Abstract
An embryonic stem (ES) cell/OP9 coculture system for the effective production of functional neutrophils is described. A 3-step differentiation strategy was developed that uses liquid culture, enabling reliable and abundant production of neutrophils at high purity without the need of sorting for isolation of mature neutrophils. Use of the OP9 stromal cell line significantly enhances the number, percentage, and duration of differentiated neutrophils produced from embryonic stem cells. Effective and sustained differentiation of ES cells into neutrophils provides a useful model system for studying neutrophil differentiation and function and the factors that regulate them. Morphologic and functional evaluation of these ES-derived neutrophils indicates that large numbers of mature neutrophils can be produced from pluripotent ES cells in vitro. Specifically, their morphology, ability to produce superoxides, flux calcium, undergo chemotaxis in response to macrophage inflammatory protein 2 (MIP-2), stain for the granulocyte-specific marker–specific chloroacetate esterase, and contain the neutrophil-specific markers Gr-1 and the mouse neutrophil-specific antigen indicates that they are comparable with purified mouse bone marrow neutrophils. They also express gelatinase and lactoferrin granule proteins. During the differentiation of these ES-derived neutrophils, regional areas of neutrophil production can be identified that have been designated as neutrophil generating regions (NGRs).
Introduction
In humans, approximately 16 × 1010 neutrophils are produced daily.1-3 Furthermore, this number can be rapidly increased 5- to 10-fold in response to infection. This requirement necessitates a large pool (∼18 × 1011) of neutrophil progenitors and neutrophils in the bone marrow, as well as fine control of other processes of differentiation. Due to the rapid induction of neutrophils in response to inflammatory stimuli, it is clear that understanding the role(s) of neutrophils in disease requires a better appreciation of their differentiation. Although the complexity of the marrow microenvironment has been described and modeled, little is known about the numerous factors and interactions that are involved in normal homeostasis as well as disease.4,5
Using an in vitro model system to study neutrophil development enables more controlled assessment of the many factors involved in granulopoiesis. The in vitro differentiation of bone marrow–derived cells into granulocytes has been used for more than 20 years.2,6 However, use of bone marrow cultures to produce granulocytes has several inherent limitations. First, as bone marrow contains multiple cell types at several stages of differentiation, it is difficult to evaluate the developmental progression of neutrophils. This heterogeneity in turn limits assessment of growth factors on neutrophil differentiation. Second, genetic manipulation of bone marrow–derived cells is complex, thus making it difficult to evaluate the importance of particular genes in granulopoiesis. Several promyelocytic leukemia cell lines such as HL-60 and PLB-985 can be induced to differentiate into neutrophil-like cells and are frequently used to study neutrophils.7 However, these cell lines are transformed and are not functionally analogous to normal mature neutrophils.7,8
The in vitro differentiation of embryonic stem (ES) cells into hematopoietic cells provides an excellent model system for studying distinct lineages. Importantly, several studies have demonstrated that the in vitro differentiation of ES cells recapitulates the early stages of murine hematopoiesis.9-11 This includes the responsiveness of the ES cell–derived hematopoietic progenitors to various cytokines that have been demonstrated to be critical for embryonic hematopoietic development. In addition, the timing of hematopoiesis in ES cells is very similar to that seen in vivo, with neutrophils developing rather late. As ES cells are pluripotent and can be genetically manipulated, they can be used to assess the effects of gene modification in vitro or in vivo. In addition, they provide a system that enables evaluation of genetic changes that would be embryonically lethal if studied in vivo. However, to date, no consistent and effective method for inducing differentiation of functional neutrophils from ES cells has been described.
Neutrophils develop in the context of bone marrow stroma, and stromal elements have been found to be important for embryonic and adult hematopoiesis.12,13 Use of bone marrow–derived stroma to enhance ES-derived hematopoiesis in vitro has been shown by several investigators,12-16 but effective production of neutrophils has not been reported.
In the present study, we have used a 3-step differentiation strategy involving initiation of embryoid body (EB) formation in liquid culture, followed by replating onto semiconfluent OP9 stromal cells for secondary and tertiary differentiation. We specifically selected the OP9 stromal cell line as a method to support granulopoiesis, as this stromal line is derived from the osteopetrotic mouse that lacks functional macrophage colony-stimulating factor (M-CSF) production.10,17,18 Unlike the majority of stromal cell lines that produce significant quantities of M-CSF, we anticipated that the OP9 stromal cells would be well suited to promoting neutrophil production rather than macrophages.10 After effectively differentiating neutrophils from ES cells in vitro, we have begun to characterize these cells with respect to their morphology and function. We also demonstrate the usefulness of this differentiation system for studying the effects of specific genetic alterations on neutrophil differentiation and function using mitogen-activated protein (MAP)/extracellular signal-related kinase (ERK) kinase kinase 1 (MEKK1) knock-out ES cells.
Materials and methods
Routine culture of CCE ES cells
CCE ES cells at passages 17 to 24 were used for these studies. Cells were grown in Dulbecco's modified Eagle medium (DMEM)–ES medium containing DMEM (GIBCO BRL, Rockville, MD), 2 mM l-glutamine, 15% fetal calf serum (FCS; Summit Biotech, Ft Collins, CO), 1% leukemia inhibitory factor (LIF) supernatant, 1.5 × 10–4 monothioglycerol (MTG) (Sigma, St Louis, MO), and 100 U/mL penicillin and 100 μg/mL streptomycin (GIBCO BRL) on gelatinized plates. At 2 days prior to using the ES cells for differentiation experiments, they were split into gelatinized T-25 flasks at a density of .5 × 105 cells/mL into Iscoves modified Dulbecco medium (IMDM)–ES medium, which contained the same components as the DMEM-ES medium except IMDM was used.
Use of an ES/OP9 coculture differentiation system for efficient and sustained production of mature neutrophils from ES cells
OP9 mouse bone marrow stromal cells. OP9 mouse bone marrow stromal cells were grown in alpha minimum essential medium (GIBCO BRL) containing 20% fetal bovine serum (FBS) and .75 × 10–4 M MTG (Sigma). Cells were normally passaged every other day to avoid excessive density.
ES/OP9 coculture system for the in vitro production of neutrophils. As outlined in Figure 1, a 3-step differentiation strategy was used to efficiently produce mature neutrophils from ES cells.
ES/OP9 coculture system for the in vitro production of neutrophils. An outline of the steps used to produce neutrophils from embryonic stem cells is shown.
ES/OP9 coculture system for the in vitro production of neutrophils. An outline of the steps used to produce neutrophils from embryonic stem cells is shown.
Primary differentiation for day-8 embryoid bodies (EBs). For induction of differentiation, ES cells were plated at a density of 800 to 1000 cells/mL into a nontreated petri dish (Fisher Scientific Limited, Nepean, Ontario, Canada) containing 5 mL primary differentiation mix. The primary differentiation mix contained IMDM (GIBCO BRL), 15% pretested heat-inactivated FCS (Summit Biotech), 2 mM l-glutamine, 4.5 × 10–4 M MTG (Sigma), 50 μg/mL ascorbic acid (Sigma), 5% protein-free hybridoma medium (PFHM-II; GIBCO BRL), 78% by volume IMDM, and 100 U/mL penicillin and 100 μg/mL streptomycin (GIBCO BRL).
gp-130 secondary differentiation medium. After primary differentiation for 8 days, the EBs were trypsinized for 5 minutes at room temperature and disaggregated into a cell suspension. The cells were washed in 20 mL IMDM + MTG containing 10% FBS, centrifuged, and resuspended in the gp-130 secondary differentiation medium and plated onto semiconfluent OP9 cells. The gp-130 secondary differentiation mix contained 10% pretested heat-inactivated FBS (Summit Biotech), 10% horse serum (Biocell Laboratories, Rancho Dominguez, CA), 5% protein-free hybridoma medium (GIBCO BRL), 25 ng/mL oncostatin M (OSM), 10 ng/mL basic fibroblast growth factor (FGF), 5 ng/mL interleukin-6 (IL-6), 1% kit ligand (KL) supernatant (conditioned medium from Chinese hamster ovary [CHO] cells transfected with an expression vector generously provided by the Genetics Institute, Cambridge, MA), 5 ng/mL IL-11, and 1 ng/mL rLIF (all recombinant cytokines from R&D Systems, Minneapolis, MN) in 74% by volume IMDM containing 100 U/mL penicillin and 100 μg/mL streptomycin and 1.5 × 10–4 M MTG. The FBS was tested for its ability to form and support day-7 to day-14 EBs and for the ability of hematopoietic progenitors to form secondary colonies of the myeloid lineage including neutrophils. After 24 hours, the adherent cells associated with the monolayers were trypsinized and replated in the same medium onto new semiconfluent OP9 monolayers along with the cells in suspension to reduce monocyte/macrophage and fibroblast-like contaminants.
Tertiary neutrophil differentiation mix. After 3 days in the gp-130 secondary differentiation mix, cells were transferred onto a semiconfluent OP9 monolayer at a concentration of approximately 4 × 105 cells/mL into a tertiary neutrophil differentiation mix containing 10% platelet-depleted serum (Animal Technologies, Tyler, TX), 2 mM l-glutamine, 88% by volume IMDM, 100 U/mL penicillin, 100 μg/mL streptomycin, 1.5 × 10–4 M MTG, 60 ng/mL granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA), 3 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), and 5 ng/mL IL-6 (all recombinant cytokines from R&D). Differentiating hematopoietic cells were harvested in the same manner as described for harvesting cells in the gp-130 mix. After 4 to 20 days, the cells were harvested for assays.
Isolation of morphologically mature murine bone marrow neutrophils
Femurs and tibias of C57Bl/6 mice were dissected, the marrow flushed with Hanks buffered saline solution (HBSS, GIBCO BRL), and layered on a 3-step Percoll (Pharmacia, Piscataway, NJ) gradient (72%, 64%, and 52%), which was centrifuged at 1060g for 30 minutes.19,20 Cytospin samples of the 72%:64% interface revealed 10% to 30% red blood cells and 70% to 90% morphologically mature-appearing neutrophils (MMN), with minimal apoptosis (< 5%) by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay (Boehringer Mannheim, Mannheim, Germany).
Retrieval and staining of differentiating neutrophils for histologic evaluation
For assessing the overall number and percentage of neutrophils produced, the wells containing differentiating neutrophils were harvested, cytospun, counted in a hemacytometer, and stained with a Hema 3 Staining Kit (Fisher Scientific, Pittsburgh, PA). Cells were harvested using 3 methods: (1) gentle harvesting to enable continued granulopoiesis, (2) thorough harvesting using 3 5-mL washes of phosphate-buffered saline (PBS; GIBCO BRL) to retrieve the majority of hematopoietic cells, and (3) gentle and thorough harvesting followed by trypsinization of the stroma to assess the entire composition of the wells.
To evaluate the relative maturity of the cells superficially associated with the OP9 stroma, cells associated with the neutrophil-generating regions were sterilely harvested by fine needle aspiration under a dissecting microscope and cytospun, and the relative number and percentage of neutrophils were scored based on morphology.
Chemotaxis assay
A Zigmond chamber chemotaxis assay was used to assess the chemotaxis of neutrophils derived from both wild-type and MEKK1–/– ES cells in response to 25 ng/mL macrophage inflammatory protein 2 (MIP-2).21 The relative morphology, position, orientation, and locomotion of the cells were evaluated using videomicroscopy. Cell tracings were made of each field over time and the mean and peak migratory rates were calculated. In addition, from these tracings the mean path length and net displacement of the cells toward the chemoattractant were also calculated to enable assessment of relative chemotaxis and nondirectional migration by calculating the McCutcheon Index.22 For photomicrography, a × 40 objective was used with a final microscope magnification of × 320.
Superoxide assay
Mouse bone marrow neutrophils (35 000) that were Percoll gradient purified19 and ES-derived neutrophils were loaded into a 96-well plate at a concentration of 350 000 cells/mL, and superoxide production was assessed by use of the Amplex Red assay (Molecular Probes, Eugene, OR), which quantifies hydrogen peroxide production.23
Staining for lactoferrin and gelatinase
Cytospins of purified bone marrow– and ES/OP9-derived neutrophils fixed with 70% acetone/30% MeOH were stained for lactoferrin and gelatinase using a goat antilactoferrin antibody (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) or a rabbit antigelatinase (matrix metalloproteinase-9) antibody (1:100; Affinity BioReagents, Golden, CO), respectively. A rabbit antigoat immunoglobulin G (IgG, 1:300; Molecular Probes) was used as a secondary antibody for lactoferrin, while a goat antirabbit IgG (1:300; Molecular Probes) was used as the secondary antibody for antigelatinase staining. Primary antibody staining was done at 4°C overnight followed by staining with secondary antibodies at room temperature for one hour. Cells were photographed using an Olympus Vanox microscope (Tokyo, Japan) with × 60 and × 100 objectives.
FACS analysis of ES cell–derived neutrophils
Cells were blocked for the Fc receptor using a Caltag (Burlingame, CA) mouse antimouse CD16/32 Fc blocking antibody at a 1:20 dilution in PBS + 20% FBS (catalog no. MM7400) for 20 minutes at room temperature, or, for the anti-CD14 antibody, normal mouse IgG, was used to block Fc receptors. Isotype primary antibody controls were used to assess nonspecific staining. Primary antibodies used were against Gr-1 (BD-Pharmingen, San Diego, CA; clone RB6-8C5), mouse neutrophil-specific antigen clone 7/4 (Serotec no. MCA771; Serotec, Raleigh, NC), Ter119/erythroid (BD-Pharmingen catalog no. 553672), and CD14 R-phycoerythrin (BD-Pharmingen catalog no. 553740). Staining was done for 30 minutes at room temperature for all antibodies. A Becton Dickinson FACSCAN and FacsCaliber flow cytometer were used for fluorescence-activated cell sorter (FACS) analysis (Becton Dickinson, San Jose, CA). Data were analyzed using the Cell Quest Pro program (Becton Dickinson Immunocytometry Systems, San Jose, CA). Dead cells were excluded from FACS analysis by gating out cells with low forward and side scatter profiles. Broad gating was set based on the forward scatter/side scatter plot of gradient-purified mouse bone marrow neutrophils, which will also include red blood cells and a low percentage of lymphocytes and macrophages.
Results
Initial experiments, using a primary/secondary differentiation strategy,11 and the hemangioblast system developed by Kennedy et al24 and Choi et al25 produced a variable and transient number of neutrophil colonies, which were neither pure nor present in sufficient quantities for functional studies (data not shown). As IL-6 and IL-6 mimics (which interact with receptors of the gp-130 cytokine family) have previously been found to support the expansion of a variety of hematopoietic progenitors, we investigated its effectiveness in enhancing neutrophil production in vitro from ES cells.26-29 Accordingly, a 3-step differentiation strategy in which hematopoietic progenitors produced in EBs during primary differentiation were expanded in a secondary differentiation medium containing ligands that interact with gp-130 and matured in a tertiary neutrophil differentiation mix (“Materials and methods”) was assessed. This approach, although more effective than the previous 2, did not enable sustained production of neutrophils. In addition, functional studies using neutrophils derived from hemangioblasts and the gp-130–based differentiation system indicated that the majority of these neutrophils were not mature (data not shown). As the gp-130–based differentiation strategy was initially the most effective in producing neutrophils, we began by integrating the use of OP9 stromal cells into this system (Figure 1).
The number and duration of neutrophil production from the in vitro differentiation of ES cells is enhanced by the presence of OP9 stromal cells
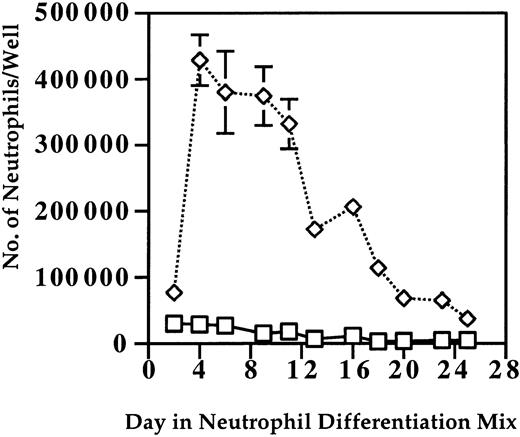
Initially, evaluation of the optimal time for the use of OP9 cells in supporting neutrophil differentiation and maturation was done. Use of OP9 cells starting in the neutrophil differentiation mix slightly enhanced the number of mature neutrophils produced (data not shown). However, when OP9 cells were used starting in the gp-130 mix and continuing through the neutrophil differentiation mix, a marked increase in the number and duration of neutrophils was seen. In Figure 2, the effects of OP9 cells on the number and duration of neutrophils produced in a single well (9.5 cm2 growth area) of a 6-well plate over 19 days from a representative experiment are shown.
Use of the OP9 stromal cell line enhances the number and duration of neutrophils produced. The effects of OP9 cells on the number and duration of neutrophils produced in a single well (9.5 cm2 growth area) of a 6-well plate over 26 days from a representative experiment is shown. At the indicated times in the neutrophil differentiation mix, 1 mL of cells in suspension following gentle flushing was retrieved and counted using a hemacytometer as described in “Materials and methods.” Multiple wells were assayed in sequence to enable evaluation of each well every 8 days, which eliminated any effect of cell removal on the cell number. In the OP9 group, cells were differentiated in the presence of the OP9 cells starting at the gp-130 differentiation medium (□, –OP9; ⋄, + OP9 cells). Error bars represent standard deviations.
Use of the OP9 stromal cell line enhances the number and duration of neutrophils produced. The effects of OP9 cells on the number and duration of neutrophils produced in a single well (9.5 cm2 growth area) of a 6-well plate over 26 days from a representative experiment is shown. At the indicated times in the neutrophil differentiation mix, 1 mL of cells in suspension following gentle flushing was retrieved and counted using a hemacytometer as described in “Materials and methods.” Multiple wells were assayed in sequence to enable evaluation of each well every 8 days, which eliminated any effect of cell removal on the cell number. In the OP9 group, cells were differentiated in the presence of the OP9 cells starting at the gp-130 differentiation medium (□, –OP9; ⋄, + OP9 cells). Error bars represent standard deviations.
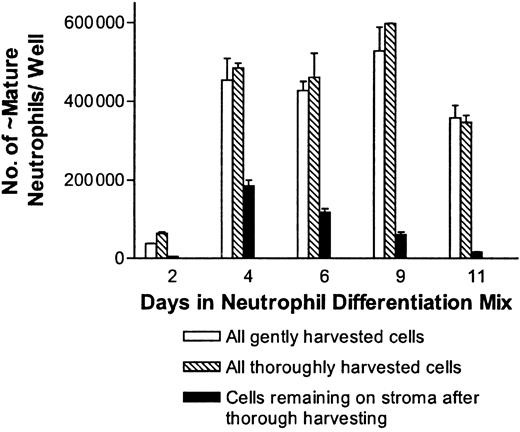
Use of OP9 stromal cells enhanced neutrophil production from 5- to 10-fold compared with cultures without stroma. From 80 000 pluripotent ES cells, approximately 6 × 106 neutrophils were obtained for 7 to 14 days using this approach. The results shown in Figure 2 represent a conservative estimate of the production of neutrophils, as in some experiments peak neutrophil production approached 8 × 105 cells/well. As 10 to 12 wells of differentiating neutrophils can be derived from 80 000 embryonic stem cells, peak neutrophil production can approach 1 × 107 cells, which represents an expansion of 125-fold. The numbers obtained are a conservative (likely about 50% of the total) estimate of the total number of neutrophils, as the wells were gently flushed prior to harvest and many neutrophils associated with the stroma were not in suspension. In experiments where thorough harvesting of the wells was done, the total number of bands to mature neutrophils recovered was typically 1.1 to 1.25 times the number when gentle harvesting was done as shown in Figure 3.
Effects of harvesting methods on neutrophil yields in the ES/OP9 coculture system. Cells from day 9 in the neutrophil differentiation mix were either gently or thoroughly harvested, as described in “Materials and methods,” at the indicated times. For this figure, the data are expressed as the total number of bands to polysegmented neutrophils recovered by gentle harvesting or by thorough harvesting. In addition, the number of cells remaining associated with the stroma after thorough harvesting is indicated. Error bars represent standard deviations.
Effects of harvesting methods on neutrophil yields in the ES/OP9 coculture system. Cells from day 9 in the neutrophil differentiation mix were either gently or thoroughly harvested, as described in “Materials and methods,” at the indicated times. For this figure, the data are expressed as the total number of bands to polysegmented neutrophils recovered by gentle harvesting or by thorough harvesting. In addition, the number of cells remaining associated with the stroma after thorough harvesting is indicated. Error bars represent standard deviations.
Use of the OP9 stroma during in vitro differentiation of ES cells into neutrophils enhances the purity of the neutrophil population produced
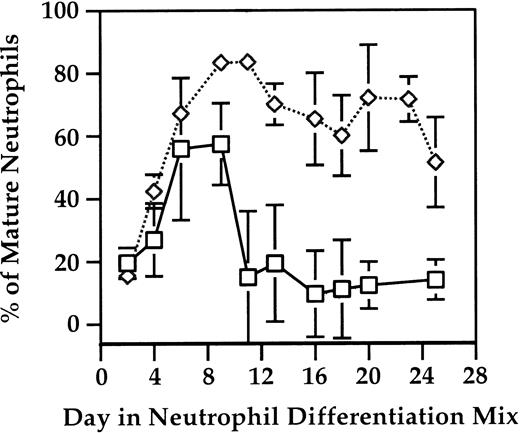
As shown in Figure 4, OP9 cells significantly enhance the percentage of mature neutrophils produced during the in vitro differentiation of ES cells into neutrophils. During peak periods of neutrophil production, which is maintained for at least a week, 75% to 96% of the cells appear to be neutrophils, with the majority of the cells being bands to mature neutrophils. This enables effective retrieval of mature neutrophils without the need of cell sorting or another selection method.
Use of the OP9 stromal cell line enhances the percentage of neutrophils produced. Cells were harvested as described in Figure 2 and stained with the Hema 3 staining kit (Fisher Scientific). The percentage of bands and mature neutrophils was scored based on morphology (□, –OP9; ⋄, + OP9 cells). Error bars represent standard deviations.
Use of the OP9 stromal cell line enhances the percentage of neutrophils produced. Cells were harvested as described in Figure 2 and stained with the Hema 3 staining kit (Fisher Scientific). The percentage of bands and mature neutrophils was scored based on morphology (□, –OP9; ⋄, + OP9 cells). Error bars represent standard deviations.
The production of neutrophils from embryonic stem cells occurs in regional areas that are reminiscent of areas of granulopoiesis found in long-term mouse bone marrow–derived cultures
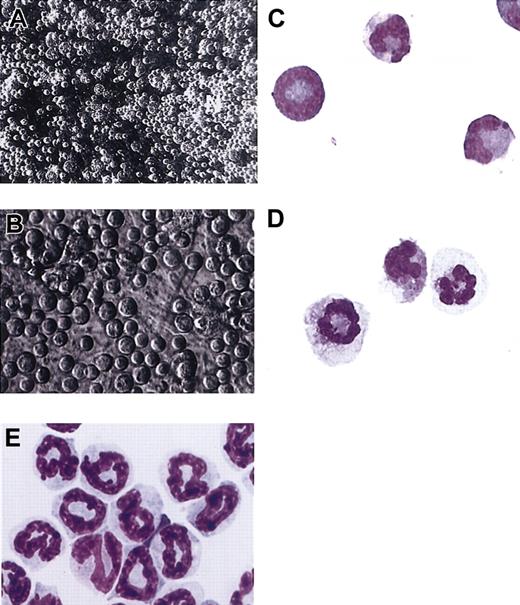
By day 3 in the neutrophil differentiation mix, distinct areas of apparent neutrophil production were seen, characterized by clusters of neutrophils at multiple stages of maturation. Phase contrast microscopy of these areas after 5 days in the tertiary neutrophil differentiation mix indicates that cells with the appearance of mature neutrophils are located on the surface of these regions and released into the medium (Figure 5A-B). For verification, under phase contrast microscopy loosely associated neutrophil-appearing cells were harvested by fine needle aspiration. In Figure 5C the majority of cells that were harvested by fine needle aspiration have the characteristic size, nuclear morphology, and staining pattern of mature mouse neutrophils. In addition, cells recovered from gently flushed wells of cultures after 7 days in the neutrophil differentiation mix were approximately 60% to 70% mature neutrophils, while cells that were directly harvested over the clusters by fine needle aspiration were approximately 80% to 85% mature neutrophils. Thus, it appears that as neutrophils mature they assume a more superficial position and are eventually released into the medium. In Figure 5D, ES-derived neutrophils from cultures from day 7 in the neutrophil differentiation mix were harvested to evaluate whether neutrophils with polysegmented nuclei can be obtained. Generally, regional areas of neutrophil production from day 7 in the neutrophil differentiation mix contain more neutrophils with polysegmented nuclei than analogous areas seen at day 5.
Neutrophil-generating regions (NGRs) contain differentiating neutrophils at all stages of maturation intimately associated with the OP9 cells. The composition of day-5 and day-7 NGRs was evaluated using fine needle aspiration to harvest loosely associated cells on the surface of these colonies. The aspirated cells were cytospun and stained with the Hema 3 staining kit (Fisher Scientific). Purified mouse bone marrow band 3 neutrophils were isolated as described in “Materials and methods.” (A) Low-power phase contrast photograph of NGRs from differentiating cells grown for 5 days in the neutrophil differentiation mix (× 180 final magnification). (B) At a higher magnification, differentiating neutrophils at multiple stages of maturation can be seen (× 400 final magnification). (C) Photograph of neutrophils harvested by fine needle aspiration from the same colony shown in panel A and stained with the Hema 3 staining kit (× 1500 final magnification). (D) Neutrophils harvested by fine needle aspiration from the surface of a day-7 NGR demonstrating polysegmented nuclei (× 1500 final magnification). (E) Hema 3–stained mouse bone marrow band 3 neutrophils (× 1500 final magnification).
Neutrophil-generating regions (NGRs) contain differentiating neutrophils at all stages of maturation intimately associated with the OP9 cells. The composition of day-5 and day-7 NGRs was evaluated using fine needle aspiration to harvest loosely associated cells on the surface of these colonies. The aspirated cells were cytospun and stained with the Hema 3 staining kit (Fisher Scientific). Purified mouse bone marrow band 3 neutrophils were isolated as described in “Materials and methods.” (A) Low-power phase contrast photograph of NGRs from differentiating cells grown for 5 days in the neutrophil differentiation mix (× 180 final magnification). (B) At a higher magnification, differentiating neutrophils at multiple stages of maturation can be seen (× 400 final magnification). (C) Photograph of neutrophils harvested by fine needle aspiration from the same colony shown in panel A and stained with the Hema 3 staining kit (× 1500 final magnification). (D) Neutrophils harvested by fine needle aspiration from the surface of a day-7 NGR demonstrating polysegmented nuclei (× 1500 final magnification). (E) Hema 3–stained mouse bone marrow band 3 neutrophils (× 1500 final magnification).
ES cell–derived neutrophils express neutrophil-specific markers
ES-derived neutrophils stain positively for the neutrophil granule constituents lactoferrin and gelatinase as well as the surface markers Gr-1 and the neutrophil-specific antigen in a similar manner to mouse bone marrow–derived neutrophils.
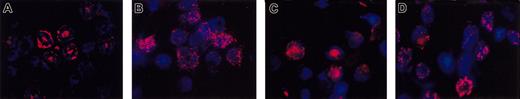
As shown in Figure 6, ES-derived neutrophils frequently stain positively for the neutrophil granule markers lactoferrin and gelatinase. As expected, only a subset (∼80% of gradient purified and ∼70% of day 5 in the neutrophil differentiation mix, respectively) of the bone marrow– (Figure 6A) and ES/OP9-derived (Figure 6C) neutrophils stain positively for gelatinase, which is a tertiary granule protein whose expression is associated with end-stage neutrophil maturation.30 In contrast, a higher percentage (∼90% and ∼80%, respectively) of bone marrow– (Figure 6B) and ES/OP9-derived (Figure 6D) neutrophils stain for lactoferrin, which is a secondary granule protein in neutrophils.
ES/OP9-derived neutrophils contain gelatinase and lactoferrin-positive granules similar to mouse bone marrow–derived PMNs. Bone marrow–derived (A-B) and ES/OP9-derived neutrophils (C-D) were stained for gelatinase (A,C) and lactoferrin (B,D) as described in “Materials and Methods.” Original magnification × 900.
ES/OP9-derived neutrophils contain gelatinase and lactoferrin-positive granules similar to mouse bone marrow–derived PMNs. Bone marrow–derived (A-B) and ES/OP9-derived neutrophils (C-D) were stained for gelatinase (A,C) and lactoferrin (B,D) as described in “Materials and Methods.” Original magnification × 900.
In Figure 7 the results of FACS staining for the neutrophil differentiation marker Gr-1 are shown for marrow-derived (A) and ES cell–derived (B) neutrophils. FACS analysis indicates that approximately 85% of gated mouse bone marrow neutrophils and 80% of ES-derived neutrophils express the granulocyte-specific antigen Gr-1. Since the expression of the Gr-1 antigen increases with neutrophil maturation, the staining histogram for the purified marrow-derived neutrophils is sharper (Figure 7A) than for the ES-derived neutrophils shown in Figure 7B. A significantly sharper histogram profile was seen for ES-derived neutrophils stained for the neutrophil-specific antigen (clone 7/4; Figure 7D) compared with Gr-1 (Figure 7B). Staining for this neutrophil-specific antigen indicated that 85% of gated purified mouse bone marrow cells and 90% of ES-derived neutrophils stained positively for this antigen. Use of primary isotype control antibodies yielded minimal background staining, demonstrating specificity of the primary antibodies (data not shown).
By FACS analysis, ES-derived neutrophils express the granulocyte-specific antigens, Gr-1, and the mouse neutrophil-specific antigen. For FACS analysis, 100 000 bone marrow (BM)– and ES-derived neutrophils were stained per well as described in “Materials and methods.” Panels A and C are of purified mature mouse bone marrow–derived neutrophils, while panels B and D are of ES-derived neutrophils harvested from day-9 cultures in the neutrophil differentiation mix. Histograms of FACS staining for Gr-1 and the neutrophil-specific antigens (Ag) are shown in A-B and C-D, respectively.
By FACS analysis, ES-derived neutrophils express the granulocyte-specific antigens, Gr-1, and the mouse neutrophil-specific antigen. For FACS analysis, 100 000 bone marrow (BM)– and ES-derived neutrophils were stained per well as described in “Materials and methods.” Panels A and C are of purified mature mouse bone marrow–derived neutrophils, while panels B and D are of ES-derived neutrophils harvested from day-9 cultures in the neutrophil differentiation mix. Histograms of FACS staining for Gr-1 and the neutrophil-specific antigens (Ag) are shown in A-B and C-D, respectively.
FACS analysis using antibodies against CD14 (for identification of monocytes/macrophages) and Ter119 (for identification of erythroid cells) was done to assess other cellular constituents of the ES/OP9 coculture system. In addition, the OP9 stromal cells were prestained with the vital dye carboxyfluorescein diacetete succinimidyl ester (CFDA SE; Molecular Probes) to enable identification of stromal cells. From Table 1 it is clear that mature neutrophils account for the majority of hematopoietic cells in the ES/OP9 coculture system.
Mature neutrophils account for the majority of hematopoietic cells in the ES/OP9 coculture system
. | Flushed wells* . | . | Flushed and trypsinized wells† . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Average no.‡ . | % recovered . | Average no.‡ . | % recovered . | ||
| Mature neutrophils | 500 000 | 79.7% ± 4.6% | 617 500 | 34.4%§ ± 7.5% | ||
| Monocytes/macrophages | 75 000 | 12%§∥ | 80 000 | 4.4% ± .35% | ||
| Erythroid | 50 000 | 8%§ | 254 556 | 14% ± .5% | ||
| Stroma | 2 500 | .4% | 850 000 | 47.2% ± .3% | ||
. | Flushed wells* . | . | Flushed and trypsinized wells† . | . | ||
|---|---|---|---|---|---|---|
| Cell type . | Average no.‡ . | % recovered . | Average no.‡ . | % recovered . | ||
| Mature neutrophils | 500 000 | 79.7% ± 4.6% | 617 500 | 34.4%§ ± 7.5% | ||
| Monocytes/macrophages | 75 000 | 12%§∥ | 80 000 | 4.4% ± .35% | ||
| Erythroid | 50 000 | 8%§ | 254 556 | 14% ± .5% | ||
| Stroma | 2 500 | .4% | 850 000 | 47.2% ± .3% | ||
As described in “Materials and methods,” wells from cultures from day 9 in the neutrophil differentiation mix were thoroughly flushed or flushed and trypsinized to include stromal cells, and the number and cell type evaluated using FACS and morphology as indicated. For FACS analysis, neutrophils, monocytes/macrophages, and erythroid cells were identified using antineutrophil Ag and Gr-1, CD-14, and Ter119 antibodies, respectively. For identification of stromal cells using FACS; CFDA SE staining of the OP9 cells was done before hematopoietic cells were plated onto them at the secondary and tertiary differentiation stages. Data are shown ± standard deviations.
Cells from a single flushed well were recovered from gentle and thorough harvesting.
Cells from a single trypsinized well were recovered following gentle and thorough harvesting.
Based on hemacytometer counting.
Based on morphology.
Based on morphology. Between 70% to 85% of these cells are mature neutrophils, while monocytes/macrophages generally account for approximately 12% to 20% of the nontrypsinized cells at day 9 in the neutrophil differentiation mix.
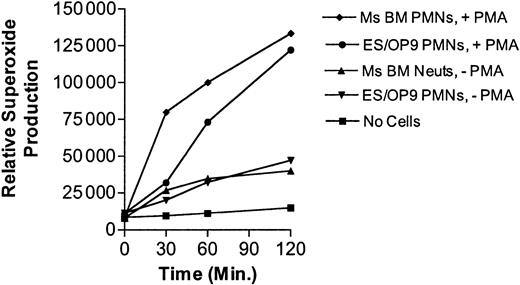
ES-derived neutrophils produce superoxide in response to PMA similarly to mouse bone marrow neutrophils
In response to specific stimuli, neutrophils from the myelocyte stage and onward characteristically produce superoxide, a critical component of their bactericidal ability.31 Consequently, the ability of ES-derived neutrophils to produce superoxide in response to phorbol myristate acetate (PMA) was compared with purified mouse bone marrow cells (Figure 8). A 2- to 3-fold increase in superoxide production was seen when the bone marrow– and ES/OP9-derived neutrophils were exposed to PMA.
Comparison of superoxide production in bone marrow– and ES cell–derived neutrophils in response to 10 ng/mL PMA Amplex Red (Molecular Probes) was used to assess the production of hydrogen peroxide, which is a reflection of superoxide production. For this assay, 35 000 Amplex Red–loaded cells per well of a low adherence 96-well plate were used as described in “Materials and methods.” Error values were too low to resolve.
Comparison of superoxide production in bone marrow– and ES cell–derived neutrophils in response to 10 ng/mL PMA Amplex Red (Molecular Probes) was used to assess the production of hydrogen peroxide, which is a reflection of superoxide production. For this assay, 35 000 Amplex Red–loaded cells per well of a low adherence 96-well plate were used as described in “Materials and methods.” Error values were too low to resolve.
The chemotactic response of ES-derived neutrophils is similar to that of mouse bone marrow–derived neutrophils
Neutrophils acquire the ability to chemotactically respond to a specific stimulus late in their development.32 Consequently, the chemotactic response of cells to specific stimuli is a useful assay to assess the functional capacity and maturation of neutrophils.33 In Table 2, the relative chemotactic response of mouse bone marrow– and ES/OP9-derived neutrophils is compared. The values shown in Table 2 are from a total of 6 representative experiments (3 experiments per group) evaluating purified mouse bone marrow– and ES-derived neutrophils. Cells classified as unresponsive did not move in response to chemoattractant, and cells were designated as nondirectionally migrating (NDM) if their mean McCutcheon Index (MI) was less than .6. The McCutcheon Index is a measure of directional movement toward a gradient.22 Cells were considered to be chemotactic if their MI was .6 or greater, indicating directional movement.
ES/OP9-derived neutrophils display similar migratory behavior as mouse bone marrow-derived PMNs
. | . | Migratory rate, μm/min . | . | . | ||
|---|---|---|---|---|---|---|
| Cells . | % of total . | Mean . | Peak . | MI . | ||
| Unresponsive BM PMNs | 41.7 ± 11 | NA | NA | NA | ||
| Unresponsive ES PMNs | 18.7 ± 13 | NA | NA | NA | ||
| NDM BM PMNs | 19.4 ± 14.4 | 6.1 ± 4.6 | 11.6 ± 6.8 | -.1 | ||
| NDM ES PMNs | 30.3 ± 17.2 | 10.4 ± 7.8 | 16.8 ± 11.4 | .03 | ||
| Chemotactic BM PMNs | 38.7 ± 12.1 | 8.1 ± 5.1 | 14.5 ± 6.5 | .85 | ||
| Chemotactic ES PMNs | 50.6 ± 16.4 | 11.9 ± 7.2 | 16.6 ± 9.3 | .84 | ||
. | . | Migratory rate, μm/min . | . | . | ||
|---|---|---|---|---|---|---|
| Cells . | % of total . | Mean . | Peak . | MI . | ||
| Unresponsive BM PMNs | 41.7 ± 11 | NA | NA | NA | ||
| Unresponsive ES PMNs | 18.7 ± 13 | NA | NA | NA | ||
| NDM BM PMNs | 19.4 ± 14.4 | 6.1 ± 4.6 | 11.6 ± 6.8 | -.1 | ||
| NDM ES PMNs | 30.3 ± 17.2 | 10.4 ± 7.8 | 16.8 ± 11.4 | .03 | ||
| Chemotactic BM PMNs | 38.7 ± 12.1 | 8.1 ± 5.1 | 14.5 ± 6.5 | .85 | ||
| Chemotactic ES PMNs | 50.6 ± 16.4 | 11.9 ± 7.2 | 16.6 ± 9.3 | .84 | ||
MI indicates net displacement of cells towards chemoattractant/total path length of cells (a negative MI indicates that the net cell migration was away from the chemoattractant); NDM, nondirectional migration in the presence of a gradient; and NA, not applicable. Data are shown ± standard deviations.
ES cell–derived neutrophils responded to MIP-2 in a similar fashion to purified mouse bone marrow–derived neutrophils in terms of their ability to undergo nondirectional migration or chemotaxis. As shown in Table 2, in representative experiments, 39% of purified mouse bone marrow–derived neutrophils and 51% of ES cell–derived neutrophils were chemotactic, while 19% of mouse bone marrow–derived neutrophils and 30% of ES cell–derived neutrophils exhibited nondirectional migration in response to a gradient.
Peak chemotactic rates of 14.5 ± 6.5 μm/min and 16.6 ± 9.3 μm/min were seen for mouse bone marrow–derived and ES-derived neutrophils, respectively. The McCutcheon Index was .85 for chemotactic mouse bone marrow–derived neutrophils, and .84 for ES-derived neutrophils. Thus, the overall chemotactic response of ES-derived neutrophils in response to 25 ng/mL MIP-2 was, if anything, greater than that seen for mouse bone marrow–derived neutrophils.
Embryonic stem cell–derived neutrophils can be used to assess the effects of mutations on neutrophil function
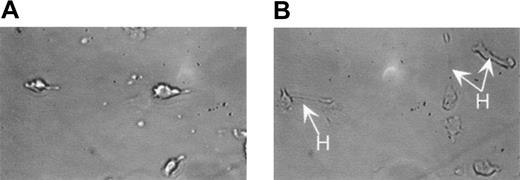
We have previously demonstrated that MEKK1 is activated by G-protein–coupled receptors in neutrophils.34 In order to demonstrate the role of specific gene products and as a technique to create genetically modified neutrophils, we have begun studies to evaluate the importance of MEKK1 on neutrophil differentiation and function. Neutrophils were differentiated from MEKK1–/– ES cells as described above. As shown in Figure 9B, MEKK1–/–-derived neutrophils display altered cell migratory behavior, with more frequent periods of hyperextension related to impaired release of their posterior. Interestingly, Yujiri et al found impaired migratory behavior in MEKK1–/– mouse embryonic fibroblasts and epithelial cells.35 Importantly, the observation of an observable phenotypic change in MEKK1–/– ES-derived neutrophils indicates the usefulness of the ES/OP9 differentiation system for studying neutrophil development and function. Ongoing studies are directed to further evaluate this altered migratory behavior.
MEKK1–/–-derived neutrophils display altered cell migratory behavior, with periods of hyperextension related to impaired release of their trailing edge. The migratory behavior of wild-type and MEKK1–/– ES/OP9-derived neutrophils was assessed by videotaping cell migration using a Zigmond chamber. As seen in panel A, neutrophils are migrating toward the gradient, which is on the left with a normal morphology. In panel B, the majority of MEKK1–/–-derived neutrophils are also migrating toward the left, frequently exhibiting impaired tail release and hyperextension (H). Original magnification × 280.
MEKK1–/–-derived neutrophils display altered cell migratory behavior, with periods of hyperextension related to impaired release of their trailing edge. The migratory behavior of wild-type and MEKK1–/– ES/OP9-derived neutrophils was assessed by videotaping cell migration using a Zigmond chamber. As seen in panel A, neutrophils are migrating toward the gradient, which is on the left with a normal morphology. In panel B, the majority of MEKK1–/–-derived neutrophils are also migrating toward the left, frequently exhibiting impaired tail release and hyperextension (H). Original magnification × 280.
Discussion
This report represents the first description of effective and sustained production of relatively pure populations of functional neutrophils from the in vitro differentiation of ES cells. Unlike previous studies using the OP9 stromal cell line during initial progenitor formation, our approach evaluated the effectiveness of this cell line in enhancing the establishment of neutrophil progenitors, as well as their effectiveness in promoting neutrophil maturity in vitro.
The experimental approach described here differs from previously published reports in the cytokine strategy used, the stage-specific timing of the differentiation mixes, and the use of a subconfluent OP9 stromal cell monolayer at early times and for a sustained period to enhance the establishment, proliferation, and differentiation of neutrophil progenitors into functional neutrophils. Within this system, appearance of localized regions of neutrophil differentiation is associated with increased production of mature and functional neutrophils, based on a number of criteria.
As OP9 cells have been found to express IL-7 and stem cell factor,36 which can promote granulopoiesis,37 and do not produce functional M-CSF, they are well suited to support the in vitro differentiation of ES cells into neutrophils. In addition, OP9 cells have been found to produce significant quantities of IL-6, which enhances hematopoietic progenitor expansion and survival.17,38
Initial studies attempted to determine the optimal time for the use of the OP9 cells for enhancing neutrophil production and maturation. For these studies, hematopoietic progenitor cells from disaggregated day-6 and day-8 EBs were plated onto subconfluent OP9 monolayers starting from the neutrophil differentiation mix and working back to the gp-130 mix. Use of day-6 EBs, which was most effective in the original gp-130 system, resulted in a significant number of erythroid cells. Since granulocyte precursors develop in EBs after erythroid precursors, it was reasoned that use of more mature EBs might contain a higher percentage of neutrophil progenitors.11
The results of these studies clearly indicate that day-8 EBs contain a significantly greater number of granulocyte progenitors than day-6 EBs, based on the appearance of neutrophil-generating regions derived from these EBs, which were otherwise generally not seen (data not shown). In addition, OP9 cells appear to support neutrophil progenitors, since plating of EB-derived hematopoietic progenitors directly onto the OP9 cells starting from the secondary gp-130 mix is more effective for producing neutrophils than using the OP9 monolayers beginning at the tertiary neutrophil differentiation mix.
The in vitro differentiation protocol described in “Materials and methods” consistently enables production of significant numbers of morphologically and functionally mature neutrophils from ES cells for 10 to 20 days, with peak production occurring from day 5 to 15 in the neutrophil differentiation mix. Using this differentiation strategy, neutrophils at a concentration of approximately 5 to 8 × 104/mL or greater in a 9.5 cm2 well were produced approximately 16 days after initiating the experiment, which is several days earlier than we obtained using the primary secondary EB-based differentiation strategy and the 3-stage gp-130 differentiation strategy without use of the OP9 cells. As 10 to 12 wells of differentiating neutrophils can be derived from 80 000 embryonic stem cells, peak neutrophil production can approach 1 × 107 cells, which represents an expansion of 125-fold. In addition, the OP9/ES coculture system enabled sustained production of functional neutrophils for at least 10 days; previous approaches have yielded neutrophil production limited to a period of 1 to 3 days.
Based on a variety of functional and morphologic criteria, pluripotent embryonic stem cells can be induced to differentiate into mature and functional neutrophils. Morphologically mature ES-derived neutrophils can be obtained that are approximately 7 to 12 μm in diameter, possess ring-like segmented nuclei, and stain like mature mouse bone marrow or peripheral blood neutrophils with purple nuclei and pale cytoplasm when stained with eosin and methylene blue stains. In addition, these ES-derived neutrophils stain positively for the neutrophil-specific antigen39 and the granulocyte differentiation antigen (Gr-1), which are specific markers for neutrophils.40 Costaining with these antibodies indicates that 75% of the cells that were thoroughly harvested using flushing are double-positive for these markers at day 9 in the neutrophil differentiation mix (data not shown). Interestingly, a subpopulation of the ES-derived neutrophils stains more intensely for Gr-1 than the marrow-derived neutrophils, possibly indicating a later maturational state than the purified “mature” bone marrow neutrophils. In contrast, expression of the mouse neutrophil-specific antigen recognized by clone 7/4 has not been reported to be associated with neutrophil maturity.39
ES-derived neutrophils differentiated in the presence of OP9 cells also appear to be functionally analogous to mouse neutrophils. Functional analysis of chemotaxis and calcium flux (data not shown) in response to MIP-2, superoxide production in response to PMA, and specific chloroacetate esterase staining (data not shown) were all similar to murine bone marrow neutrophils. In addition, they stain positively for lactoferrin and gelatinase, which are components of secondary and tertiary neutrophil granules, respectively. Expression of secondary granule proteins, including lactoferrin,41 is granulocyte specific.30 As gelatinase is a marker for neutrophil maturation and is expressed at the band to polysegmented stage, positive staining in the majority of ES/OP9-derived neutrophils indicates that these cells are mature. Interestingly, a greater percentage of morphologically mature ES/OP9-derived neutrophils stained brightly for gelatinase than mouse bone marrow–derived polymorphonuclear leukocytes (PMNs). Similarly, as the ability of neutrophils to undergo chemotaxis is acquired only in mature neutrophils, it can be used to assess functionality.32
Preliminary studies indicate that the composition and morphology of the neutrophil clusters appear to closely resemble areas of neutrophil production seen in long-term bone marrow cultures described by Allen and Dexter,3 Allen,12 and Moore et al2 wherein neutrophils are found at all stages of maturation (data not shown). In addition, the differentiation of ES cells into neutrophils using the ES/OP9 system apparently goes through similar stages of development as occurs in vivo42 and in long-term bone marrow–derived cultures originally described by Allen and Dexter.6
Effective and sustained, though not fully synchronized, production of neutrophils from embryonic stem cells provides an excellent model system for studying neutrophil development, maturation, and function. For example, this approach can be used to evaluate the cytokines and timing required for optimal neutrophil production, maturation, and function. This information is relevant to reducing the period of neutropenia in cancer and bone marrow transplant patients, as well as understanding the roles of neutrophils in inflammatory disease. In addition, the vast resource of genetically altered embryonic stem cells used for the production of transgenic mice will be useful for studying the importance of targeted genes in neutrophil differentiation and function. Our preliminary studies evaluating neutrophils differentiated from MEKK1–/– embryonic stem cells by the methods described in this study indicate that overt and more subtle phenotypic abnormalities can be resolved using this in vitro differentiation system.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1030.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Dr William L. Stanford, PhD, for generously providing the OP9 stromal cells.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal