We read with great interest the recently published article by Wagner et al1 illustrating the constitutive expression of granzyme B (GzmB) and perforin in human polymorphonuclear neutrophils (PMNs). The authors reported for the first time that GzmB and perforin are expressed not only in natural killer (NK) and cytotoxic T cells but also in human PMNs. Studies by our group (data not shown) confirm the findings by Wagner et al,1 but we were also able to identify constitutive expression of granzyme A (GzmA) in human PMNs.
It is well known that GzmA, GzmB, and perforin are cytolytic molecules that are found in acidic granules of NK and cytotoxic T cells. GzmA and GzmB independently and synergistically induce cell death in a perforin-dependent manner. Although it is not entirely clear whether perforin is necessary for the granzymes (Gzm's) to get into the cells, perforin is required for the release of Gzm's from the endocytic compartment into the cytosol and for trafficking to the nucleus. Whereas GzmB can induce apoptosis via the caspase-dependent as well as -independent pathways, GzmA uses a caspase-independent pathway to induce rapid cell death. So far as it has been described, GzmA seems to focus on disrupting the mitochondrial transmembrane potential by unknown mechanisms (reviewed in Lieberman2 ).
Since cell death occurs quite rapidly in PMNs undergoing phagocytosis,3 suggesting a cell death receptor-independent pathway, we tested whether perforin, GzmB, and GzmA are expressed in human PMNs. Therefore, we isolated PMNs by density gradient centrifugation and hypotonic lysis. Immediately after isolation, PMNs were pretreated with saponin and stained intracellularly with a monoclonal antibody recognizing human GzmA (clone: CB9; BD PharMingen, San Diego, CA) and analyzed using Epics XL-MCL Flow Cytometry System (Beckman Coulter, Fullerton, CA). According to a standard protocol, Western blotting was performed using a polyclonal antibody against human GzmA (clone: H-96; Santa Cruz Biotechnology, Santa Cruz, CA). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using the following primers for linear amplification of GzmA (GenBank accession no. NM 006144): forward 5′-ttt ctg gca tcc tct ctc tca-3′ and reverse 5′-ggg tca tag cat gga tag gg-3′ (yielding a 304-bp product).
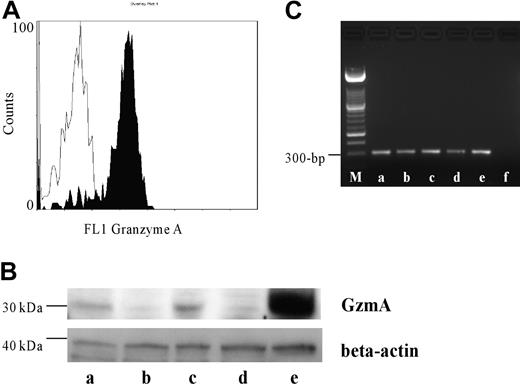
For the first time a wide variation of constitutive GzmA expression was detected by flow cytometry (GzmA-positive cells: 43.2%-89.5%; n = 5) as well as by Western blotting (Figure 1). This is contradictory to findings of Sayers et al,4 but can be explained by a methodological problem in their experimental settings (storage of specimens at -70°C) and by a substantial interindividual variation in the amount of GzmA in PMNs. Preliminary data suggest an inverse relation between the amount of GzmA expression and the rate of phagocytosis-induced apoptosis of PMNs (data not shown). In summary, we provide new information regarding GzmA as a cytotoxic weapon of PMNs, which will lead to a further understanding of the cytotoxic and apoptotic capacity of human PMNs.
Expression of GzmA in human PMNs. (A) PMNs of 5 different donors were intracellularly stained with antihuman GzmA (black profile) and the respective isotype control (white profile). A representative histogram is shown. (B) Western blotting revealed a strong band between 29 and 35 kDa in PMNs and peripheral blood mononuclear cells (PBMCs), which served as a positive control, typically for GzmA. The blot was reblotted with a mouse antihuman beta-actin antibody to demonstrate that comparable amounts of protein were used. Lanes a through d show human PMNs of 4 different donors, and lane e shows human PBMCs. (C) RT-PCR was performed as described above. Lane a shows human PBMCs as positive control; lanes b through e, PMNs from 5 different donors; and lane f, negative control. M indicates length marker.
Expression of GzmA in human PMNs. (A) PMNs of 5 different donors were intracellularly stained with antihuman GzmA (black profile) and the respective isotype control (white profile). A representative histogram is shown. (B) Western blotting revealed a strong band between 29 and 35 kDa in PMNs and peripheral blood mononuclear cells (PBMCs), which served as a positive control, typically for GzmA. The blot was reblotted with a mouse antihuman beta-actin antibody to demonstrate that comparable amounts of protein were used. Lanes a through d show human PMNs of 4 different donors, and lane e shows human PBMCs. (C) RT-PCR was performed as described above. Lane a shows human PBMCs as positive control; lanes b through e, PMNs from 5 different donors; and lane f, negative control. M indicates length marker.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal