anti-CD41), and the ordinate shows either FL-2H (phycoerythrin (PE)–anti-CD14) (A-C) or cell size, FSC (D). Cells within gate R2 are considered CD41+ based on the results of FITC–MOPC-21 control experiments.
In vitro megakaryocyte production in normal plasma. CD34+-rich cells were cultured in media containing rHuMGDF and control plasma. Cultures were harvested on day 5 (A), day 7 (B), or day 10 (C-D). The abscissa shows data from FL-1H (FITC–anti-CD41), and the ordinate shows either FL-2H (phycoerythrin (PE)–anti-CD14) (A-C) or cell size, FSC (D). Cells within gate R2 are considered CD41+ based on the results of FITC–MOPC-21 control experiments.
ITP plasma. Of the plasmas tested from 18 patients with chronic ITP, 12 significantly suppressed in vitro megakaryocyte production (Figure 2) but did not affect the numbers of non-megakaryocytic cells (FITC–anti-CD41– cells). The degree of suppression ranged from more than 90% to 26%. Serial dilutions of 1 positive plasma from an ITP patient (ITP-1) caused less suppression of megakaryocyte production as smaller quantities of ITP plasma were added to the cultures (Figure 3).
Suppression of megakaryocyte production by ITP plasma. CD34+-rich cells were cultured in media containing rHuMGDF and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.
Suppression of megakaryocyte production by ITP plasma. CD34+-rich cells were cultured in media containing rHuMGDF and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.
Suppression of megakaryocyte production by ITP plasma dilutions. CD34+-rich cells were cultured in media containing rHuMGDF and serially diluted plasma from patient ITP-1. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.
Suppression of megakaryocyte production by ITP plasma dilutions. CD34+-rich cells were cultured in media containing rHuMGDF and serially diluted plasma from patient ITP-1. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.
Because some ITP patients underwent transfusion, we evaluated the effect of anti-HLA antibodies on megakaryocyte production. We tested plasma from a patient with myelodysplasia (5q–) who had received more than 600 U of red blood cells and had high levels of anti-HLA antibodies against most HLA antigens, including those of the pheresis donor. This plasma did not suppress megakaryocyte production (data not shown).
Role of antibody
To evaluate the role of antibody in the suppression of megakaryocyte production, we studied the effects of patient and control IgG on in vitro megakaryocyte production and adsorption of autoantibody from patient plasma.
Effect of IgG. Cultures were prepared in the same manner as described earlier, except that 100 μL media containing 120 μg patient or control IgG was added. A significant reduction in megakaryocyte production was noted in cultures containing IgG from patients ITP-5 (P = .003) and ITP-6 (P = .006) when compared with cultures containing control IgG (Figure 4).
Suppression of megakaryocyte production by IgG from ITP plasma. Cultures contained CD34+ cells, rHuMGDF, and normal plasma, as described, except that purified IgG (100 μL of 1.2 mg/mL) from either ITP patients (ITP-5 or ITP-6) or control subjects was added. Total megakaryocytes were calculated as described in Figure 3. Results reflect the mean (± SD) of 6 separate cultures for each IgG. Control results were calculated as a group.
Suppression of megakaryocyte production by IgG from ITP plasma. Cultures contained CD34+ cells, rHuMGDF, and normal plasma, as described, except that purified IgG (100 μL of 1.2 mg/mL) from either ITP patients (ITP-5 or ITP-6) or control subjects was added. Total megakaryocytes were calculated as described in Figure 3. Results reflect the mean (± SD) of 6 separate cultures for each IgG. Control results were calculated as a group.
Effect of autoantibody adsorption. To further evaluate the role of antibody, we adsorbed plasma containing high-titer antibodies to GPIIb-IIIa with immobilized GPIIb-IIIa. The effect on antibody removal is shown in Figure 5. Significantly more megakaryocytes were present in cultures containing ITP plasma that had been adsorbed with immobilized GPIIb-IIIa when compared with cultures containing unadsorbed plasma. Adsorbed control plasmas gave similar results to the unadsorbed material.
Effect of autoantibody adsorption with immobilized GPIIb-IIIa. Biotinylated murine monoclonal anti-GPIIb (2A9), coupled to avidin-Sepharose beads, was incubated with normal platelet lysate to allow GPIIb-IIIa binding. After washing, test plasmas (patient plasmas containing anti-GPIIb/IIIa antibody [ITP-1, ITP-5] and control plasmas [Cont-1, Cont-2]) were incubated with 2 separate aliquots of the immobilized GPIIb-IIIa, followed by centrifugation. Adsorption removed more than 80% of the autoantibody, as demonstrated in binding assays. Unadsorbed and adsorbed (ads) plasmas were tested, and the total numbers of megakaryocytes present after 10 days were determined.
Effect of autoantibody adsorption with immobilized GPIIb-IIIa. Biotinylated murine monoclonal anti-GPIIb (2A9), coupled to avidin-Sepharose beads, was incubated with normal platelet lysate to allow GPIIb-IIIa binding. After washing, test plasmas (patient plasmas containing anti-GPIIb/IIIa antibody [ITP-1, ITP-5] and control plasmas [Cont-1, Cont-2]) were incubated with 2 separate aliquots of the immobilized GPIIb-IIIa, followed by centrifugation. Adsorption removed more than 80% of the autoantibody, as demonstrated in binding assays. Unadsorbed and adsorbed (ads) plasmas were tested, and the total numbers of megakaryocytes present after 10 days were determined.
Effect of ITP plasma on ploidy distribution
The effect of ITP plasma on ploidy distribution was studied in 6 plasmas that showed suppressed megakaryocyte production and 1 that did not; examples are shown in Figure 6 and Table 2. ITP plasmas (ITP-1 and ITP-5) that suppressed in vitro megakaryocyte production not only reduced the total number of megakaryocytes produced during the culture period but also impaired megakaryocyte maturation. This resulted in a reduction in the percentages of 4N, 8N, and 16N megakaryocytes. This effect became progressively less evident as plasma was serially diluted and was not seen when patient antibody was adsorbed with immobilized antigen (data not shown). By contrast, plasma from patient ITP-18, which did not show suppressed megakaryocyte production, had no effect on ploidy distribution.
Effect of plasma on megakaryocyte ploidy distribution. Histograms show the cell ploidy distribution of CD34+-rich cells cultured for 10 days in the following plasmas (left to right): control, ITP-1, ITP-5 and ITP-18. Cells cultured in control or ITP-18 plasma (no evident suppression of in vitro megakaryocyte production) show 4 distinct peaks, 2N, 4N, 8N, 16N, while cells cultured in ITP-1 or ITP-5 plasma (both suppressed in vitro megakaryocyte production) show primarily 2N cells.
Effect of plasma on megakaryocyte ploidy distribution. Histograms show the cell ploidy distribution of CD34+-rich cells cultured for 10 days in the following plasmas (left to right): control, ITP-1, ITP-5 and ITP-18. Cells cultured in control or ITP-18 plasma (no evident suppression of in vitro megakaryocyte production) show 4 distinct peaks, 2N, 4N, 8N, 16N, while cells cultured in ITP-1 or ITP-5 plasma (both suppressed in vitro megakaryocyte production) show primarily 2N cells.
Effect of ITP plasma on megakaryocyte ploidy distribution
. | Ploidy distribution, % ± SD . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Assay . | 2N . | 4N . | 8N . | 16N . | |||
| Day 5 | |||||||
| Control plasma | 39.5 ± 1.8 | 54.3 ± 2.3 | 5.0 ± 0.6 | 1.3 ± 0.2 | |||
| ITP-1 plasma | 86.2* | 12.9* | 0.9* | 0* | |||
| ITP-5 plasma | 66.5* | 31* | 2.2* | 0.4* | |||
| ITP-18 plasma | 51.9* | 43.6* | 3.7* | 0.9 | |||
| Day 7 | |||||||
| Control | 36.9 ± 7.0 | 49.9 ± 4.8 | 10.6 ± 1.8 | 2.4 ± 0.4 | |||
| ITP-1 | 90.4* | 22.8* | 0.5* | 0.1* | |||
| ITP-5 | 73.8* | 9.0* | 3.1* | 0.4* | |||
| ITP-18 | 52.3* | 39.0* | 7.3 | 1.4* | |||
| Day 10 | |||||||
| Control | 39.5 ± 4.5 | 42.7 ± 3.2 | 14.1 ± 1.5 | 3.2 ± 0.7 | |||
| ITP-1 | 85.8* | 9.7* | 0.4* | 0* | |||
| ITP-5 | 90.0* | 12.8* | 1.6* | 0.2* | |||
| ITP-18 | 44.5 | 40.4 | 12.3 | 1.4 | |||
. | Ploidy distribution, % ± SD . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| Assay . | 2N . | 4N . | 8N . | 16N . | |||
| Day 5 | |||||||
| Control plasma | 39.5 ± 1.8 | 54.3 ± 2.3 | 5.0 ± 0.6 | 1.3 ± 0.2 | |||
| ITP-1 plasma | 86.2* | 12.9* | 0.9* | 0* | |||
| ITP-5 plasma | 66.5* | 31* | 2.2* | 0.4* | |||
| ITP-18 plasma | 51.9* | 43.6* | 3.7* | 0.9 | |||
| Day 7 | |||||||
| Control | 36.9 ± 7.0 | 49.9 ± 4.8 | 10.6 ± 1.8 | 2.4 ± 0.4 | |||
| ITP-1 | 90.4* | 22.8* | 0.5* | 0.1* | |||
| ITP-5 | 73.8* | 9.0* | 3.1* | 0.4* | |||
| ITP-18 | 52.3* | 39.0* | 7.3 | 1.4* | |||
| Day 10 | |||||||
| Control | 39.5 ± 4.5 | 42.7 ± 3.2 | 14.1 ± 1.5 | 3.2 ± 0.7 | |||
| ITP-1 | 85.8* | 9.7* | 0.4* | 0* | |||
| ITP-5 | 90.0* | 12.8* | 1.6* | 0.2* | |||
| ITP-18 | 44.5 | 40.4 | 12.3 | 1.4 | |||
Significant at P < .05.
Discussion
In the 1950s and 1960s the studies of Harrington et al1 and Shulman et al2 showed that many ITP patients had plasma antibodies capable of destroying platelets in vivo. Following these studies, it was concluded that platelet destruction was the sole mechanism causing thrombocytopenia in patients with chronic ITP. However, autologous platelet survival studies in the 1980s, showing that most ITP patients had normal or decreased platelet turnover, suggested that platelet production in chronic ITP may also be impaired.3-6 This hypothesis is supported by early morphologic studies of ITP bone marrow showing normal or increased numbers of megakaryocytes with a shift to younger forms that lacked evidence of cytoplasmic granularity or platelet formation and manifested degenerative changes in the nucleus and cytoplasm.13,14 Subsequent studies, using electron microscopy, showed 50% to 75% of ITP megakaryocytes had extensive damage consisting primarily of abnormalities of the demarcation membrane system. In some cases, damaged cells showed attached monocytes, which appeared to phagocytose megakaryocyte fragments.15 It is not surprising that ITP autoantibody might affect platelet production because it has been well shown that the common ITP autoantigens, GPIIb-IIIa and GPIb-IX, are expressed on the surfaces of megakaryocytes7 and that ITP autoantibody binds to megakaryocytes.16,17
Attempts to use megakaryocyte colony formation to study thrombopoiesis in ITP have given contradictory results. Some groups, culturing ITP bone marrow, have found increased numbers of megakaryocyte colony-forming units (CFU-Ms),18,19 whereas others have found decreased numbers of CFU-Ms.20 Similarly, one group21 reported that ITP plasma did not affect CFU-M formation by normal cells, yet another group18 noted increased CFU-M formation in the presence of ITP serum.
Chang et al10 recently reported that plasma from some patients with childhood ITP suppresses in vitro megakaryocyte production. They cultured cord blood cells as a source of CD34+ cells, with a thrombopoietic growth factor and plasma from either control subjects or ITP patients. After 8 days of culture, approximately 16% of the cells were megakaryocytes. They compared the effect of plasma from the following groups: control plasma, antibody-positive ITP plasma (anti-GPIb, anti–GPIIb-GPIIIa, or both), and antibody-negative ITP plasma. They noted that the number of megakaryocytes at day 8 was significantly reduced in cultures containing anti-GPIb antibodies but that no significant suppression occurred in the presence of ITP antibody-negative plasma or ITP plasma containing only anti–GPIIb-IIIa antibody.
The present results in patients with adult chronic ITP are similar, in some ways, to those of Chang et al10 in childhood ITP, but there are also differences. Using cells from healthy subjects, obtained by leukapheresis and CD34+ cell selection, we show that the plasma from 12 of 18 patients with adult chronic ITP significantly suppressed in vitro megakaryocyte production. Unlike the results of Chang et al10 in childhood ITP, who concluded that only anti-GPIb antibodies cause suppression, we noted that suppression can be induced by plasma containing anti–GPIIb-IIIa, anti–GPIb-IX, or both antibodies. Why the apparent differences between these study findings? First, the patients in the Chang et al10 study were all children, and most had acute ITP (44 of 53), not chronic ITP (9 of 53). These 2 types of ITP almost certainly have different pathogenetic mechanisms. Second, Chang et al10 studied only 5 patients with antibodies to GPIIb-IIIa (2 chronic ITP, 3 acute ITP), and, as they acknowledged, they could not rule out the importance of antibodies to GPIIb-IIIa because of their small numbers. In addition, their 2 human monoclonal antibodies that inhibited megakaryocyte production in vitro were directed to the GPIIb-IIIa complex (2E7 against GPIIb and 5E7 against GPIIIa), which would support the importance of antibodies to this complex as well as those directed to GPIb-IX.
The importance of autoantibody in suppressing in vitro megakaryocyte production is supported by the suppression of megakaryocyte production by purified patient IgG and the reduced suppression after plasma adsorption with the specific autoantigen. The observation that some residual suppression of megakaryocyte production remained, despite 2 adsorptions with antigen (Figure 5), is best explained by residual antibody. It is difficult to remove all antibody by adsorption and, as shown in Figure 3, a 1:16 dilution of ITP-1 plasma (6.5% of undiluted antibody) still results in the significant suppression of megakaryocyte production. Although our studies support the role of autoantibody, it is possible that other factors in ITP plasma may contribute to this suppression of in vitro megakaryocyte production, such as differences in cytokine concentrations and quantities of transforming growth factor-β (TGF-β).
It would be interesting and informative to relate the quantity of plasma autoantibody that binds to megakaryocytes with the degree of in vitro suppression of megakaryocyte production and to determine at what level of megakaryocyte maturation autoantibody binding is most prominent. Unfortunately, we have been unable to quantitate plasma antibody binding to megakaryocytes for technical reasons. Because of the high IgG concentration in plasma, multiple washes are required to eliminate nonspecific IgG binding. Because of their fragility and tendency to clump, the megakaryocytes are either irreversibly clumped or are lysed by the time washing is complete, despite the use of additives to prevent clumping (eg, high bovine serum albumin (BSA) concentrations, PGE1).
Is the titer of autoantibody or the epitope the most critical factor in determining megakaryocyte suppression? As noted, we have no data on the degree of megakaryocyte sensitization with antibody, but all plasmas studied (those that suppressed and those that did not) had extremely high levels of antiplatelet antibody, inferring high levels of antibody binding to megakaryocytes. Because some plasmas suppress and others do not, it seems likely that epitope location is more important than antibody titer.
Antibodies causing suppression not only resulted in a decrease in the total number of megakaryocytes produced in vitro, they also inhibited the maturation of megakaryocytes into cells of higher ploidy. This effect was not seen in the studies of Chang et al,10 though this difference may be attributed to their earlier harvest time (8 days) and to the known slower in vitro maturation of megakaryocytes into cells of higher ploidy when cord blood is used as a source of CD34+ cells.22
Residual medication in the plasma of patients with ITP must be considered as a possible cause of the suppression of in vitro thrombopoiesis because most of our patients were taking some form of medication. This possibility seems unlikely, however, for several reasons: (1) 3 of the positive plasmas came from patients not on therapy; (2) most of the other positive plasmas were from patients receiving corticosteroids or intravenous gamma globulin, as were some of the negative plasmas; (3) one negative and one positive plasma were observed in patients receiving azathioprine; and (4) plasma adsorption with antigen probably would be unlikely to remove a medication.
Potential mechanism(s) involved in the suppression of megakaryocyte production that must be considered include antibody-induced destruction of megakaryocytes by phagocytic cells (the CD34+-rich populations from unstimulated donors contained significant numbers of phagocytic cells, particularly monocytes), autoantibody-induced activation of complement, induction of apoptosis (a recently published abstract described significant morphologic features, consistent with para-apoptosis, in 83% of megakaryocytes and 64% of megakaryoblasts from ITP marrows, whereas no such features were seen in marrow from healthy controls).19 Future studies will be directed toward evaluating these potential mechanisms.
In summary, plasma autoantibody from some adult patients with chronic ITP suppresses the in vitro production and maturation of megakaryocytes, suggesting that similar suppression may occur in vivo. These results also suggest that thrombocytopenia in adult patients with chronic ITP may result from the destruction of peripheral platelets and the suppression of platelet production.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-08-2672.
Supported by United States Public Health Service grants HL61809 and HL-66947.
J.N. and J.P. are employees of Amgen Inc, which donated the marrow growth and development factor (MGDF) used in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
This is publication number 15939-MEM from The Scripps Research Institute.

![Figure 2. Suppression of megakaryocyte production by ITP plasma. CD34+-rich cells were cultured in media containing rHuMGDF and either ITP or control plasma. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-08-2672/6/m_zh80040456710002.jpeg?Expires=1767699224&Signature=K7PO-fhbBtqRzVf5ENuZtKSmYNMDgAhWMpi7hbPiTbZXWll-A2EKBam3xmYjeEPrGE7mIY3Rcgw~CEOqOTw8-cd~e84ezHn14pWmzL9VsuaR9cTz8NreeBLjDK8UYAkzXHSqZPqt0iR1CfAEE8GkV239qADtpdKw57Hs5cKeGbYZ9D6JwzNtuM-sbhsg-QhAzrTKcvNOvDOCuJ6RxvL3ca~s2R5DGs5BTGiepalD-f~6spVju2hdYLzfs1fWdmNCRPs1jS-hC2A-V3on2letcFJ8qT5KwwkcvoBPLwMH6JS0Z5fflxk~mMAu~CwLfM~rxxE3e3tCJ5nUR~X619jPyg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Suppression of megakaryocyte production by ITP plasma dilutions. CD34+-rich cells were cultured in media containing rHuMGDF and serially diluted plasma from patient ITP-1. After harvesting the cultures on day 10, the total number of megakaryocytes was determined [cell number (determined by direct counting of each culture) × % megakaryocytes (determined from FACS data)]. % Control megakaryocytes = mean total megakaryocytes in ITP cultures/mean total megakaryocytes in control cultures × 100.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-08-2672/6/m_zh80040456710003.jpeg?Expires=1767699224&Signature=UacWi7Nvw62KjsQixUKJHm2sn~UxT6hVvet-mblwCIAL3GTyisPye4SciR5EBjF3pyaVZZMljMoXvKjmo9DEL1yWT1Xb4qhqoe66AkOee9kOruq-Jv~qCM9Y3jO8Pl4FBp9gBQgPvcj1eYXPKnRcKZg1I1hpHM4HrX3nnt962b1Fcoc5FmovvTOLzwrYE42dDrdbAnkt8REB-ACEDbumSJK4uxY9H02w3CrKF6a4pD9ptbYiymN3ymw22zqep6g9ck1f4TMYRYuXF5AviMQGhHaHTY2ag0eJ~b2YUk5YKyGd4ynTnKroXLMgWbf6QdCrHeYcg3yx9BpBrXjnW6K-4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Effect of autoantibody adsorption with immobilized GPIIb-IIIa. Biotinylated murine monoclonal anti-GPIIb (2A9), coupled to avidin-Sepharose beads, was incubated with normal platelet lysate to allow GPIIb-IIIa binding. After washing, test plasmas (patient plasmas containing anti-GPIIb/IIIa antibody [ITP-1, ITP-5] and control plasmas [Cont-1, Cont-2]) were incubated with 2 separate aliquots of the immobilized GPIIb-IIIa, followed by centrifugation. Adsorption removed more than 80% of the autoantibody, as demonstrated in binding assays. Unadsorbed and adsorbed (ads) plasmas were tested, and the total numbers of megakaryocytes present after 10 days were determined.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/4/10.1182_blood-2003-08-2672/6/m_zh80040456710005.jpeg?Expires=1767699224&Signature=IdGzdHV8rdz6lDecX0xYN9wl-1iPjSMza4c--iPxlE1FBrYc0nRcxSzAjxpMxwZjoBHGh8HqNIw5XXERU5W5GkoDfkWzzL0IrRGydkBDVRIseafrsVS7xJcnu62A9~9vk4Kl9~rzstuQxr9A64nipOhloptA37pc6XDm66CIFbTQZbJ-94CvIjSL8OJWKVTwcybYuuhQ~2pBQTqyb63sh3LqwSTBZY7dq1K20FccI0sqFgfgtvkG69CXssmhGRG4QXtohafshH7CLDmnLFhBuDc~oS6mFlh5yn7N20gVhe~4XUQNBUMuHKLeJP26UQycWVK8YhmZbCrA6X1AKN4pgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal