Abstract
Based on the favorable safety profile and the independent activity of rituximab in B-cell lymphoma, we evaluated its efficacy and toxicity after high-dose therapy (HDT) and autologous hematopoietic cell transplantation (HCT). Thirty-five patients with diffuse large cell (25 patients), mantle cell (3 patients), transformed (3 patients), or other (4 patients) subtypes of B-cell lymphoma received HDT followed by a purged autologous graft. The rituximab schedule was 4 weekly infusions (375 mg/m2) starting at day 42 after HCT and, for patients 5 to 35, a second 4-week course 6 months after HCT. All planned therapy was completed in 29 patients. With 30 months' median follow-up, the 2-year event-free survival (EFS) rate was 83% and the overall survival (OS) rate was 88%. For 21 patients with relapsed or refractory large cell lymphoma, the EFS rate was 81% and the OS rate was 85%. Grades 3 to 4 neutropenia occurred in 19 (54%) patients. A prospective study of immune reconstitution included measurements of lymphocyte subsets, immunoglobulins, and response to vaccination. Serious infections were not observed despite delayed B-cell recovery in all patients and suppressed immunoglobulin G (IgG) levels and low pneumococcus antibody titers in a subset. Rituximab after HDT and HCT is feasible, and these phase 2 data support the current US Intergroup phase 3 trial in recurrent/refractory diffuse large cell lymphoma.
Introduction
Although 40% to 50% of patients with diffuse aggressive non-Hodgkin lymphoma (NHL) will be cured of their disease by initial combination chemotherapy, standard dose chemotherapy is effective in salvaging no more than 10% of patients with recurrent or refractory disease. Based largely on the randomized Parma trial and other phase 2 trials, high-dose therapy (HDT) followed by autologous hematopoietic cell transplantation (HCT) has become standard treatment for patients with chemosensitive, relapsed, or refractory aggressive NHL.1-4 In this setting, HCT renders approximately 35% to 40% of patients free of disease on a long-term basis. Relapse is the primary cause of failure after HCT. In addition to residual disease after HCT, the reinfusion of tumor cells in the autologous graft may contribute to relapse.5,6 A number of strategies, including in vivo and in vitro purging, have been used to purify autologous grafts and to minimize the risk of reinfusing tumor cells.5,7-9 Efforts to address resistant disease by increasing the intensity of HDT, performing double transplantation, or incorporating immunotherapy in the form of myeloablative allogeneic transplantation or posttransplantation cytokines have been hampered by increased toxicity and, to date, have not demonstrated improvements in overall survival.10-12
In the fall of 1997, rituximab, a chimeric anti-CD20 monoclonal antibody, was approved for use in the United States for patients with relapsed indolent lymphoma. In the pivotal study, single-agent rituximab was tolerated well with minimal marrow toxicity, less than 1% incidence of grades 3 to 4 neutropenia, and less than 1% incidence of grades 3 to 4 thrombocytopenia.13 Responses were observed in 11 (37%) of 30 patients with relapsed or refractory diffuse large B-cell lymphoma (DLCL).14 There is evidence that rituximab can be added to combination chemotherapy regimens without significant increases in hematologic toxicity.15-18 Adding rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) resulted in significantly longer overall and event-free survival times compared with CHOP alone in older patients with DLCL.17 On this basis, we initiated a prospective trial in 1998 on the hypothesis that rituximab would be tolerated by patients early in their recovery from HCT and would have a favorable impact on the minimal residual disease that leads to relapse.
Patients, materials, and methods
Inclusion criteria were recurrent or refractory B-cell NHL, CD20 expression on the most recent biopsy specimen, age 16 years or older, adequate marrow recovery (absolute neutrophil count 1.5 × 109/L or higher and platelet count exceeding 50 × 109/L on day 42 after transplantation), preserved kidney and liver function, and autologous peripheral blood HCT after carmustine (BCNU)–based preparation. During the course of the study, 2 modifications in eligibility took place to allow the participation of patients receiving total body irradiation (TBI)–based HDT and patients in first complete remission (CR) after induction chemotherapy who had features that put them at high risk for relapse. Under these amendments, 3 patients with mantle cell lymphoma in first CR undergoing TBI-based HDT and 4 patients with high-intermediate or high-risk DLCL (age-adjusted international prognostic index) in first CR were enrolled. To enrich the study for DLCL, the last 10 places in the study were reserved for patients with that histology. Refractory disease was defined as failure to achieve CR after the initial chemotherapy regimen.
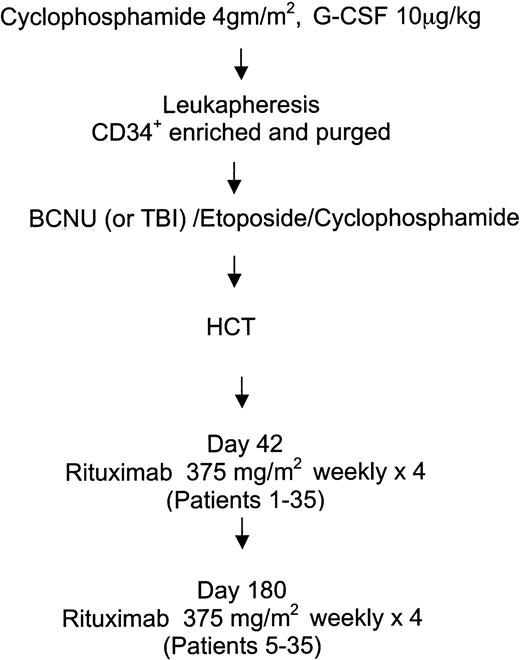
Patients meeting inclusion criteria for histology and the preparatory regimen (n = 92) were screened for eligibility. Patients with Eastern Cooperative Oncology Group (ECOG) performance status 2 or higher, active infection, active pneumonitis (n = 3), concurrent steroid use, progressive disease after transplantation (n = 2), posttransplantation radiotherapy (n = 3), prior exposure to rituximab (n = 4), marrow graft source (n = 3), primary central nervous system (CNS) (n = 1), equivocal CD20 expression (n = 1), or failure to meet hematologic criteria (n = 21) were ineligible according to the protocol. The major reasons 19 eligible patients did not participate were concern about the investigational nature of the study or refusal to comply with the additional follow-up required. The treatment schema for HCT and adjuvant rituximab is outlined in Figure 1. All patients were mobilized with a single dose of cyclophosphamide 4 g/m2 and granulocyte–colony-stimulating factor (G-CSF). The peripheral blood progenitor cell product was enriched for CD34+ cells and purged with a panel of anti–B-cell antibodies, as previously described.7 Thirty-two patients received carmustine, etoposide, and cyclophosphamide, and 3 patients received TBI in place of BCNU as previously described.2,3 At day 42 after HCT, all study patients were scheduled to receive 4 weekly infusions of rituximab 375 mg/m2. All patients received premedication with acetaminophen and diphenhydramine 30 minutes before rituximab administration. As part of the feasibility evaluation of this trial, if relative safety of a 4-week course of rituximab was documented in the first 4 patients, the remaining patients (patients 5-35) were scheduled to receive a second course of rituximab 375 mg/m2 weekly for 4 weeks beginning 6 months after HCT.
Patients were followed up monthly for 6 months with complete blood counts (CBCs), serum chemistry profiles, and physical examination for evidence of toxicity or relapse. Patients receiving a second course of rituximab continued to have CBCs taken monthly until 1 year after HCT. For the second year after HCT, patients were followed up every 3 months with physical examination, CBCs, and serum chemistry profiles and thereafter were evaluated every 6 to 12 months. As part of routine posttransplantation care but not prespecified by the study, computed tomography of the chest, abdomen, and pelvis and bone marrow biopsy were repeated at 6 and 12 months after HCT and then yearly unless clinically indicated.
Immune reconstitution was assessed with quantitative immunoglobulins and lymphocyte subsets at 6 and 12 months. Based on a lack of B-cell recovery in patients at 12 months, unplanned assessments of lymphocyte subsets and quantitative immunoglobulins were collected for patients at 18 and 24 months from HCT when possible. Flow cytometry with fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies was used to assess numbers of CD3+, CD4+, CD8+, CD16+, CD56+, CD45+, CD19+, and CD20+ cells. Vaccines against tetanus, Haemophilus influenzae, and pneumococcus were administered to patients at 6 and 9 months after their last rituximab infusion (12 and 15 months after transplantation). Vaccines were selected to assess different mechanisms of action in eliciting an antibody response: pneumococcus for a T-cell–independent pathway, tetanus for a T-cell–dependent pathway, and H influenzae for a combined B-cell/T-cell pathway by the addition of a protein conjugate. Antibody titers were assessed immediately before the initial vaccinations (baseline) and 3 months after each set of vaccinations. Antibody titers were graded as protective based on absolute values or an observed 4-fold or higher increase over baseline.
The major study end point was toxicity with an emphasis on acute side effects, infections over a 2-year period, and description of immune reconstitution. Treatment was considered feasible if the incidence of treatment-related mortality did not exceed 20% and the incidence of nonhematologic grades 3 to 4 toxicity did not exceed 30%. The sample size was increased from 24 to 35 patients to increase the number of DLCL patients. Results are reported on an intent-to-treat basis for all enrolled patients, regardless of whether they received all planned therapy. OS and EFS were calculated from the date of HCT. For the end point of EFS, the earlier event of relapse or death in remission was considered the date of failure. Survivals were estimated using the Kaplan-Meier method.19
Results
From March 1998 to December 2000, 35 patients met the eligibility criteria and consented to participate in the study. Their characteristics are shown in Table 1. The median age was 51 years (range, 28-70 years), and most had DLCL. Eighteen patients had relapses after the initial CR, 10 (8 with DLCL) were refractory to their first chemotherapy regimen, and 7 (4 DLCL, 3 mantle cell) were considered at high risk in first CR. The median number of prior chemotherapy regimens was 2; 7 patients received only 1 regimen, and another 7 patients received 3 or more prior regimens. Prior regimens included CHOP (n = 33), DHAP (n = 17; dexamethasone, cytarabine, cisplatin); ESHAP (n = 5; etoposide methylprednisolone, cytarabine, cisplatin), and CVP (n = 3; cyclophosphamide, vincristine, prednisone). One or 2 patients each received a variety of other regimens. As described in Table 1, responses to the last regimen given before HCT were CR or minimal disease (n = 25)—defined as no nodal mass larger than 2 cm, a 75% reduction in disease, or both—partial remission (n = 9), and a minor response classified as stable disease in 1 patient.
Patient characteristics
Characteristic . | N . | % . |
|---|---|---|
| Sex | ||
| Male | 23 | 66 |
| Female | 12 | 34 |
| Histology | ||
| Diffuse large B-cell | 25 | 71 |
| Mantle cell | 3 | 9 |
| Transformed | 3 | 9 |
| Other B-cell* | 4 | 11 |
| Disease status at autologous HCT | ||
| Relapsed | 18 | 51 |
| Refractory | 10 | 29 |
| First remission | 7 | 20 |
| Response to last chemotherapy | ||
| CR/minimal disease† | 25 | 71 |
| PR | 9 | 26 |
| Stable disease | 1 | 3 |
Characteristic . | N . | % . |
|---|---|---|
| Sex | ||
| Male | 23 | 66 |
| Female | 12 | 34 |
| Histology | ||
| Diffuse large B-cell | 25 | 71 |
| Mantle cell | 3 | 9 |
| Transformed | 3 | 9 |
| Other B-cell* | 4 | 11 |
| Disease status at autologous HCT | ||
| Relapsed | 18 | 51 |
| Refractory | 10 | 29 |
| First remission | 7 | 20 |
| Response to last chemotherapy | ||
| CR/minimal disease† | 25 | 71 |
| PR | 9 | 26 |
| Stable disease | 1 | 3 |
Other histologies were follicular grade 2 (mixed) lymphoma, marginal zone lymphoma, chronic lymphocytic leukemia, and a diffuse small cell lymphoma that could not be further classified.
Minimal disease is defined as a 75% reduction in tumor volume and no mass larger than 2 cm.
Thirty-two patients received HDT with BCNU, etoposide, and cyclophosphamide, and 3 patients with mantle cell lymphoma received TBI with etoposide and cyclophosphamide. The median CD34+ cell dose of the purged autografts was 6.8 × 106/kg (range, 1.9-11.7 × 106/kg). Twenty-nine patients received all planned rituximab infusions. Reasons for the 6 who did not included progressive disease (2 patients), death from pneumonitis after the first rituximab course (1 patient), neutropenia during rituximab administration (1 patient), severe rituximab infusional reaction (1 patient), and disseminated herpes zoster at the time of the second rituximab course (1 patient).
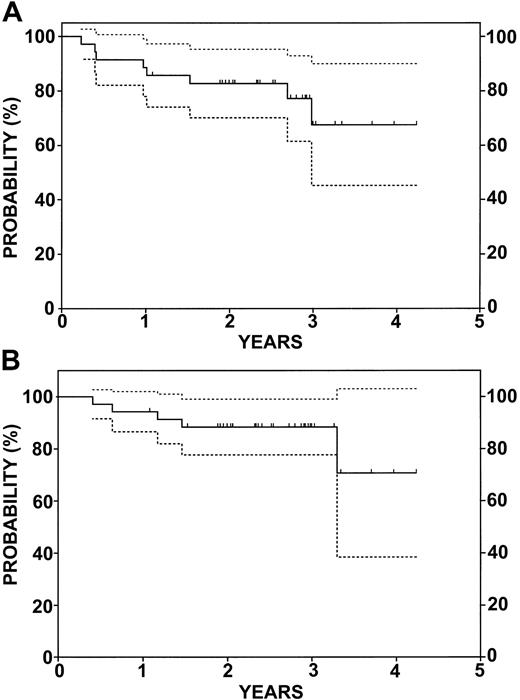
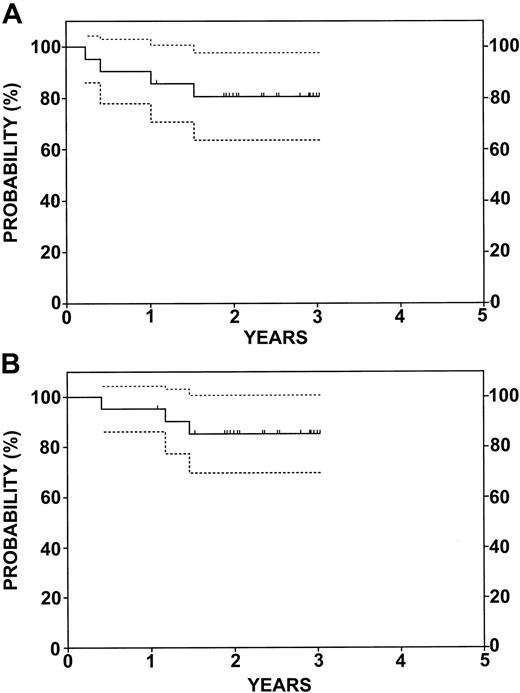
With a median follow-up of 30 months, the estimated 2-year EFS rate was 83% (confidence interval [CI], 70%-95%), and the 2-year estimated OS rate was 88% (CI, 78%-99%) for all patients assessed on an intent-to-treat basis (Figure 2). Five patients died, 4 of progressive disease and 1 in remission from pneumonitis. Among 7 patients in relapse, 2 received a single course of rituximab. Relapses occurred in patients with DLCL (3 patients), transformed NHL (2 patients), and mantle cell (2 patients). Figure 3 shows the EFS and OS for the 21 patients with relapsed (13 patients) or refractory (8 patients) DLCL. At HCT, 13 had minimal disease, 7 were in partial remission (PR), and 1 had stable disease. The results are similar to that of the whole group. With a median follow-up of 28 months, the estimated 2-year EFS rate was 81% (CI, 64%-98%), and the estimated 2-year OS rate was 85% (CI, 70%-100%). As described above, 3 of these patients had relapses and 2 died. A third patient, in CR, died of BCNU pneumonitis.
EFS and OS for 35 patients enrolled in the study. (A) EFS. (B) OS. Dotted lines represent 95% CI. Tic marks represent censored data.
EFS and OS for 35 patients enrolled in the study. (A) EFS. (B) OS. Dotted lines represent 95% CI. Tic marks represent censored data.
EFS and OS for 21 patients with relapsed or refractory diffuse large B-cell lymphoma. (A) EFS. (B) OS. Dotted lines represent 95% CI. Tic marks represent censored data.
EFS and OS for 21 patients with relapsed or refractory diffuse large B-cell lymphoma. (A) EFS. (B) OS. Dotted lines represent 95% CI. Tic marks represent censored data.
Table 2 describes the hematologic toxicity observed in our study patients after the initial rituximab dose. Neutropenia (Common Toxicity Criteria) was the most common toxicity, with 21 patients developing at least 1 episode of grades 2 to 4 neutropenia. Grades 3 to 4 neutropenia were recorded in 19 (54%) patients who experienced a total of 46 episodes, including 13 patients with more than 1 episode. Neutropenia was noted as early as the fourth week of the initial rituximab infusion but also occurred late, as evidenced by episodes occurring more than 300 days after HCT. There was no characteristic pattern of neutropenia: total white blood cell (WBC) counts ranged from low normal (3.2-4 × 106/μL) with isolated depression of neutrophils (6%-8%) to low absolute (0.7-0.9 × 106/μL), with normal percentages of neutrophils (57%-62%). All episodes either resolved spontaneously within 7 days (n = 14) or responded to 2 to 4 days of G-CSF (n = 32). The decision to administer G-CSF was according to physician choice. Bone marrow biopsies performed on 8 patients while they had neutropenia showed normocellular findings with the presence of granulocyte precursors. One episode of fever and no serious infections resulted from the neutropenia. We were unable to associate neutropenia with prior treatment, marrow involvement, CD34+ cell dose, and engraftment kinetics. Other hematologic toxicities were limited to 3 patients with grades 2 to 3 thrombocytopenia and 4 patients with grades 2 to 3 anemia. These events primarily occurred within the first 100 days of transplantation; in 2 patients, they occurred simultaneously with neutropenia.
Hematologic toxicity
. | . | . | No. episodes by grade . | . | . | ||
|---|---|---|---|---|---|---|---|
| Parameter . | No. patients . | % . | 2 . | 3 . | 4 . | ||
| Neutropenia | 21 | 60 | 26 | 25 | 21 | ||
| Thrombocytopenia | 3 | 9 | 2 | 2 | 0 | ||
| Anemia | 4 | 11 | 4 | 1 | 0 | ||
. | . | . | No. episodes by grade . | . | . | ||
|---|---|---|---|---|---|---|---|
| Parameter . | No. patients . | % . | 2 . | 3 . | 4 . | ||
| Neutropenia | 21 | 60 | 26 | 25 | 21 | ||
| Thrombocytopenia | 3 | 9 | 2 | 2 | 0 | ||
| Anemia | 4 | 11 | 4 | 1 | 0 | ||
Grading was in accordance with the Common Toxicity Criteria.
Nonhematologic toxicities included BCNU-related pneumonitis in 8 patients (Table 3). This clinical syndrome was based on low-grade fever, dyspnea, failure to thrive, or general malaise and a decrease in diffusing capacity of at least 20% from pretransplantation levels. Only 2 patients displayed classic pulmonary infiltrates, hypoxia, or respiratory compromise. All 7 treated patients responded to corticosteroid therapy, whereas 1 patient died after a delayed diagnosis. Twenty patients participated in a double-blind phase 3 trial of a novel herpes zoster vaccine. Six of these 20 patients acquired herpes zoster, which was disseminated in 3, during follow-up. None of the 15 patients who did not participate in the study acquired herpes zoster. Other toxicities included community-acquired pneumonia in 4 patients, all of which occurred without neutropenia and resolved with oral outpatient antibiotics. Pleural effusion developed in 1 patient, who was subsequently noted to have a decreased cardiac ejection fraction. A final unexpected adverse event, amyotrophic lateral sclerosis, was diagnosed in a patient more than 3 years after HCT. The diagnosis was made by neurologic evaluation on the basis of typical clinical findings, magnetic resonance imaging, and nerve conduction studies.
Nonhematologic toxicity
Toxicity . | No. patients . | % . |
|---|---|---|
| Pneumonitis | 8 | 23 |
| Herpetic infection | 6 | 17 |
| Respiratory infection | 4 | 11 |
| Pleural effusion | 1 | 3 |
| Infusional toxicity | 1 | 3 |
| Amyotrophic lateral sclerosis | 1 | 3 |
Toxicity . | No. patients . | % . |
|---|---|---|
| Pneumonitis | 8 | 23 |
| Herpetic infection | 6 | 17 |
| Respiratory infection | 4 | 11 |
| Pleural effusion | 1 | 3 |
| Infusional toxicity | 1 | 3 |
| Amyotrophic lateral sclerosis | 1 | 3 |
Pneumonitis was fatal in 1 patient.
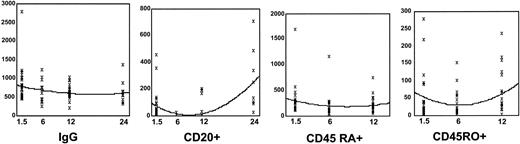
Lymphocyte subsets and quantitative immunoglobulins were measured in follow-up, some of which are shown in Figure 4. Quantitative immunoglobulins appeared to be only modestly affected by the addition of rituximab in the posttransplantation period. Immunoglobulin G (IgG) levels were below the normal range in 14 of 35 patients before their first rituximab infusion. Mean values declined after rituximab infusions at day 42 and 6 months after HCT. IgG levels remained suppressed in 11 (33%) of 33 and 9 (30%) of 30 patients at 6 and 12 months, respectively, after transplantation. IgA and IgM levels appeared less affected because mean values for the group were in the normal range. Nonetheless, 10 and 9 patients had levels of IgA below normal at 6 and 12 months, respectively, and 11 and 9 patients showed similar modest deficiencies in IgM at the same time points. B-cell counts, measured by CD20+ and CD19+ cells, were low in absolute number in 27 of 35 patients at study entry and declined to zero in all patients receiving rituximab, as measured by flow cytometry at 6 months after HCT. The only patients with measurable B-cell counts (3 patients) at 12 months received a single course of rituximab. By 18 to 24 months after HCT, B-cell numbers were recovering toward the normal range. Because it had not been planned that lymphocyte subsets would be measured at this time, the data are less complete, but in 14 of 20 patients evaluated, absolute numbers of CD19+ and CD20+ cells recovered to the normal range.
Serial assessment of IgG and lymphocyte subsets. Time is measured in months since transplantation. IgG levels are measured in mg/dL, and CD20+, CD45 RA+, and CD45RO+ numbers are expressed as number of cells ×106/μL. Each patient sample is represented by X. Median values are represented by the line.
Serial assessment of IgG and lymphocyte subsets. Time is measured in months since transplantation. IgG levels are measured in mg/dL, and CD20+, CD45 RA+, and CD45RO+ numbers are expressed as number of cells ×106/μL. Each patient sample is represented by X. Median values are represented by the line.
Mean CD3+ numbers were only mildly depressed and remained so during the follow-up period. There was wide interpatient variability in the absolute numbers of CD3+ cells with normal (n = 12), decreased (n = 16), or elevated (n = 6) values at study entry. Rituximab had no consistent effect on total T-cell numbers. Mean CD4+ cell numbers were decreased throughout the first year after HCT but recovered by 24 months. CD8+ cell numbers were largely normal at study entry and declined slightly after the first rituximab infusion. The CD4+/CD8+ ratio was 0.4 at study entry, 0.46 at 6 months, and 0.6 at 12 months. As with the total T-cell numbers, there was wide interpatient variability in T-cell subsets. Less recovery was observed among the CD4+CD45RA+ population of naive T cells. These cells were present in low numbers after HCT, in contrast to the persistently higher numbers of CD4+CD45RO+-activated or memory T cells. Natural killer (NK) cells, as identified by the CD56+/CD16+ subset of lymphocytes, were present in normal quantities from study entry throughout the first year of follow-up.
Antibody titers against tetanus, H influenzae, and pneumococcus were measured at baseline, 3 months after the first vaccination set, and 3 months after the second vaccination set. Complete data are available on 22 patients. At baseline, 6 (27%) patients had protective antibodies against H influenzae, 7 (32%) had them against pneumococcus, and 12 (55%) had them against tetanus. After the first and second vaccinations, 16 (73%) and 17 (77%) patients, respectively, showed protective antibody titers to H influenzae. For tetanus, 14 (64%) and 15 (68%) patients had demonstrable protective antibody titers after the first and second vaccinations, respectively. However, for pneumococcus, only 9 (41%) patients had protective titers after both vaccinations.
Discussion
HDT with autologous HCT is a standard treatment for patients with chemosensitive relapsed and refractory DLCL, yet 40% to 60% of patients successfully undergoing HCT experience disease relapse. The objective of our study was to test the feasibility of adjuvant rituximab after HCT, a potential strategy to address the minimal residual disease that remains in many patients after transplantation and that destines them to relapse. This strategy has been pursued with interferon and interleukin-2, but neither has the specificity, favorable toxicity profile, or independent efficacy of rituximab.13,20,21 Subsequent to the initiation of our trial, other groups have reported their experiences with rituximab in combination with HCT.5,22-25 In these studies, rituximab was used as an in vivo purging strategy before and after HCT. Treatment regimens for these trials are listed in Table 4, and their experiences are discussed here in relation to our trial.
Rituximab and autologous HCT
. | . | . | No. of rituximab . | . | . | |
|---|---|---|---|---|---|---|
| Author/reference . | NHL histology . | N . | Mobilization . | After HCT . | Median follow-up, mo . | |
| Flinn11 | Indolent | 25 | 1 | 1 | 3 | |
| Magni25 | Indolent and mantle cell | 15 | 4 | 2 | 10 | |
| Ladetto26 | Multiple | 32 | 2-4 | 2 | 14 | |
| Mangel27 | Mantle cell | 13 | 1 | 8* | 8 | |
| Rapoport28 | Multiple (22 DLCL) | 33 | - | 4 | 26 | |
| Horwitz | Multiple (25 DLCL) | 35 | - | 8† | 28 | |
. | . | . | No. of rituximab . | . | . | |
|---|---|---|---|---|---|---|
| Author/reference . | NHL histology . | N . | Mobilization . | After HCT . | Median follow-up, mo . | |
| Flinn11 | Indolent | 25 | 1 | 1 | 3 | |
| Magni25 | Indolent and mantle cell | 15 | 4 | 2 | 10 | |
| Ladetto26 | Multiple | 32 | 2-4 | 2 | 14 | |
| Mangel27 | Mantle cell | 13 | 1 | 8* | 8 | |
| Rapoport28 | Multiple (22 DLCL) | 33 | - | 4 | 26 | |
| Horwitz | Multiple (25 DLCL) | 35 | - | 8† | 28 | |
Variable schedule after HCT.
First 4 patients received only 4 doses.
The rates of 83% EFS and 88% OS rates at 2 years compare favorably with historical series, including our own.3 Our data are particularly noteworthy in the subgroup of 21 patients with relapsed or refractory DLCL for whom rates of relapse and survival are well characterized. The 2-year EFS and OS rates were 58% and 62%, respectively, in 203 patients with NHL who underwent transplantation at Stanford University from 1994 to 2000 and who underwent similar preparatory regimens and in vitro purging of the autologous grafts. However, our trial was primarily designed to test the feasibility of this approach and was not intended as a comparison with standard high-dose regimens. In addition, as noted above, the study patients were selected from a group of 92 patients who underwent transplantation within the time period, 57 of whom were not eligible or chose not to participate. Among these 57 patients who were not recruited to the study, 55 had chemosensitive disease compared with 34 of 35 study patients.
The excellent outcomes for most of our patients suggest value in testing this strategy further. It is of interest that a higher response rate (78% vs 43%; P < .01) was seen for patients treated with prior HCT compared with prior conventional therapy in the rituximab pivotal trial.13 Anecdotally, rituximab was surprisingly effective as a single agent in patients with DLCL who had relapses after HCT.26 However, Rapaport et al25 reported a 2-year EFS rate of 30% and an OS rate of 35% in the only other trial of rituximab and HCT conducted primarily in patients with DLCL. These differences emphasize the requirement for a phase 3 trial to determine whether the addition of rituximab contributes meaningfully to the overall success of HCT.
As in patients who did not undergo transplantation, we found rituximab to be tolerated well after HCT. Of the 35 patients treated, only 1 had significant infusional toxicity. Despite the requirement for adequate engraftment, we observed grades 3 to 4 neutropenia in 54% of our patients; many experienced multiple episodes that occurred up to 1 year from transplantation. Flinn et al9 reported transient neutropenia in 6 of 25 patients with indolent NHL who received rituximab as part of an in vivo purging strategy before HCT and a single dose of rituximab after engraftment. Rapaport et al25 described decreased white blood cell counts in only 4 of 26 patients, whereas Mangel et al24 and Ladetto et al23 did not report neutropenia with rituximab after HCT in 13 and 32 patients, respectively. In a retrospective review of 28 patients treated at our institution with the same HDT followed by HCT with a purged graft, we found no cases of documented delayed neutropenia. However, the patients in the current study were under closer surveillance, and there was more opportunity to detect neutropenia. Other hematologic toxicities were rare in our trial.
Papadaki et al27 reported on 2 occurrences of neutropenia in rituximab-treated patients who showed evidence of large granular lymphocytes (LGLs) as characterized by a predominance of CD3+CD8+CD57+CD28– T cells in blood and bone marrow. These authors postulated a mechanism whereby LGLs induce neutropenia, as has been described by CD95-triggered apoptosis from Fas and Fas-ligand secreted by LGLs and Fas/Fas-ligand–independent mechanisms.28,29 Although LGL infiltration was not noted in our bone marrow specimens or peripheral blood analyses, none of our samples were evaluated by flow cytometry. Another hypothesis regarding the cause of neutropenia concerns the role of tumor necrosis factor (TNF). Voog et al30 found that high circulating levels of the 75-kDa TNF receptor were associated with a greater incidence and a longer duration of grade 4 neutropenia among 101 patients treated with chemotherapy alone. Investigators described higher levels of TNF in a subset of patients receiving rituximab with CHOP compared with patients treated with CHOP alone.31 Further evaluation of these potential mechanisms is recommended for future study of rituximab-treated patients in whom neutropenia develops.
The complete absence of B cells 1 year after HCT in all patients receiving 2 courses of rituximab was notable. B-cell recovery was seen during the next 12 months, with a return to normal values in most patients evaluated at 24 months after transplantation. This delayed B-cell recovery is different from the experiences of patients who underwent HCT without rituximab, in whom B-cell recovery occurred between 3 and 6 months.32-34 Early experience with a single course of rituximab in patients who did not undergo transplantation showed prompt B-cell depletion, with recovery beginning at 6 months and recovery to the normal range occurring by 9 to 12 months after therapy.13,35 T-cell recovery was not clearly influenced by the addition of rituximab to HCT. Consistent with the literature, T-cell recovery in our study demonstrated relatively normal numbers of CD8+ cells, with CD4+ numbers remaining low for a year or longer, resulting in a persistently inverted CD4+/CD8+ ratio.36,37 Similarly, CD45+RO+ memory cell numbers far exceeded CD45+RA+ naive T-cell numbers during the first year. This phenomenon has been noted previously in patients recovering from HCT and suggests that T-cell recovery represents the expansion of mature T cells and a limited T-cell repertoire.32,37 We observed normal rather than elevated NK cell numbers but might have missed early elevations because our baseline measurements were taken 6 weeks after HCT.
Mean immunoglobulin levels were normal or near normal at study initiation and remained in this range during the follow-up period. However, despite normal mean values, immunoglobulin levels were suppressed in many patients throughout follow-up. Our results are not different from those for patients who undergo HCT without rituximab.37-39 In the course of this study, we evaluated the ability of patients to mount secondary humoral immune responses to recall antigens. Few patients were able to mount a new humoral immune response or to show evidence of a boosted humoral response to the T-cell–independent pathway required for pneumococcal vaccination. Better humoral responses were found for the conjugate H influenzae vaccine and for the T-cell–dependent tetanus vaccine. Because Storek et al40 found poor T-cell–independent B-cell responses up to 2 years after bone marrow transplantation, it is unclear whether rituximab adversely influenced humoral responses. However, van der Kolk et al41 found 11 patients to have significantly decreased responses to recall antigen, tetanus, and polio immunization and no responses to primary antigen, keyhole limpet hemocyanin (KLH), and hepatitis A immunization after rituximab monotherapy. It is possible that the extended elimination of B cells by rituximab could further blunt or even abrogate humoral immune responses after HCT. From a practical standpoint, we did not observe serious infections in our study. Other series using rituximab in patients who underwent HCT described divergent results, ranging from limited infections seen by Flinn et al9 and Mangel et al9,24 to fatal pneumonia and cytomegalovirus (CMV) reactivation reported by Ladetto et al,23 to septic deaths and CMV infections in the series of Rapoport et al25 and Goldberg et al.42 As described in Table 4, there are differences in rituximab scheduling in each of these studies, but it is not obvious that these subtle differences would result in highly disparate toxicity.
Results from our study illustrate that rituximab is a feasible adjuvant to HCT. Although delayed and severe neutropenia was observed, this toxicity did not result in significant adverse consequences, and patients responded rapidly to granulocyte-stimulating factors. Optimal dosing and scheduling for rituximab use during transplantation have not been defined. The ability of rituximab to effect the in vivo purging of circulating lymphoma cells and the molecular remission of follicular lymphoma advocates for the further study of this drug during mobilization. Alternatively, our study provides intriguing data regarding the use of rituximab after autologous ACHT. It is important to establish whether rituximab use during transplantation can improve cure rates in patients with relapsed or refractory DLCL. To that end, we strongly support the US Intergroup randomized, controlled trial comparing standard HCT with HCT that incorporates rituximab therapy.
Prepublished online as Blood First Edition Paper, August 7, 2003; DOI 10.1182/blood-2003-04-1257.
Supported by grants from the National Institutes of Health (CA 49605) and Genentech, Inc.
Presented in abstract form at the 43rd Annual Meeting of the American Society of Hematology, December 10, 2001, Orlando, FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal