Abstract
Relatively little is known about the modulators of the vascular endothelial growth factor A (VEGF-A)/Flk1 signaling cascade. To functionally characterize this pathway, VEGF-A stimulation of endothelial cells was performed. VEGF-A–mediated Flk1 activation resulted in increased translocation of the endogenous Fps/Fes cytoplasmic tyrosine kinase to the plasma membrane and increased tyrosine phosphorylation, suggesting a role for Fps/Fes in VEGF-A/Flk1 signaling events. Addition of a myristoylation consensus sequence to Fps/Fes resulted in VEGF-A–independent membrane localization of Fps/Fes in endothelial cells. Expression of the activated Fps/Fes protein in Flk1-deficient embryonic stem (ES) cells rescued their contribution to the developing vascular endothelium in vivo by using ES cell–derived chimeras. Activated Fps/Fes contributed to this rescue event by restoring the migratory potential to Flk1 null progenitors, which is required for movement of hemangioblasts from the primitive streak region into the yolk sac proper. Activated Fps/Fes in the presence of Flk1 increased the number of hemangioblast colonies in vitro and increased the number of mesodermal progenitors in vivo. These results suggest that Fps/Fes may act synergistically with Flk1 to modulate hemangioblast differentiation into the endothelium. We have also demonstrated that activated Fps/Fes causes hemangioma formation in vivo, independently of Flk1, as a result of increasing vascular progenitor density.
Introduction
Mesodermal progenitors known as hemangioblasts give rise to the blood islands of the yolk sac.1 Blood islands consist of elongated peripheral endothelial precursor cells called angioblasts that surround more centrally localized hematopoietic progenitors or hemoblasts.1 The coalescence of these blood islands to form primitive vascular channels is termed vasculogenesis.1 Concomitant with this initial plexus formation, the vascular endothelium proliferates and sprouts to give rise to new vessels in a process termed angiogenesis.1 These processes are repeated in the embryo proper. The immature vessels undergo further remodeling and maturational events that include the recruitment of smooth muscle cells and the development of supporting basement membranes.2-4
Vascular endothelial growth factor A (VEGF-A) signaling has been implicated in virtually all aspects of cardiovascular system formation, including heart development, hematopoiesis, vasculogenesis, angiogenesis, and endothelial survival.5,6 VEGF-A binds to 2 receptors, Flk1 (kinase domain receptor [KDR] or VEGFR-2) and Flt1 (VEGFR-1), that are members of a family of receptor tyrosine kinases that also includes the platelet-derived growth factor (PDGF) and macrophage colony-stimulating factor (M-CSF) receptors.7 Studies aimed at finding signaling molecules that act downstream of Flk1 have identified several src homology 2 (SH2) domain–containing proteins that either interact directly with this receptor and/or become tyrosine phosphorylated on VEGF-A stimulation. These putative substrates and downstream affecter molecules include the adapter protein Nck, direct p21Ras modulator proteins (Shc, Grb2, and p120GAP), phospholipase C σ (PLCσ), members of the Src family of cytoplasmic tyrosine kinases, focal adhesion kinase (FAK), and the tyrosine phosphatases SHP1 and SHP2.8-12 As a result of these interactions, mitogen-activated protein (MAP)–kinase and phosphatidylinositol 3 (PI3)–kinase-Akt pathways become activated and are thought to be involved in regulating endothelial proliferation/differentiation, survival, and cell motility.7-10,13
The essential nature of VEGF-A and its receptors in cardiovascular development has been demonstrated by gene targeting studies in the mouse. Targeted deletion of a single VEGF-A allele resulted in haploinsufficiency and embryonic lethality because of abnormal blood vessel development around embryo day (E) 9 to 9.5.14,15 Flk1 receptor null embryos died at E8.5 as a result of a failure of mesodermal precursor cells to differentiate into functional vascular endothelial cells, endocardium, and hematopoietic cells.16
To further understand the role of Flk1 in vasculogenesis and hematopoiesis, the developmental potential of Flk1 null embryonic stem (ES) cells was studied in an ES cell-chimera approach.17 These chimeras showed that Flk1 signaling was essential for the migration of mesodermal progenitor cells from the primitive streak region to the yolk sac, where they form the blood islands.17 This in vivo work has been substantiated by in vitro differentiation studies, showing that Flk1 null ES cells could differentiate into hematopoietic and endothelial cells in vitro, although at a reduced frequency.18,19 Hematopoietic/endothelial progenitors, therefore, require Flk1 signaling for their subsequent migration and expansion and not for their initial development/differentiation.
Aberrant VEGF-A/Flk1 signaling has also been implicated in pathologic conditions, including proliferative retinopathies, tumorigenesis, rheumatoid arthritis, psoriasis, as well as vascular lesions such as hemangiomas that are the most common tumors of infancy.20,21 Recently, specific mutations have been found in 2 VEGF-A receptors (KDR and Flt 4) in human hemangiomas.22
A mouse model for hemangioma formation that relied on transgenic expression of an activated form of Fps/Fes cytoplasmic tyrosine kinase (hereafter referred to as Fps) was previously reported.23 Activation of the Fps kinase was achieved by incorporating coding sequences for a Src-like amino-terminal myristoylation consensus sequence into the human fps transgene (fpsMF). The resulting tissue-specific expression of myristoylated Fps (MFps) caused widespread hypervascularity, progressing to multifocal hemangiomas.23 The fps proto-oncogene encodes a 92-kDa cytoplasmic protein tyrosine kinase that belongs to a unique tyrosine kinase subfamily composed of itself and Fer kinase (for reviews see Smithgall et al24 and Greer25 ). The human Fps protein tyrosine kinase is 822 amino acids in length and consists of a unique amino-terminal half, followed by an SH2 and carboxyl-terminal kinase domain.24,25 Fps is expressed at high levels in cells of the myeloid lineages and has been implicated in signaling pathways downstream from several members of the cytokine receptor superfamily.26-30 Fps has also been implicated in the regulation of survival and differentiation of granulomonocytic progenitor cells.31,32 Fps is also expressed at high levels in vascular endothelial cells23,33,34 where it might regulate FGF-2–induced chemotaxis of murine brain capillary endothelial cells35 and Angiopoietin-2–induced endothelial migration and tube formation.36
The expression of fps in angioblast progenitors33 and its ability to selectively transform the vascular endothelium23,34 has prompted us to investigate whether this kinase may also play a role in VEGF-A/Flk1 signaling. Here, we show that cellular Fps undergoes increased plasma membrane localization and exhibits increased tyrosine phosphorylation on VEGF-A stimulation of endothelial cells in vitro. An activated, plasma membrane localized MFps can functionally substitute for direct signaling events downstream of Flk1 and/or complement parallel pathways responsible for endothelial formation in vivo.
Materials and methods
Immunofluorescence, antiphosphotyrosine blots, and Western analysis
The eEND.237 and c166 endothelial cells34 were maintained as previously described.34,38 The c166 cells were derived from yolk sacs of fpsMF transgenic mice, and they overexpress the MFps.23,34 Prior to immunofluorescence and Western analysis the eEND.2 cells were starved in Dulbecco modified Eagle medium (DMEM) with 0.5% fetal calf serum (FCS) for 48 hours, followed by a 5-minute stimulation with 10 ng/mL recombinant VEGF-A at 37°C (R&D, Vienna, Austria). Immunofluorescence analysis of cells was performed as previously described33 by using the FpsQE primary rabbit polyclonal antibody33 and a cy3-conjugated antirabbit secondary antibody (Becton Dickinson Immunocytochemistry Systems, San Jose, CA). Western analysis and antiphosphotyrosine blots were performed as described previously9,33 by using the PY20 antiphosphotyrosine antibody (Transduction Laboratories, Lexington, KY). Protein concentrations from ES cell lysates were determined by using the BCA protein assay kit (Pierce, Rockford, IL). Fps protein levels from Western blots were standardized against β-actin levels by using a monoclonal antibody raised against β-actin (Sigma, St Louis, MO).
ES cell culture and generation of MFps-expressing ES clones
The Flk1-lacZ16 ES cells were maintained as previously described.39 The Flk1-green fluorescent protein (GFP)–targeted R1 cells were generated by using an essentially identical targeting strategy as was performed previously to knock lacZ in-frame into the start codon of exon 1 in the Flk1 genomic locus.16 The PCAGI-puro chicken β-actin promoter-based construct used to overexpress MFps was provided by Dr Austin Smith (Edinburgh, Scotland). The complementary DNA (cDNA) encoding myristoylated Fps (MFps) was cloned upstream of the internal ribosome entry site (IRES)–Puro selectable cassette as an EcoR1 fragment. For selection of MFps-expressing puromycin-resistant R1 ES cell clones, 1 × 107 cells were electroporated at 260 V and 500 μF with a gene pulser (Biorad, Hercules, CA). Cells were then plated at a density of 2 × 106 per 10-cm dish. Selection was started 24 to 48 hours after electroporation with 1.25 μg/mL puromycin (Sigma) for a week. ES cell clones were expanded and analyzed by Western blot analysis.
Embryoid body formation and hemangioblast colony assays
For in vitro differentiation of ES cells into embryoid bodies, subconfluent R1 cells were trypsinized, and 5 × 106 cells were seeded into Petri dishes (VWR International, West Chester, PA) containing 10 mL ES cell media without leukemia inhibitory factor (LIF) and 20% fetal bovine serum (FBS), 1 mM monothioglycerol instead of β-mercaptoethanol. Flk1-GFP cells were differentiated for 8 days, then lightly trypsinized and plated on gelatin-coated tissue culture plates and allowed to grow for an additional 4 days. Randomly selected fields were chosen for analysis, and representative images are shown. Blast colony assays were performed as previously described.40 Briefly, day 2.75 embryoid bodies were trypsinized in prewarmed 0.25% trypsin and 1.1 mM EDTA (ethylenediaminetetraacetic acid) for 3 minutes and passed 4 times through a 20-gauge needle to make a single-cell suspension. Four thousand cells were replated in methylcellulose (55%) medium containing preselected 10% FBS, ascorbic acid (0.25%), l-glutamine (2 mM), transferrin (1%), monothioglycerol (MTG), D4T-conditioned media (15%), VEGF-A (5 ng/mL), and c-kit ligand (KL; 1%). Blast colonies were counted after 4 days. Two separate ES cell clones per genotype were plated in triplicate wells. The average of 2 separate set of triplicate experiments is presented.
Flow cytometry analysis
Single-cell suspensions were generated by dissociation of E11.5 embryos and yolk sacs by treatment with 0.08% dispase/collagenase solution (Boehringer-Mannheim, Indianapolis, IN) in 20% FBS for 2 hours. Samples were prepared by pooling cells obtained from 3 separate nonchimeric, Flk1GFP/GFP null, or MFps Flk1GFP/GFP null chimeric embryos or yolk sacs. Samples were incubated with a phycoerythrin (PE)–conjugated anti-PECAM (platelet endothelial cell adhesion molecule) antibody (Pharmingen, San Diego, CA), PE-conjugated c-Kit antibody (Pharmingen), or a PE-conjugated nonspecific antirabbit secondary antibody (Pharmingen) for 15 minutes at 4°C in the dark. Cells were washed twice with Hanks buffered saline salt (HBSS) containing 1% FBS, strained with a 40-μm nylon mesh before analysis on an Epics cell sorter.
Generation of mice by ES cell aggregation with diploid and tetraploid embryos
Diploid embryo-ES cell chimeras were generated by injecting C57/BL6 blastocysts with Flk1lacZ/+ heterozygous or Flk1lacZ/lacZ null R1 ES, and Flk1GFP/+- or Flk1GFP/GFP-derived chimeras were generated by ES cell aggregation with diploid host embryos as previously described.41 Chimeras were either dissected at E11.5 to 12.5 or were allowed to develop to term. Completely ES cell–derived embryos were generated by aggregation of ES cell lines with tetraploid embryos as described39 and dissected at E7.0 to 7.5.
Histology, X-gal staining, and GFP visualization
Flk1lacZ ES cell–derived chimeric embryos and yolk sacs were dissected in ice-cold phosphate-buffered saline (PBS) and stained with X-gal as previously described.16 Hemangioma samples and X-gal–stained embryos were fixed overnight at 4°C in 4% paraformaldehyde (PFA). Samples for histologic analysis were dehydrated through a graded alcohol series and embedded in paraffin wax as previously described.42 Sections (7 μm) were deparaffinized and either stained with Harris hematoxylin and/or eosin. Flk1lacZ ES cell–derived embryoid bodies were X-gal stained as previously described.43 Flk1GFP ES cell–derived embryos and dispersed embryoid bodies were visualized as described.44
Results
Wild-type Fps protein is responsive to VEGF-A–induced signaling
To determine if Fps is a downstream target of Flk1, biochemical experiments were performed by using middle-T–transformed endothelial cell lines (Figure 1A-F). Middle-T–transformed endothelioma cells have been shown previously to express Flk1.45 The eEND.2 cell line, derived from an embryonic hemangioma,46 was starved and stimulated with recombinant VEGF-A protein. Fps localization in these cells was analyzed by indirect immunofluorescence. eEND.2 cells were incubated with a pre-immune rabbit serum and showed only low levels of background fluorescence (Figure 1A). With the use of the FpsQE rabbit polyclonal antibody,33 Fps protein was localized to vesicle-like structures in the cytoplasm of unstimulated cells (Figure 1B), as previously reported in several other cell types.33,47 An increased amount of Fps was observed at the plasma membrane following VEGF-A stimulation (arrows in Figure 1C-D). To determine whether VEGF-A stimulation led to increased Fps activation, eEND.2 cells were starved and then lysed before and after VEGF-A stimulation. Cell lysates were immunoprecipitated with the polyclonal FpsQE antibody followed by Western blot analysis with a monoclonal antiphosphotyrosine antibody to determine how VEGF-A stimulation changed the phosphorylation status of Fps protein. A 92- to 94-kDa phosphorylated protein was found to be present in the FpsQE immunoprecipitates following VEGF stimulation but not in unstimulated lysates (Figure 1E). This blot was stripped and reprobed with the FpsQE antibody to demonstrate that Fps protein was immunoprecipitated in both starved and stimulated eEND.2 lysates (Figure 1F).
Endogenous cellular Fps protein is responsive to VEGF-A–induced signaling. (A-D) Indirect immunofluorescence analysis of eEND.2 endothelial cell lines. Original magnification × 1000. (A) Control eEND.2 cells were incubated with pre-immune serum, whereas cells in panels B-D were incubated with the FpsQE antibody. (B) Unstimulated eEND2 cells show Fps protein localized to vesicular and cytoskeletal compartments of the cytoplasm. (C-D) VEGF-A–stimulated eEND.2 cells showed increased plasma membrane localization of endogenous Fps protein. (E) Antiphosphotyrosine immunoblot (IB) of FpsQE immunoprecipitates (IPs) performed on eEND.2 whole cell lysates show increased Fps tyrosine phosphorylation on VEGF-A stimulation (+ lane in E). Immunoblots were striped and reprobed to demonstrate the presence of Fps protein in stimulated and unstimulated Fps IPs (F). (G) Unstimulated c166 cells demonstrated increased membrane localization of the MFps protein. (H) VEGF-A stimulation of c166 cells does not lead to further relocalization of MFps protein to the plasma membrane. Original magnification × 630 (G-H).
Endogenous cellular Fps protein is responsive to VEGF-A–induced signaling. (A-D) Indirect immunofluorescence analysis of eEND.2 endothelial cell lines. Original magnification × 1000. (A) Control eEND.2 cells were incubated with pre-immune serum, whereas cells in panels B-D were incubated with the FpsQE antibody. (B) Unstimulated eEND2 cells show Fps protein localized to vesicular and cytoskeletal compartments of the cytoplasm. (C-D) VEGF-A–stimulated eEND.2 cells showed increased plasma membrane localization of endogenous Fps protein. (E) Antiphosphotyrosine immunoblot (IB) of FpsQE immunoprecipitates (IPs) performed on eEND.2 whole cell lysates show increased Fps tyrosine phosphorylation on VEGF-A stimulation (+ lane in E). Immunoblots were striped and reprobed to demonstrate the presence of Fps protein in stimulated and unstimulated Fps IPs (F). (G) Unstimulated c166 cells demonstrated increased membrane localization of the MFps protein. (H) VEGF-A stimulation of c166 cells does not lead to further relocalization of MFps protein to the plasma membrane. Original magnification × 630 (G-H).
As a positive control for these stimulation studies the c166 murine endothelial cell line was used.23,34 This cell line was previously derived from the yolk sacs of fpsMF transgenic mice.23,34 The Src-like myristoylation sequence directs cotranslational fatty modification of the MFps protein, which targets it to membrane-enriched cellular compartments and increases its kinase activity, apparently as a result of its constitutive plasma membrane localisation.23,48 As a result, VEGF-A stimulation of c166 cells did not result in further observable increases in Fps plasma membrane relocalization (Figure 1G-H), as was demonstrated in the case of VEGF-A–stimulated eEND2 cells (Figure 1C-D).
On the basis of these observations, we hypothesized that Fps might be a downstream mediator of Flk1 signaling which is normally recruited and activated by the VEGF-A–engaged receptor. The apparent constitutive plasma membrane localization of MFps suggested that activation of MFps might be VEGF-A–independent, and that it might, therefore, be able to rescue certain hematopoietic and/or endothelial differentiation defects in a Flk1 null genetic background. In vivo genetic complementation experiments were, therefore, performed to obtain further support for our hypothesis that Fps is a downstream modulator of VEGF-A/Flk1 signaling.
Activated Fps restored migration and sprouting response to Flk1 null progenitors in vitro
A panel of MFps-expressing R1 ES cell clones was generated which was either heterozygous or homozygous null for Flk1. The Flk1 null allele was obtained by the in-frame targeting of lacZ16 or enhanced green fluorescence protein (EGFP) into the Flk1 genomic locus. The fpsMF transgene used in these experiments consisted of a full-length fps cDNA construct that contained the Src-like myristoylation sequence under the transcriptional control of the ubiquitously expressed chicken β-actin promoter together with an IRES-puromycin bi-cistronic selectable marker (Figure 2A). Of the 11 MFps-expressing Flk1lacZ/lacZ null ES clones selected after electroporation of the fpsMF construct, 10 were found to express the MFps protein at 2- to 3-fold higher levels than the endogenous Fps protein in control puromycin-resistant Flk1lacZ/lacZ cells (Figure 2B). Several parental Flk1lacZ/+ (Figure 2C-D), control puromycin-resistant Flk1lacZ/lacZ null (Figure 2F-G), and MFps-expressing Flk1lacZ/lacZ null (Figure 2I-J) ES clones were differentiated in vitro into embryoid bodies (EBs) and were X-gal stained and analyzed for vascular channel formation after 8 days of differentiation (Figure 2C,D,F,G,I,J). Similarly, Flk1GFP/+ (Figure 2E), Flk1GFP/GFP (Figure 2H), and MFps-expressing Flk1GFP/GFP ES clones (Figure 3K) were differentiated for 8 days, lightly trypsinized, and replated on gelatin-coated tissue culture dishes. These cells were allowed to grow for a further 4 days in culture (Figure 2E,H,K). GFP-expressing cells were visualized by using direct fluorescence microscopy. Control Flk1lacZ/+ ES cells formed X-gal–positive endothelial cells in embryoid bodies that exhibited sprouting behavior, motility, and vascular channel formation by day 8 (arrows in Figure 2C-D). GFP-positive vascular endothelial cells derived from Flk1GFP/+ embryoid bodies formed extensive vascular channels and demonstrated a high degree of sprouting angiogenic activity (arrow in Figure 3F). Flk1lacZ/lacZ EBs showed few X-gal–positive cells localized to limited areas with no signs of migratory activity after 8 days of differentiation (Figure 2F-G). Similarly, GFP-positive cells derived from Flk1GFP/GFP ES cells showed no sprouting activity or channel formation (arrowheads in Figure 2H). MFps-expressing Flk1lacZ/lacZ EBs showed a higher density of X-gal–stained cells than the Flk1lacZ/lacZ EBs (asterisk in Figure 2I and data not shown). In addition, the MFps-expressing Flk1GFP/GFP EBs formed some channel-like structures, whereas Flk1GFP/GFP EBs did not (Figure 2K). However, the number of sprouts and extent of branching was not as great as that observed in the Flk1GFP/+ EB-derived controls (Figure 2E). In addition, morphologically aberrant sprouting structures were also evident in the MFps-expressing Flk1GFP/GFP EB-derived samples (asterisk in Figure 2K). To confirm that endothelial cells were in fact present in the Flk1 null ES-derived cell populations, a separate PECAM immunofluorescence analysis was performed and detected PECAM-positive cells present in both MFps-expressing and nonexpressing Flk1 null EB cell–derived populations (Figure 2M-N). MFps-expressing, Flk1 null-derived PECAM-positive cells, however, showed greater protrusive activity (arrows in Figure 2N) than the more rounded PECAM-positive cells derived from the Flk1 null ES cells (Figure 2M).
Expression of the MFps protein partially restores migration and sprouting response of Flk1 null progenitors in vitro. (A) Schematic depiction of chicken–β-actin promoter-based construct used to overexpress the fpsMF cDNA in ES cells. (B) Western blot analysis of Flk1lacZ/lacZ null ES cell clones demonstrated a 2- to 3-fold increase in MFps in 10 of 11 puro-resistant clones compared with endogenous wild-type Fps protein levels in Flk1 null clones transfected with the empty puromycin construct (protein concentrations were standardized against β-actin levels [lower blot in B]). (C-D) Flk1lacZ/+ embryoid bodies showed endothelial migration and vascular channel formation, indicated by arrows. (E) Flk1GFP/+ embryoid bodies demonstrated angiogenic sprouting activity, indicated by arrows. (F-G) Flk1lacZ/lacZ embryoid bodies demonstrated dramatically reduced numbers of X-gal–stained cells and little migration or sprouting (arrowheads). (H) Similarly, Flk1GFP/GFP embryoid body–derived cells showed little EGFP expression and no migration or sprouting activity (arrowheads). (I-J) MFps-expressing Flk1lacZ/lacZ embryoid bodies showed an increased number of X-gal–stained cells (asterisk in I) and increased sprouting activity (arrow in J). (K) MFps-expressing Flk1GFP/GFP embryoid body–derived cells displayed increased EGFP expression, increased sprouting (arrow), and evidence of aberrant cellular morphology (asterisk). (L) Control Flk1lacZ/lacZ null EB-derived cells incubated with no primary antibody. Indirect PECAM immunofluorescence staining of Flk1lacZ/lacZ EB-derived cells (M) and MFps-expressing, Flk1lacZ/lacZ EB-derived cells (N).
Expression of the MFps protein partially restores migration and sprouting response of Flk1 null progenitors in vitro. (A) Schematic depiction of chicken–β-actin promoter-based construct used to overexpress the fpsMF cDNA in ES cells. (B) Western blot analysis of Flk1lacZ/lacZ null ES cell clones demonstrated a 2- to 3-fold increase in MFps in 10 of 11 puro-resistant clones compared with endogenous wild-type Fps protein levels in Flk1 null clones transfected with the empty puromycin construct (protein concentrations were standardized against β-actin levels [lower blot in B]). (C-D) Flk1lacZ/+ embryoid bodies showed endothelial migration and vascular channel formation, indicated by arrows. (E) Flk1GFP/+ embryoid bodies demonstrated angiogenic sprouting activity, indicated by arrows. (F-G) Flk1lacZ/lacZ embryoid bodies demonstrated dramatically reduced numbers of X-gal–stained cells and little migration or sprouting (arrowheads). (H) Similarly, Flk1GFP/GFP embryoid body–derived cells showed little EGFP expression and no migration or sprouting activity (arrowheads). (I-J) MFps-expressing Flk1lacZ/lacZ embryoid bodies showed an increased number of X-gal–stained cells (asterisk in I) and increased sprouting activity (arrow in J). (K) MFps-expressing Flk1GFP/GFP embryoid body–derived cells displayed increased EGFP expression, increased sprouting (arrow), and evidence of aberrant cellular morphology (asterisk). (L) Control Flk1lacZ/lacZ null EB-derived cells incubated with no primary antibody. Indirect PECAM immunofluorescence staining of Flk1lacZ/lacZ EB-derived cells (M) and MFps-expressing, Flk1lacZ/lacZ EB-derived cells (N).
Chimeric analysis of Flk1lacZ/+ and Flk1lacZ/lacZ ES cells expressing the MFps protein. (A) E12.5 chimeric embryo containing Flk1lacZ/+ ES-derived cells showing X-gal–stained vasculature throughout. (B) E12.5 chimeric embryo-derived from Flk1lacZ/lacZ ES cells shows a lack of Flk1 null (X-gal stained) cell contribution to the vasculature. (C-D) Low-degree chimeric contribution of MFps-expressing; Flk1lacZ/+ ES cells leads to normal vascular channel formation (arrows in D point to superficial abdominal and limb vasculature). (E-F) High-degree chimeric contribution of MFps-expressing; Flk1lacZ/+ ES cells leads to aberrant vascular development in the yolk sac and vascular hemorrhage (arrowhead in F). (G-H) Hemangioma isolated from the small intestine of a 3-week-old MFps-expressing Flk1lacZ/+ chimeric adolescent (arrowhead in G). Higher-power magnification of the hemangioma revealed extensive hemorrhaging and hypervascularity in the surrounding tissue of the intestine (arrow in H). (I-K) Low-degree chimera contribution of MFps-expressing Flk1lacZ/lacZ ES cells rescued vascular contribution (arrows) to intraembryonic (I) and yolk sac sites (J-K). X-gal–staining MFps-expressing; Flk1lacZ/lacZ-derived endothelial cells were found in intersomitic vessels in the embryo (arrow in I) and in morphologically normal yolk sac blood vessels (arrow in J). Histologic analysis shows X-gal–stained endothelial cells surrounding hematopoietic cells in the yolk sac vasculature (arrow in K). (L-N) High-degree chimera contribution of MFps-expressing Flk1lacZ/lacZ ES cells lead to aberrant vascular development and hemangioma formation in the yolk sac (L-M). (N) Histologic analysis of the hemangioma seen in panel M demonstrated the MFps-expressing; Flk1lacZ/lacZ null endothelial cells lining the blood filled hemangioma cavity (arrows in M-N). Panels G and H were stained by hematoxylin and eosin; panels K and N were stained by eosin. Original magnification × 8 (A-C, E); × 12 (D, F, L); × 20 (I, J, M); and × 200 (G, H, K, N).
Chimeric analysis of Flk1lacZ/+ and Flk1lacZ/lacZ ES cells expressing the MFps protein. (A) E12.5 chimeric embryo containing Flk1lacZ/+ ES-derived cells showing X-gal–stained vasculature throughout. (B) E12.5 chimeric embryo-derived from Flk1lacZ/lacZ ES cells shows a lack of Flk1 null (X-gal stained) cell contribution to the vasculature. (C-D) Low-degree chimeric contribution of MFps-expressing; Flk1lacZ/+ ES cells leads to normal vascular channel formation (arrows in D point to superficial abdominal and limb vasculature). (E-F) High-degree chimeric contribution of MFps-expressing; Flk1lacZ/+ ES cells leads to aberrant vascular development in the yolk sac and vascular hemorrhage (arrowhead in F). (G-H) Hemangioma isolated from the small intestine of a 3-week-old MFps-expressing Flk1lacZ/+ chimeric adolescent (arrowhead in G). Higher-power magnification of the hemangioma revealed extensive hemorrhaging and hypervascularity in the surrounding tissue of the intestine (arrow in H). (I-K) Low-degree chimera contribution of MFps-expressing Flk1lacZ/lacZ ES cells rescued vascular contribution (arrows) to intraembryonic (I) and yolk sac sites (J-K). X-gal–staining MFps-expressing; Flk1lacZ/lacZ-derived endothelial cells were found in intersomitic vessels in the embryo (arrow in I) and in morphologically normal yolk sac blood vessels (arrow in J). Histologic analysis shows X-gal–stained endothelial cells surrounding hematopoietic cells in the yolk sac vasculature (arrow in K). (L-N) High-degree chimera contribution of MFps-expressing Flk1lacZ/lacZ ES cells lead to aberrant vascular development and hemangioma formation in the yolk sac (L-M). (N) Histologic analysis of the hemangioma seen in panel M demonstrated the MFps-expressing; Flk1lacZ/lacZ null endothelial cells lining the blood filled hemangioma cavity (arrows in M-N). Panels G and H were stained by hematoxylin and eosin; panels K and N were stained by eosin. Original magnification × 8 (A-C, E); × 12 (D, F, L); × 20 (I, J, M); and × 200 (G, H, K, N).
Activated Fps partially rescues Flk1 deficiency in vivo
To determine whether MFps demonstrated similar rescue potential in vivo, the Flk1-targeted ES cells with or without the fpsMF transgene were introduced into embryos either by blastocyst injection or by aggregation with 8-cell stage embryos. Ten different MFps-expressing Flk1lacZ/+ ES cell clones were found by Western blotting analysis to express the MFps protein at similar levels (data not shown), as the MFps-expressing Flk1lacZ/lac/Z ES clones seen in Figure 2B.
Control Flk1lacZ/+ or Flk1lacZ/lacZ ES clones were injected into blastocytes, and the degree of ES cell chimerism was analyzed at E12.5 by X-gal staining or degree of eye pigmentation. Chimeric embryos derived in part from the Flk1lacZ/+ ES cells showed the characteristic X-gal staining in vascular endothelial cells (Figure 3A). The Flk1lacZ/lacZ ES cells were unable to contribute to the developing vasculature as was demonstrated by the lack of X-gal–stained blood vessels in chimeric E12.5 embryos (Figure 3B). When chimeras were made with MFps-expressing Flk1lacZ/+ cells, some embryos with relatively low ES cell contribution developed normally (Figure 3C) and exhibited X-gal–stained endothelial cells organized into normal vascular channels in the abdominal, intersomitic, and limb regions (boxed area in Figure 3C, black arrows in Figure 3D). Chimeric embryos with a high degree of MFps-expressing Flk1lacZ/+ ES cell contribution, as judged by the large number of X-gal–stained cells, showed signs of aberrant vascular development in the yolk sac and embryo (Figure 3E-F and data not shown). Higher-power magnification of one of these aberrant vascular structures revealed excessive vascular sprouting and regions of vascular hemorrhage (boxed area in Figure 3E, arrowhead in Figure 3F). Moderate chimeric juvenile mice-derived MFps-expressing Flk1lacZ/+ ES cells died at approximately 3 weeks of age as a result of internal hemorrhaging. Autopsies performed on these mice revealed disseminated vascular malformation and hemangiomas (Figure 3G-H). These experiments suggested a powerful biologic effect of the MFps protein on vasculogenesis and/or angiogenic processes. A summary table of representative chimeric embryo analysis and the observed phenotypes are presented in Supplemental Table S1, which is available on the Blood website. (See the Supplemental Data link at the top of the online article.) To determine whether MFps could modify the vasculogenic or hematopoietic phenotypes in Flk1-deficient cells, similar in vivo chimera experiments were performed with the MFps-expressing Flk1lacZ/lacZ ES cell clones (Figure 3I-N).
Similar to the chimeras derived from MFps-expressing Flk1lacZ/+ ES cells, high chimerism and ubiquitous expression of the MFps protein in the absence of Flk1 expression led to cardiovascular and craniofacial abnormalities and to aberrant vascular structures in the yolk sac (Figure 3L-N and Supplemental Figure S1). These abnormalities are reminiscent of the developmental defects previously documented in transgenic mice expressing the viral Gag-Fps protein.49,50
In embryos that demonstrated a lower degree of MFps-expressing Flk1lacZ/lacZ chimerism, X-gal–stained, Flk1-null endothelial cells were found in vascular channels in the embryo proper (Figure 3I) and in the yolk sac vasculature (Figure 3J-K). Closer morphologic and histologic analysis revealed that these aberrant vascular structures were hemangiomas consisting of blood-filled lesions lined by X-gal–stained endothelial cells derived from MFps-expressing Flk1lacZ/lacZ cells (arrowheads in Figure 3M-N). Certain chimeric embryos also showed increased amounts of X-gal–staining cells localized at specific intraembryonic hematopoietic sites (Supplemental Figure S1), as compared with chimeras generated with Flk1lacZ/lacZ cells (Figure 3B). Representative chimeric embryo analysis and their observed phenotypes are summarized in Supplemental Table S2.
To independently confirm the endothelial rescue potential of the MFps protein, ES cell clones in which the Flk1 locus was targeted with the EGFP reporter were then used to facilitate lineage analysis by flow cytometry by using endothelial-specific markers. A similar number of Flk1GFP embryos were examined, and similar results were obtained as in the Flk1lacZ chimeras presented in Supplemental Tables S1 and S2. Control chimeras generated using Flk1GFP/+ ES cells showed endothelial-specific EGFP expression (Figure 4A), whereas chimeras generated with Flk1GFP/GFP ES cells once again showed no contribution of Flk1 null ES cells to the vascular endothelial lineage, as judged by EGFP expression (Figure 4B). However, we did observe strong contribution and EGFP expression of the Flk1GFP/GFP ES cells to the developing heart (asterisk in Figure 4B). Moderate to high chimerism of MFps-expressing Flk1GFP/GFP ES cells was once again associated with vascular hemorrhage in the chimeric embryos and hemangioma formation in the yolk sac vasculature (arrows in Figure 4C and arrowheads in Figure 4G-H). PECAM-positive cells were seen in both embryos and yolk sacs from Flk1GFP/GFP chimeras, but very few EGFP single-positive or PECAM/EGFP double-positive cells were observed (Figure 4I-J, middle panels). In contrast, there were higher levels of PECAM/EGFP double-positive cells in both embryos and yolk sacs from MFps-expressing Flk1GFP/GFP chimeras (Figure 4I-J, right panels). In terms of the percentage of labeled cells present in the yolk sac there was 7.5% PECAM-positive cells in the MFps-expressing Flk1GFP/GFP-derived samples. Of the 7.5% PECAM positive cells, 1.16% was also EGFP-positive. Therefore, 15.5% of the PECAM-positive population in the yolk sac also expressed EGFP. In the MFps-expressing Flk1GFP/GFP-derived chimeric embryonic samples, 3.76% of the cells were PECAM labeled, and of this 0.16% were also EGFP-positive (4% of PECAM-positive cells were also EGFP-positive). This finding is in contrast to the negative control Flk1GFP/GFP ES cell–derived chimeras that had only 0.5% EGFP-positive cells of the PECAM-positive population (0.05% of 9.34%) in the yolk sac and virtually no double-positive cells in the embryo proper. The higher percentage of MFps-expressing Flk1GFP/GFP-derived PECAM/EGFP double-positive cells present in the yolk sac compared with the embryo proper was consistent with the higher numbers of X-gal–stained cells observed in the yolk sacs compared with embryos in the MFps-expressing Flk1lac-Z/lac-Z–derived chimeras (Figure 3 and data not shown).
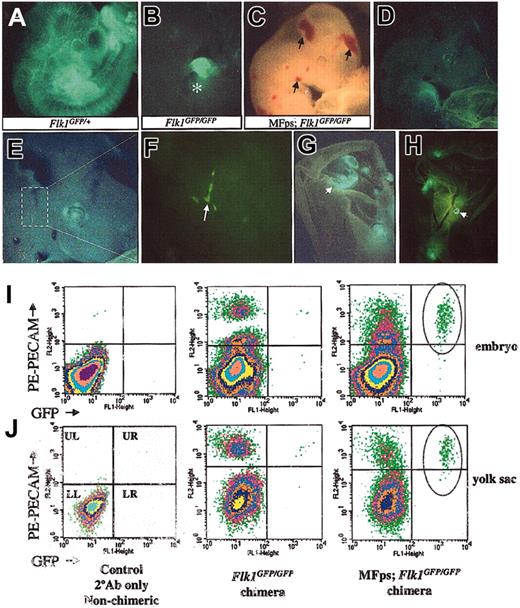
Chimera and flow cytometry analysis of MFps-expressing Flk1GFP/GFP ES cell–derived embryos confirmed the endothelial rescue potential of the MFps protein. (A) E10.5 Flk1GFP/+ chimera showing strong ES cell contribution to the vascular endothelial lineage as judged by EGFP expression analysis by direct fluorescence microscopy. (B) E11.5 Flk1GFP/GFP null chimera demonstrates a lack of contribution of Flk1 null ES cells to vascular lineage; however, this chimera shows strong contribution of Flk1 null cells to the heart as observed by the high levels of EGFP expression (asterisk in B). (C-H) E11.5 MFps-expressing Flk1GFP/GFP chimeras show signs of vascular hemorrhage in head and neck (arrows in C) and hemangioma formation in the yolk sac (arrowheads in G-H). (F) Higher-power magnification of head region in panel E, showing EGFP-positive vascular-like channel formation. (I-J) Flow cytometry analysis of single-cell suspensions obtained from the embryo (I) and yolk sac (J) of control non-PECAM–stained embryos (left panels), PECAM-labeled Flk1GFP/GFP chimeras (middle panels), and MFps-expressing Flk1GFP/GFP chimeras (right panels). Substantial numbers of EGFP/PECAM double-positive endothelial cells were detected in the MFps-expressing Flk1GFP/GFP-derived chimeric embryo and yolk sacs (circles in upper right quadrant of right panels) but not in the Flk1GFP/GFP-derived populations (middle panels). Original magnification × 12 (A, C, G, H); × 20 (B, D); × 40 (E); and × 200 (F).
Chimera and flow cytometry analysis of MFps-expressing Flk1GFP/GFP ES cell–derived embryos confirmed the endothelial rescue potential of the MFps protein. (A) E10.5 Flk1GFP/+ chimera showing strong ES cell contribution to the vascular endothelial lineage as judged by EGFP expression analysis by direct fluorescence microscopy. (B) E11.5 Flk1GFP/GFP null chimera demonstrates a lack of contribution of Flk1 null ES cells to vascular lineage; however, this chimera shows strong contribution of Flk1 null cells to the heart as observed by the high levels of EGFP expression (asterisk in B). (C-H) E11.5 MFps-expressing Flk1GFP/GFP chimeras show signs of vascular hemorrhage in head and neck (arrows in C) and hemangioma formation in the yolk sac (arrowheads in G-H). (F) Higher-power magnification of head region in panel E, showing EGFP-positive vascular-like channel formation. (I-J) Flow cytometry analysis of single-cell suspensions obtained from the embryo (I) and yolk sac (J) of control non-PECAM–stained embryos (left panels), PECAM-labeled Flk1GFP/GFP chimeras (middle panels), and MFps-expressing Flk1GFP/GFP chimeras (right panels). Substantial numbers of EGFP/PECAM double-positive endothelial cells were detected in the MFps-expressing Flk1GFP/GFP-derived chimeric embryo and yolk sacs (circles in upper right quadrant of right panels) but not in the Flk1GFP/GFP-derived populations (middle panels). Original magnification × 12 (A, C, G, H); × 20 (B, D); × 40 (E); and × 200 (F).
Similar experiments were performed to address the hematopoietic rescue potential of the MFps protein by using a PE-conjugated anti-c-kit antibody (specific for hematopoietic precursors). However, despite finding X-gal–stained Flk1 null cells in hematopoietic organs in certain chimeric embryos and yolk sacs (Supplemental Figure S1), no EGFP/c-Kit double-positive cells were observed in chimeric samples examined irrespective of the presence of the MFps protein (data not shown).
MFps increased hemangioblast migration and differentiation
ES cells aggregated with tetraploid embryos give rise to a completely ES cell-derived embryo proper39 (hereafter, these embryos are denoted as tetraploid ↔ ES cell line–derived embryos). This approach was used to eliminate the variability of ES cell contribution of Flk1-targeted vascular progenitors seen in the diploid chimera analysis. Hemangioblast colony assays were then used to substantiate the apparent increased number of Flk1-labeled progenitors seen in the diploid chimera experiments using ES cells expressing MFps.
Tetraploid ↔ Flk1lacZ/+ ES cell–derived embryos showed normal X-gal staining in mesodermal progenitors in an E7.5 embryo (Figure 5A).17 Flk1lacZ/lacZ cells had been previously shown to possess a limited migratory potential in vivo and were limited to the primitive streak region of diploid chimeric embryos.17 This limited migration of X-gal–stained hemangioblasts was also seen in tetraploid ↔ Flk1lacZ/lacZ ES cell–derived embryos at E7.0 to 7.5 (Figure 5B-C) compared with parallel experiments using Tetraploid ↔ Flk1lacZ/+ ES cells (Figure 5A). MFps partially restored the migratory potential of the X-gal–stained tetraploid ↔ MFps-expressing Flk1lacZ/lacZ cells in E7.0 to 7.5 derived embryos (Figure 5E-F), as can be seen by the migration of mesodermal progenitors away from the primitive streak region and into the yolk sac proper. Tetraploid ↔ MFps-expressing Flk1lacZ/+ cells also showed increased migration in comparison with tetraploid ↔ Flk1lacZ/+ ES cells (Figure 5, compare A and D), and there was an increase in the density of X-gal–stained progenitors (asterisk in Figure 5D).
Tetraploid ↔ ES cell–derived embryos and hemangioblast colony analysis of Flk1 heterozygous, Flk1 null, and MFps-expressing Flk1 null ES cells. (A-F) X-gal analysis of tetraploid ↔ ES cell–derived embryos. E7.0 to E7.5 embryos derived totally from Flk1lacZ/+ ES cells (A), Flk1lacZ/lacZ ES cells (B-C), MFps-expressing Flk1lacZ/+ ES cells (D), or MFps-expressing;Flk1lacZ/lacZ ES cells (E-F). Flk1lacZ/lacZ hemangioblast progenitors showed limited migratory potential (B-C). In contrast, MFps-expressing Flk1lacZ/lacZ-derived hemangioblast progenitors showed enhanced migratory activity (E-F). MFps-expressing Flk1lacZ/+ cells demonstrated the largest increases in X-gal–stained hemangioblast progenitors (D). Hemangioblast colony formation analysis was conducted on Flk1GFP/+ and MFps-expressing Flk1GFP/+ ES cell–derived embryoid bodies (G), as well as Flk1GFP/GFP and MFps-expressing Flk1GFP/GFP ES cell–derived embryoid bodies (H). C-chorion membrane, lines in panels B, C, E, and F represent extent of X-gal staining progenitor cell migration. The numbers in panels G and H represent the ES clone number used in the analysis. Original magnification × 20 (A-F).
Tetraploid ↔ ES cell–derived embryos and hemangioblast colony analysis of Flk1 heterozygous, Flk1 null, and MFps-expressing Flk1 null ES cells. (A-F) X-gal analysis of tetraploid ↔ ES cell–derived embryos. E7.0 to E7.5 embryos derived totally from Flk1lacZ/+ ES cells (A), Flk1lacZ/lacZ ES cells (B-C), MFps-expressing Flk1lacZ/+ ES cells (D), or MFps-expressing;Flk1lacZ/lacZ ES cells (E-F). Flk1lacZ/lacZ hemangioblast progenitors showed limited migratory potential (B-C). In contrast, MFps-expressing Flk1lacZ/lacZ-derived hemangioblast progenitors showed enhanced migratory activity (E-F). MFps-expressing Flk1lacZ/+ cells demonstrated the largest increases in X-gal–stained hemangioblast progenitors (D). Hemangioblast colony formation analysis was conducted on Flk1GFP/+ and MFps-expressing Flk1GFP/+ ES cell–derived embryoid bodies (G), as well as Flk1GFP/GFP and MFps-expressing Flk1GFP/GFP ES cell–derived embryoid bodies (H). C-chorion membrane, lines in panels B, C, E, and F represent extent of X-gal staining progenitor cell migration. The numbers in panels G and H represent the ES clone number used in the analysis. Original magnification × 20 (A-F).
The ability of MFps to increase hemangioblast numbers was also assessed by using an in vitro hemangioblast colony assay (Figure 5G-H). Two independent ES cell clones of the 4 different genotypes (Flk1GFP/+, MFps-expressing Flk1GFP/+, Flk1GFP/GFP, or MFps-expressing Flk1GFP/GFP) were differentiated in methylcellulose under previously established culture conditions for hemangioblast colony formation51,52 (Figure 5G-H). The results of this analysis confirm that blast colony formation is reduced in the absence of Flk1; however, in either the presence or absence of Flk1, MFps expression led to a 5- to 10-fold increase in the number of blast colonies. Highest numbers of hemangioblast colonies were generated from MFps-expressing Flk1GFP/+ ES cells in this system (Figure 5G). This observation was consistent with the highest density of X-gal–stained mesodermal progenitors observed in tetraploid ↔ MFps-expressing Flk1lacZ/+ ES cell–derived embryos (Figure 5D).
Discussion
In this study we have demonstrated that the endogenous cellular Fps protein undergoes increased plasma membrane localization on VEGF-A stimulation in endothelial cells. Concomitant with this translocation event, Fps becomes tyrosine phosphorylated and may transduce cellular signals downstream of Flk1. Although there has been no experimental evidence showing a direct interaction between Fps and Flk1 to date, there is compelling indirect evidence that these 2 proteins may activate similar downstream substrates. Viral and cellular Fps proteins have been demonstrated to interact with the Flk1 substrates p120GAP and Shc-A.53-56 Interestingly, gene inactivation studies of both Shc-A and p120GAP resulted in embryonic lethality because of cardiovascular abnormalities.57,58 More recently, Fps has been demonstrated to take part in semaphorin3A signaling that is involved in neuronal outgrowth and axonal guidance.59 In that study, Fps was shown to modulate neuropilin-1/plexinA1 complex formation and signaling. Neuropilin-1 has also been demonstrated to be a coreceptor for Flk1 in binding the VEGF-A164 isoform in endothelial and tumor cell lines.60 It is, therefore, possible that Fps might similarly modulate Flk1/neuropilin-1 signaling in endothelial cells.
MFps partially rescues Flk1 vascular deficiency in vitro and in vivo
Expression of activated MFps led to enhanced migratory potential of Flk1 null progenitors and increased spouting angiogenic responses. The effect of MFps expression on ES cell–derived embryoid body development or hemangioblast colony-forming cell (BL-CFC) assays demonstrated that the activated Fps protein increased hemangioblast development regardless of the presence of Flk1. Furthermore, MFps restored the ability of Flk1 null cells to contribute to the developing vasculature. Interestingly, when the chimeric contribution of MFps-expressing cells was low to moderate, normal vascular channels developed; however, when chimerism increased, hemangioma development occurred independently of the presence of Flk1. As was demonstrated previously, Flk1 null cells do not contribute to the vasculature,17 whereas MFps-expressing Flk1 null cells do contribute but at a lower frequency than that of MFps-expressing Flk1 heterozygous cells. This observation argues that MFps can at least partially restore the ability of Flk1 null cells to contribute to the vasculature; however, MFps does not completely rescue all the aspects of the vasculogenesis defects exhibited by Flk1 null cells. Furthermore, MFps increased the migratory potential of Flk1 null ES cell–derived mesodermal cells away from the primitive streak region and into the yolk sac proper in tetraploid ↔ ES cell–derived embryos. In addition, it is clear that Flk1 and MFps can act synergistically to increase hemangioblast numbers both in vitro and in vivo. Highest numbers of hemangioblast colonies were observed in vitro and increased X-gal–stained mesodermal progenitors were observed in tetraploid ↔ ES cell–derived embryo analysis in the presence of both Flk1 and MFps.
From a molecular perspective, transformation of fibroblasts by the MFps protein requires the activity of Ras, Rac, and cdc42, all of which have been implicated in cytoskeletal reorganization and cellular movement.61 Cellular Fps has also been demonstrated to interact with Cas (Crk-associated substrate) in macrophage cells,62 and viral Fps has been demonstrated to interact with connexin-43.63 Cas binds to FAK, tensin, and other components of focal adhesions, and connexin-43 is also involved in regulating cell-cell contacts. Flk1 activation of FAK as well as connexin-43 is believed to play a role in regulating endothelial migration and permeability.8,64 Therefore, in the absence of Flk1, MFps might rescue Flk1 precursor migration by activating these common substrates. Flk1 activation of the MAP-kinase and the PI3-kinase/AKT pathway are believed to play key roles in regulating endothelial proliferation/differentiation decisions as well as endothelial movement.7-10,13 Activated Fps constitutively activates the MAP-kinase pathway in several different cellular systems,61,65 and cellular Fps has been found to play a role in modulating endothelial tube formation through its interactions with PI3-kinase in both sonic hedgehog (Shh)– and stromal cell-derived factor 1-α (SDF-1α)–stimulated endothelial cells in vitro.66,67 As well, overexpression of a myristoylated PI3-kinase or a myristoylated Akt caused essentially identical endothelial lesions in chick chorioallantoic membrane (CAM) assays, as the MFps induced hemangiomas described in this study.68
Despite observing increased MFps-induced hemangioblast colony development in vitro and increased numbers of X-gal–stained hemangioblast progenitors in tetraploid ↔ ES cell–derived embryos, we have failed to definitively demonstrate the hematopoietic rescue potential of MFps on Flk1 null progenitors in vivo. Using MFps-expressing Flk1GFP/GFP ES cell–derived chimeras and c-Kit as a marker in FACS analysis, we failed to detect any c-Kit/EGFP double-positive cells. This failure is despite the fact that we did obtain some histologic evidence for hematopoietic rescue in that we observed X-gal–stained hematopoietic-like cells in the yolk sac and embryo proper in MFps-expressing Flk1lacZ/lacZ ES cell–derived chimeras. c-Kit is known to be expressed at high levels in approximately 70% of hematopoietic stem cells but becomes dramatically down-regulated in most terminally differentiated hematopoietic populations.69 Expression of an activated chimeric Fes protein, that exhibits similar increases in kinase activity as the MFps protein used in this study, has recently been demonstrated to induce terminal granulocyte differentiation.70,71 Therefore, it is conceivable that the reason we have not detected c-Kit/EGFP double-positive cells in MFps-expressing Flk1GFP/GFP ES cell–derived chimeras is that MFps causes terminal granulocyte differentiation in vivo, and c-Kit is no longer highly expressed in the EGFP-positive hematopoietic populations. We are, therefore, currently repeating this analysis by using more mature granulocyte-specific markers to address MFps' potential to rescue Flk1 hematopoietic deficiency in vivo.
Functional consequences of constitutive Flk1 signaling
Fong et al43,72 have concluded that the endothelial alterations exhibited in embryos lacking the other principle VEGF-A receptor, Flt1, was due to increased numbers of endothelial progenitors that developed in the absence of Flt1. As a consequence of Flt1 deficiency, there may be more VEGF-A available to bind Flk1. Increased VEGF-A availability led to increased Flk1 signaling and angioblast differentiation, resulting in an increased population density of endothelial cells that ultimately resulted in aberrant vascular formation.43 Although this view of Flt1 as a “VEGF-A sink” may be an underrepresentation of the actual biologic role of this receptor, this study along with others73 has demonstrated how excessive VEGF-A signaling adversely affects vascular development and endothelial behavior. MFps expression in a Flk1 heterozygous background in E7.0 to 7.5 tetraploid ↔ ES cell–derived embryos led to similar increases in hemangioblast development as was seen in Flt1 null E7.0 embryos.43 As was the case with Flt1 null ES cells, low-degree contribution of MFps-expressing Flk1 heterozygous ES cells in chimeras allowed for normal vascular development, whereas high-degree chimerism resulted in aberrant blood vessel formation. These results suggest that constitutive MFps expression might lead to similar phenotypes as is observed in the case of excessive VEGF-A/Flk1 signaling.72
Ema et al40 have recently knocked the gene encoding for the transcription factor Scl/Tal1 into the Flk1 locus to investigate whether restored Tal1 function could rescue Flk1 deficiency in vitro and in vivo. Restored Tal1 expression, under control of the Flk1 promoter, could partially rescue Flk1 null hemangioblast colony formation as well as hematopoiesis in vitro. A synergistic action of Tal1 and Flk1 in increasing endothelial numbers was also documented and shown to be VEGF-A–dependent, thereby arguing that Flk1 signaling through Tal1 regulated differentiation decisions between hematopoietic, vascular, and smooth muscle cell fates.40 We see a similar rescue potential in MFps-expressing Flk1 null ES cells in vitro, and we have documented similar synergistic effects of MFps on Flk1 signaling in terms of its effects on hemangioblast colony formation.40 We are currently using similar in vitro models to investigate whether MFps may also skew mesodermal cell fate decisions in a manner similar to that observed in Flk1/Tal1 cooperative signalling.40 Unlike the situation with the Tal1 rescue of Flk1, we observed hemangioma development in the in vivo MFps rescue. These results imply both Flk1-dependent and Flk1-independent effects of MFps.
In conclusion, we have identified Fps as a biologically relevant modulator of the VEGF-A/Flk1 signaling pathway, and through our chimeric analysis of ES cells expressing the activated Fps kinase we have gained insights into the cellular mechanisms by which activated Fps causes vascular lesions. The ability of Fps to modulate hemangioblast development and potentially modulate mesodermal cell fate differentiation decisions make this kinase a putative target in designing pharmacologic strategies aimed at stimulating or inhibiting endothelial development in pathologic settings.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-07-2343.
Supported in part by a National Cancer Institute of Canada (NCIC; grant 21335) and the Austrian Research Foundation (S74-MOB). J.H. is a recipient of an NCIC postdoctoral fellowship. The IMP is funded by Boehringer Ingelheim. A.N. is a senior Canadian Institutes of Health Research (CIHR) scientist.
Equal contributions were made by both the Mount Sinai Hospital Samuel Lunenfeld Research Institute and the Research Institute for Molecular Pathology.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Note added in proof. During the final stages of preparation of this manuscript for submission, Kanda et al74 showed that cellular Fps becomes tyrosine phosphorylated on VEGF-A stimulation in their Flk1-expressing PAE (porcine aortic endothelial) cell line.74 As well, wild-type Fps overexpression led to enhanced capillary tube formation in vitro in a VEGF-A–independent manner.74
We thank Cheryl Smith for flow cytometry analysis.


![Figure 2. Expression of the MFps protein partially restores migration and sprouting response of Flk1 null progenitors in vitro. (A) Schematic depiction of chicken–β-actin promoter-based construct used to overexpress the fpsMF cDNA in ES cells. (B) Western blot analysis of Flk1lacZ/lacZ null ES cell clones demonstrated a 2- to 3-fold increase in MFps in 10 of 11 puro-resistant clones compared with endogenous wild-type Fps protein levels in Flk1 null clones transfected with the empty puromycin construct (protein concentrations were standardized against β-actin levels [lower blot in B]). (C-D) Flk1lacZ/+ embryoid bodies showed endothelial migration and vascular channel formation, indicated by arrows. (E) Flk1GFP/+ embryoid bodies demonstrated angiogenic sprouting activity, indicated by arrows. (F-G) Flk1lacZ/lacZ embryoid bodies demonstrated dramatically reduced numbers of X-gal–stained cells and little migration or sprouting (arrowheads). (H) Similarly, Flk1GFP/GFP embryoid body–derived cells showed little EGFP expression and no migration or sprouting activity (arrowheads). (I-J) MFps-expressing Flk1lacZ/lacZ embryoid bodies showed an increased number of X-gal–stained cells (asterisk in I) and increased sprouting activity (arrow in J). (K) MFps-expressing Flk1GFP/GFP embryoid body–derived cells displayed increased EGFP expression, increased sprouting (arrow), and evidence of aberrant cellular morphology (asterisk). (L) Control Flk1lacZ/lacZ null EB-derived cells incubated with no primary antibody. Indirect PECAM immunofluorescence staining of Flk1lacZ/lacZ EB-derived cells (M) and MFps-expressing, Flk1lacZ/lacZ EB-derived cells (N).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-07-2343/6/m_zh80030456090002.jpeg?Expires=1764971062&Signature=W96KkXWpUjiWOk3NS8K-UD~zmEiP0KpX4Ies1X5j4CL4YfHQxsJBihbs767m9hNLodO9-6Ci1XkyGJ9MISXyMJv54ieV03vUEFcWbSwCegCdFqnDmMkKOEs6qiKlA8LolhavsJ3wBvdSUfz5zDrNCqFCkmMGgUc8wu-O-KPUX82Y-VGeDCS~Hd6BdypzQ2WW4-l~vYL1xO85dlfW9~m~XjPPlrPGlXvnNBq6o5L3Q2MuURvLVf3d55pfKBQsrjBW5X5ojkBnRAzBCznWIoS6xnc7J-mrosFk~RsnvzhZRBOOooqwBi05unf82B7F1RmA317aDtFphOxGUh3DN24EkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal