Cell adhesion is of vital importance for a number of cellular functions. Several molecular families of cell adhesion proteins are known, among which the most important are the integrins and their cellular and soluble ligands. Currently, 24 different integrins have been described. They are complex heterodimeric molecules and need activation to become adhesive. They are commonly grouped in subfamilies according to their β chains.
Blood cells express at least members of the β1, β2, β3, and β7 integrin families, but none are found on mature red cells. The primary cellular ligands for the leukocyte-specific β2 integrins are the intercellular adhesion molecules (ICAM); 5 have been described. Platelets are known to adhere through the αIIbβ3 integrin to fibrinogen. Among the ICAMs, ICAM-4 is unique due to its expression on erythroid cells. This molecule was originally described as a member of the LandsteinerWiener (LW) blood group of antigens, but subsequent cloning and adhesion assays showed that it is a member of the ICAM family of adhesion proteins.1 Its physiologic function has remained unknown, but it shows an interesting relationship to the Rh antigens.2 Thus, Rhnull cells lack the ICAM-4 molecule and they may be part of a large membrane protein complex. Whereas the other ICAMs specifically bind to β2 integrins, ICAM-4 has recently been found to interact with several types of integrins expressed on blood and endothelial cells.
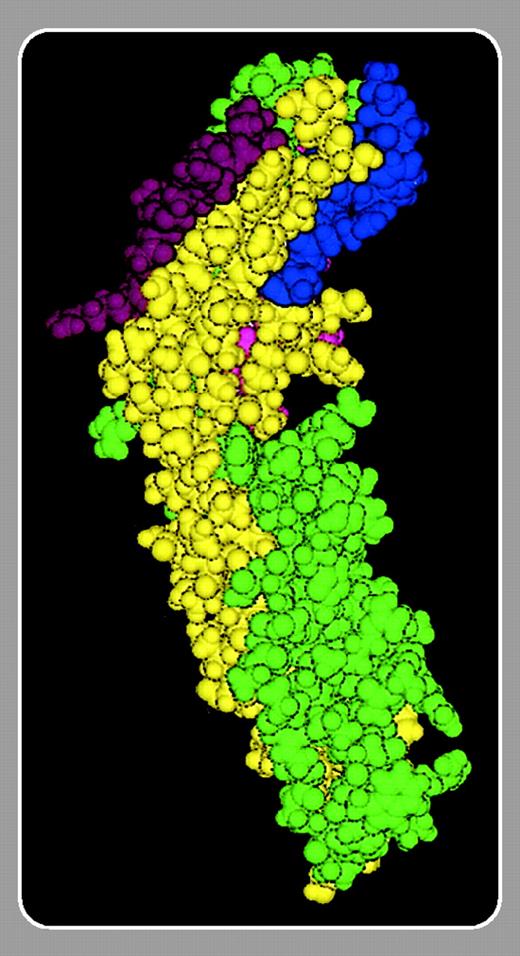
In this issue, Mankelow and colleagues (page 1503) have used mutational analysis to map the binding site on ICAM-4 for αV integrins and visualized the binding site using a structural model based on the structure of ICAM-2. Interestingly, the binding site is located in the first immunoglobulin (Ig) domain adjacent to but partially separated from those previously described for αLβ2 and αMβ2. The binding site for αLβ2 is also confined to the first Ig domain of ICAM-4, whereas the binding site for αMβ2 also involves domain 2.3 A relatively large binding site also seems to be the case for ICAM-4 binding to the platelet integrin αIIbβ3.4 The α4β1 integrin is known to bind to leucine–aspartic acid–valine (LDV) sequences and the binding site on ICAM-4 is evidently different from those of the other integrins. Based on the mutational studies, Mankelow et al synthesized peptides that inhibited the ICAM-4/αV-integrin interaction. The active peptides are clearly different from those previously described to interfere with ICAM/β2, α4β1,5 and αIIbβ3 interactions. The findings that red cells may specifically bind through ICAM-4 to integrins expressed on all major types of blood and endothelial cells indicate that it may be possible to develop reagents specific for the different ICAM-4 (red cell)/integrin interactions. Because of the pivotal role of red cells in human physiology and disease such reagents would be of great value.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal