Abstract
Deficiency of granulocyte-macrophage colony-stimulating factor (GM-CSF) in mice results in pulmonary alveolar proteinosis (PAP) from impaired surfactant catabolism by alveolar macrophages (AMs). Recently, we have shown that neutralizing anti-GM-CSF autoantibodies develop specifically in patients with idiopathic pulmonary alveolar proteinosis (iPAP). Analogous to murine PAP models, it is plausible that the autoantibodies reduce GM-CSF activity, resulting in AM dysfunction and surfactant accumulation. To examine this hypothesis, we estimated the neutralizing activity of the autoantibodies in the lungs of patients and characterized their biologic properties. GM-CSF bioactivity was completely abrogated in the bronchoalveolar lavage fluid (BALF) of patients with iPAP but not in healthy subjects. Autoantibodies were present in the alveoli in high concentrations and colocalized with GM-CSF. They recognized human GM-CSF with high avidity (KAV = 20.0 ± 7.5 pM) and high specificity, reacting with its superstructure and neutralizing GM-CSF activity to a level 4000 to 58 000 times the levels of GM-CSF normally present in the lung. Although target epitopes varied among patients, GM-CSF amino acids 78 to 94 were consistently recognized. Thus, autoantibodies bind GM-CSF with high specificity and high affinity, exist abundantly in the lung, and effectively block GM-CSF binding to its receptor, inhibiting AM differentiation and function. Our data strengthen the evidence associating anti-GM-CSF autoantibodies with the pathogenesis of this disease. (Blood. 2004;103:1089-1098)
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disorder characterized by the excessive accumulation of surfactant lipids and proteins in alveoli, resulting in impaired gas exchange and respiratory insufficiency.1-3 Clinically, PAP is divided into congenital, secondary, and idiopathic forms, with the latter comprising more than 90% of cases.2 Idiopathic PAP (iPAP) has a variable clinical course ranging from spontaneous remission to respiratory failure, and it can be complicated by secondary infections, frequently with opportunistic pathogens.2
The pathogenesis of iPAP is unknown. However, the observation that histologically similar PAP occurs in mice genetically deficient in granulocyte macrophage-colony-stimulating factor (GM-CSF) (GM-/- mice) or its receptor (GM Rβc-/- mice) suggested that an abnormality in GM-CSF signaling may be involved.4-7 Clearance of surfactant lipids and surfactant proteins by alveolar macrophages (AMs) in these mice is severely impaired, thus providing an explanation for the increase in surfactant accumulation.8 Murine PAP can be corrected by the local expression of GM-CSF in the lungs (GM-/- mice)9-12 or by bone marrow transplantation from a wild-type (GM Rβc-/-) mouse.13 AMs from GM-/- mice also display a number of molecular, morphologic, and functional abnormalities in addition to defective surfactant catabolism, all of which are corrected by the reconstitution of GM-CSF in the lungs.9-11,14,15 AMs from GM-/- mice are deficient in the macrophage-differentiation-inducing transcription factor, PU.1: the presence and level of PU.1 in AMs is determined by the presence and level of GM-CSF in the lung.12,16,17 Furthermore, retroviral-mediated PU.1 expression in cultured GM-/-AMs corrects all the observed AM defects described above, including surfactant protein catabolism.12 These data show that GM-CSF exerts its critical role over surfactant homeostasis by acting locally in the murine lung and stimulating AM terminal differentiation.12,15-17
Several lines of evidence suggest that GM-CSF bioactivity in the lung is also critical for surfactant homeostasis in humans. First, the histopathologic abnormalities of the lung in iPAP in humans strongly resemble those of GM-/- mice.1,4-7,18,19 Second, AMs in murine and human iPAP share a number of similar morphologic and functional abnormalities, including large “foamy” appearance,4,5,18,20 decreased phagocytosis,14,16,18,19,21 and reduced cellular adherence.14,18 Third, bronchoalveolar lavage fluid (BALF) and sera from patients with iPAP contain polyclonal autoantibodies (hereafter referred to as autoantibodies) that bind and neutralize human GM-CSF.22-24 Fourth, these autoantibodies are specific for iPAP and have not been detected in patients with congenital or secondary PAP, other lung diseases, or healthy persons.22,23 Because physiological levels of GM-CSF protein are normally low, including levels in the lungs that are on the order of several picograms per milliliter BALF,25 the presence of neutralizing autoantibodies might have an important effect on GM-CSF bioactivity levels in the lung.
Based on these findings in animals and humans, we hypothesized that autoantibodies in iPAP bind and neutralize GM-CSF, thus producing a functional GM-CSF deficiency in the lungs. This reduces GM-CSF bioactivity in the lungs to levels below the threshold required for the maintenance of normal AM functions, including surfactant catabolism. To address this hypothesis, we established a GM-CSF bioassay and used this to measure GM-CSF bioactivity levels in the BALF of iPAP patients and controls and to evaluate the relationship between pulmonary autoantibody levels and GM-CSF bioactivity. In addition, the avidity and specificity of binding to GM-CSF, neutralizing capacity, and GM-CSF-binding epitopes were determined for autoantibodies from a number of iPAP patients. Results indicate that GM-CSF-neutralizing capacity in the lungs is in vast excess of pulmonary GM-CSF levels, demonstrating that anti-GM-CSF antibodies are functionally important in iPAP, where they eliminate GM-CSF activity and thus GM-CSF-dependent signaling in the lung.
Patients, materials, and methods
Study subjects
All clinical samples were collected from 7 hospitals in Japan participating in the study of iPAP after written, informed consent was obtained under protocols approved by the institutional review boards of the 7 participating hospitals. A diagnosis of iPAP was suspected from historical, physical, radiographic, and laboratory data and was confirmed by biochemical analysis of BALF, pulmonary histopathologic findings, or both. None of the iPAP patients had a history of intercurrent or antecedent illnesses associated with secondary PAP,2 including hematologic disorders, infectious disease, or toxic pulmonary inhalation syndromes. None of the patients or subjects reported here have been included in prior reports on PAP from our group, and none had received treatment with exogenous GM-CSF. Sera were obtained from 107 patients with iPAP, 19 healthy control subjects, and 10 patients with other lung diseases (idiopathic pulmonary fibrosis [n = 3], sarcoidosis [n = 3], acute respiratory distress syndrome [n = 2], collagen vascular disease [n = 2]). BALF was obtained from 34 patients with iPAP, 18 healthy control subjects, and 14 patients with other lung diseases (eosinophilic pneumonia [n = 4], idiopathic pulmonary fibrosis [n = 4], sarcoidosis [n = 3], hypersensitivity pneumonitis [n = 2], eosinophilic granuloma [n = 1]). Open-lung biopsy specimens were obtained from 4 patients with iPAP and 4 patients undergoing resection of surgically operable lung cancer.
Reagents
GM-CSFs. The following recombinant GM-CSFs were kindly provided by the researchers or companies indicated: human (rhGM-CSF), Escherichia coli derived (Kirin Brewery, Takasaki, Japan), Chinese hamster ovary (CHO) cell derived (Novartis Pharma, Basel, Switzerland), yeast derived (Immunex, Seattle, WA); murine (Kirin Brewery); bovine (Dr Yuichi Yokomizo, National Institute of Animal Health, Tsukuba, Japan). Iodine-125 (125I)-Bolton-Hunter-labeled rhGM-CSF (E coli derived) was purchased from NEN Life Science Products (Boston, MA). Recombinant carboxymethylated GM-CSF was produced according to the method described previously.26 Trypsin-digested rhGM-CSF was prepared as described.27
Other cytokines. Recombinant human interleukin-3 (IL-3), IL-4, IL-10, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were purchased from R&D Systems (Minneapolis, MN).
Antibodies. A polyclonal, epitope-specific, rabbit antihuman GM-CSF antibody was obtained after immunization with an rhGM-CSF peptide comprised of amino acid residues 54 to 73 and was purified by affinity chromatography. Epitope-specific murine antihuman GM-CSF monoclonal antibody sets 4117, 1089, 3092, and 1022, which recognize amino acid residues 1 to 11, 40 to 77, 78 to 94, and 110 to 127, respectively, of rhGM-CSF28 were kindly provided by Dr Kanakura (Osaka University Medical School, Japan).
Cell line. A GM-CSF-dependent cell line, TF-1,29 was kindly provided by Dr Kitamura (University of Tokyo, Japan).
Human alveolar macrophage cultures
Using flexible bronchoscopy, human AMs were obtained from iPAP patients and controls under conscious sedation and local anesthesia (lidocaine). Briefly, three 50-mL aliquots of normal saline were instilled and suctioned sequentially from the right middle lobe or lingula. Cells were enumerated by hemocytometer, cytocentrifuge sediments were prepared and stained with Diff-Quick, and differential cell counts were performed on 500 cells per person. AMs were isolated by adhesion to plastic culture dishes for 1 hour. Adherent cells were seeded into 96-well plates (5 × 104 cells/well) in RPMI 1640 medium with or without 20% (vol/vol) cell-free BALF from either iPAP patients or healthy controls. In some experiments, the culture medium also contained rhGM-CSF (20 ng/mL). Cells were incubated for 14 days (37°C, 5% CO2). Cell viability was measured by trypan blue dye exclusion, and cell growth was measured by the 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich, St Louis, MO) assay method, as described previously.22,30
Immunohistochemical localization of GM-CSF
GM-CSF was localized in the lung by immunohistochemical staining on paraffin-embedded lung sections from 4 iPAP patients or 4 controls using a monoclonal murine antihuman GM-CSF antibody (R&D Systems), as described previously.31 Control lung tissues were obtained from the normal lung parenchyma of surgical specimens removed for the resection of lung cancer nodules. Immunohistochemical staining for surfactant apoprotein A (SP-A) was used to identify alveolar type II cells using a murine antihuman SP-A antibody (DAKO, Carpinteria, CA) as above. Color development was performed using 3-amino-9-ethyl carbazole (AEC) liquid substrate chromogen (DAKO) for GM-CSF and diaminobenzidine (DAB) (Nichirei, Tokyo, Japan) for SP-A.
Quantification of GM-CSF bioactivity
TF-1 cells (2 × 104 cells/well) were cultured (37°C, 5% CO2) in microtiter plates for 3 days in macrophage-SFM medium (Invitrogen, Carlsbad, CA) containing rhGM-CSF in concentrations ranging from 0 to 20 ng/mL without or with BALF (20% vol/vol) from iPAP patients or healthy controls. TF-1 cell survival was evaluated using the MTT assay (Sigma-Aldrich) as described previously.22,30 The percentage of cell survival was calculated using the equation, cell survival (%) = 100 × (A - B)/(C - B), where A is the absorbance of TF-1 cells grown in the presence of rhGM-CSF and BALF, B is the absorbance of TF-1 cells grown in medium only, and C is the absorbance of TF-1 cells grown without BALF but containing GM-CSF (20 ng/mL). The concentration of exogenously added rhGM-CSF that was required for 50% survival of TF-1 cells (SC50, ng/mL) in each sample was determined. GM-CSF bioactivity was determined using the equation GM-CSF bioactivity = SC50(C) - SC50(B), where GM-CSF bioactivity is expressed as nanogram equivalents of GM-CSF/mL BALF, SC50(C) is the concentration of exogenously added rhGM-CSF required for 50% survival of cells without added BALF, and SC50(B) is the concentration of exogenously added rhGM-CSF required for 50% survival of cells in the presence of BALF. Negative values for GM-CSF bioactivity in BALF are hereafter referred to as the neutralizing capacity of BALF.
Quantification and purification of anti-GM-CSF autoantibodies
Confocal microscopy
Lung tissues resected from an iPAP patient or a healthy control were fixed in periodate-lysine-paraformaldehyde (PLP).34 Sections were incubated in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), immunostained with fluorescein isothiocyanate (FITC)-labeled antihuman immunoglobulin G (IgG; BD Biosciences, San Jose, CA) or with phycoerythrin (PE)-labeled GM-CSF (R&D Systems), and examined by confocal laser microscopy (Carl Zeiss, Tokyo, Japan). Serial sections from an iPAP patient was immunostained with FITC-labeled antimurine IgG (Nichirei) and PE-labeled G-CSF (R&D Systems) to exclude nonspecific binding. Hematoxylin-eosin was used for counterstaining.
GM-CSF autoantibody immune complexes
BALF from iPAP patients or healthy controls was delipidated with 1-butanol, and the protein fraction was precipitated with cold acetone. The protein pellet was resuspended in PBS, incubated with protein-A Sepharose 4 Fast Flow (Amersham Biosciences, Buckinghamshire, United Kingdom; 4°C, overnight), and washed with PBS/0.1% Tween 20. Bound proteins were eluted with glycine-HCl (100 mM, pH 2.7) and reprecipitated with 80% cold acetone. Bound and unbound protein fractions were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA), and GM-CSF was detected using rabbit antihuman GM-CSF antibodies (1:1000; R&D Systems), horseradish peroxidase-conjugated goat antirabbit IgG antibodies (1:100; Nichirei), and ECL Plus (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).
Delipidated BALF proteins were subjected to PAGE under nonreducing conditions, followed by Western blot analysis as described. IgG in the BALF proteins was also detected by Western blot analysis using horseradish peroxidase-conjugated goat antihuman IgG (1:3000; DAKO) and ECL Plus.
BALF (100 μL) from iPAP patients or healthy controls was incubated with [125I]-GM-CSF (0.03 pmol; 2 hours, room temperature) and then subjected to PAGE under nonreducing conditions followed by autoradiography.
Characterization of autoantibodies
GM-CSF binding avidity and capacity of purified autoantibodies were determined using the [125I]-GM-CSF binding assay method of Svenson et al.35 Briefly, sera IgG from iPAP patients were isolated by protein-G Sepharose column chromatography (Amersham Biosciences) and quantified as described. Autoantibodies from each person were analyzed separately. Calculations were performed using molecular weights of 14.7 and 146 kDa for rhGM-CSF and IgG, respectively, and the results were expressed in picomolars. The binding capacity (Bmax, sites/mol IgG) was determined from the plateau value of the saturation-binding plot. The average affinity (KAV) was defined as the concentration of free GM-CSF at 50% Bmax.
The binding specificity of autoantibodies for GM-CSF was determined using sandwich enzyme-linked immunosorbent assay (ELISA). Micro-ELISA plates (Nunc, Roskilde, Denmark) were coated with rhGM-CSF produced in E coli (as a standard) or GM-CSF produced in CHO cells, the large trypsin fragment of rhGM-CSF, carboxymethylated rhGM-CSF, murine or bovine GM-CSF, or various human cytokines (all at 1 μg/mL) overnight, and then with 1% BSA/PBS. Plates were then incubated with purified autoantibody (100 ng/mL) for 1 hour. The reactivity of the autoantibodies in each well was measured by the method described above and was expressed as an OD ratio to that of E coli-derived rhGM-CSF.
The GM-CSF-neutralizing capacity of autoantibodies was determined as previously described using a bioassay based on GM-CSF-stimulated proliferation of TF-1 cells.22,30 Briefly, TF-1 cells (4 × 104 cells/well) were cultured (37°C, humidified atmosphere, 5% CO2) for 3 days in medium containing rhGM-CSF (5 ng/mL) and various concentrations of purified autoantibodies, murine antihuman GM-CSF monoclonal antibody (R&D Systems), goat antihuman GM-CSF polyclonal antibody (R&D Systems), or human IgG (Sigma-Aldrich). The percentage of growth inhibition was calculated from the following equation: Growth inhibition (%) = 100 × (A - B)/(A - C), where A, B, and C are the absorbances of TF-1 cells grown with rhGM-CSF (5 ng/mL) alone, rhGM-CSF and anti-GM-CSF antibodies or the negative control, and medium alone, respectively. The concentration of each autoantibody at 50% growth inhibition (IC50) was obtained from each growth inhibition curve. GM-CSF epitope mapping of autoantibodies was performed using a competitive binding assay using murine monoclonal antibodies that recognize specific regions of human GM-CSF.28 Purified autoantibodies (0-1 μg/mL) were mixed individually with the murine monoclonal anti-GM-CSF epitope-specific antibodies 4117, 1089, 3092, and 1022 (each at 1 μg/mL). These murine antibodies recognize rhGM-CSF residues 1-11, 40-77, 78-94, and 110-127, respectively.28 Polyclonal rabbit anti-GM-CSF peptide 54-73 was used as a positive control, and human IgG (Sigma-Aldrich) was used as a negative control. Antibody mixtures were then incubated (4°C, overnight) in PBS containing [125I]-GM-CSF (1 ng/mL), 2% BSA, and 0.1% Triton X-100. Mixtures were then transferred to individual wells of a 96-well plate (FlashPlate Plus; Perkin-Elmer, Boston, MA) that had been previously coated with goat antimouse IgG and plastic scintillant. Plates were incubated (room temperature, 2 hours) and washed in PBS/0.1% Tween 20, and bound radioactivity was counted in a TopCount NXT Microplate Scintillation and Luminescence Counter (Perkin-Elmer). The ability of autoantibodies to inhibit the binding of GM-CSF region-specific monoclonal antibodies was determined using the following formula: Binding inhibition (%) = 100 × [Cpm(A) - Cpm(B)]/[Cpm(A) - Cpm(C)], where Cpm(A) is the radioactivity in wells containing murine monoclonal antibody and [125I]-GM-CSF but no autoantibodies; Cpm(B) is the radioactivity in wells containing autoantibodies, murine monoclonal antibody, and [125I]-GM-CSF; and Cpm(C) is the radioactivity in wells containing [125I]-GM-CSF but neither murine monoclonal antibody nor autoantibodies.
Statistical analysis
Statistical analyses were performed on a microcomputer using StatView (version 4.0) (Abacus Concepts, Berkeley, CA) using the Mann-Whitney U test or the Kruskal-Wallis rank sum procedures for nonparametric data. P < .05 was considered significant.
Results
AM growth and development are inhibited by iPAP bALF
As shown in Table 1, BALF from patients with iPAP contained fewer AMs and increased the number of lymphocytes compared with that from age-matched healthy subjects. AMs isolated from iPAP BALF by plastic adhesion had “monocyte-like” morphology and were smaller than those from healthy controls (Figure 1A). To determine whether an inhibitory activity for AM growth or function is present in the iPAP lung, we incubated AMs from iPAP patients in the presence of culture medium alone or medium plus iPAP- or control-BALF in the absence or presence of GM-CSF (20 ng/mL). AMs from iPAP patients were viable but remained small after 14 days in culture with medium alone or in medium containing iPAP-BALF (Figure 1B). In contrast, iPAP-AMs developed a normal appearance and were larger in medium containing normal BALF with or without added GM-CSF. Although iPAP-AMs failed to grow and develop a normal appearance in medium plus iPAP-BALF, they did so when additional GM-CSF (20 ng/mL) was added. The effects of control-BALF or GM-CSF on iPAP-AM growth were confirmed with an MTT assay (data not shown). Cell viability in each instance was greater than 90%. These data establish that GM-CSF bioactivity is reduced in the iPAP-BALF by an inhibitory substance that can be overcome with the addition of GM-CSF.
Cellular constituents in the BALF
. | . | . | . | . | . | Median cell differentials . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Median BAL cell density, ×104/mL . | Macrophage . | . | Lymphocyte . | . | Neutrophil . | . | Eosinophil . | . | |||||||
. | No. subjects . | Sex, F/M . | Median age, y . | Median BAL recovery, % . | . | No., ×104/mL . | % . | No., ×104/mL . | % . | No., ×104/mL . | % . | No., ×104/mL . | % . | |||||||
| Patients with iPAP | 34 | 10/24 | 52 (28-79) | 70.2 (48.7-73.3) | 10.5 (2.8-55.9) | 4.2 (2.17-26.5)* | 60.0 (5.9-90.9)* | 3.2 (0.5-52.6)* | 32.5 (6.8-94.1)* | 0.3 (0.0-4.8) | 2.3 (0.0-15.0)* | 0.0 (0.0-0.3) | 0.3 (0.0-6.3) | |||||||
| Control | 18 | 10/8 | 50 (27-72) | 67.0 (59.3-77.0) | 14.0 (3.8-42.2) | 13.2 (3.3-39.8) | 93.3 (84.0-96.8) | 0.8 (0.2-3.1) | 6.0 (2.9-14.0) | 0.1 (0.0-0.4) | 0.3 (0.0-2.0) | 0.0 (0.0-0.2) | 0.0 (0.0-1.0) | |||||||
. | . | . | . | . | . | Median cell differentials . | . | . | . | . | . | . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Median BAL cell density, ×104/mL . | Macrophage . | . | Lymphocyte . | . | Neutrophil . | . | Eosinophil . | . | |||||||
. | No. subjects . | Sex, F/M . | Median age, y . | Median BAL recovery, % . | . | No., ×104/mL . | % . | No., ×104/mL . | % . | No., ×104/mL . | % . | No., ×104/mL . | % . | |||||||
| Patients with iPAP | 34 | 10/24 | 52 (28-79) | 70.2 (48.7-73.3) | 10.5 (2.8-55.9) | 4.2 (2.17-26.5)* | 60.0 (5.9-90.9)* | 3.2 (0.5-52.6)* | 32.5 (6.8-94.1)* | 0.3 (0.0-4.8) | 2.3 (0.0-15.0)* | 0.0 (0.0-0.3) | 0.3 (0.0-6.3) | |||||||
| Control | 18 | 10/8 | 50 (27-72) | 67.0 (59.3-77.0) | 14.0 (3.8-42.2) | 13.2 (3.3-39.8) | 93.3 (84.0-96.8) | 0.8 (0.2-3.1) | 6.0 (2.9-14.0) | 0.1 (0.0-0.4) | 0.3 (0.0-2.0) | 0.0 (0.0-0.2) | 0.0 (0.0-1.0) | |||||||
Numbers in parentheses are ranges.
P < .05 compared with control.
Morphology and in vitro growth of adherent AMs from a patient with iPAP. (A) Adherent AMs from a patient with iPAP and a healthy subject. iPAP AMs showed a small, monocyte-like appearance with basophilic cytoplasm (phase-contrast micrograph [i], original magnification × 200; and Wright-Giemsa staining [ii], original magnification × 1000), whereas those from control-BALF had large eosinophilic cytoplasm (phase-contrast micrograph [iii], original magnification × 200; and Wright-Giemsa staining [iv], original magnification × 1000). (B) After incubation with control-BALF for 14 days, iPAP-AM size increased markedly (ii) (original magnification × 200) compared with before incubation (Ai) or incubation with medium alone (i), and adding 20 ng/mL GM-CSF further promoted growth (iii). After incubation with iPAP-BALF, AMs remained small (iv), but adding 20 ng/mL GM-CSF restored their growth (v).
Morphology and in vitro growth of adherent AMs from a patient with iPAP. (A) Adherent AMs from a patient with iPAP and a healthy subject. iPAP AMs showed a small, monocyte-like appearance with basophilic cytoplasm (phase-contrast micrograph [i], original magnification × 200; and Wright-Giemsa staining [ii], original magnification × 1000), whereas those from control-BALF had large eosinophilic cytoplasm (phase-contrast micrograph [iii], original magnification × 200; and Wright-Giemsa staining [iv], original magnification × 1000). (B) After incubation with control-BALF for 14 days, iPAP-AM size increased markedly (ii) (original magnification × 200) compared with before incubation (Ai) or incubation with medium alone (i), and adding 20 ng/mL GM-CSF further promoted growth (iii). After incubation with iPAP-BALF, AMs remained small (iv), but adding 20 ng/mL GM-CSF restored their growth (v).
Pulmonary GM-CSF expression is not diminished in iPAP
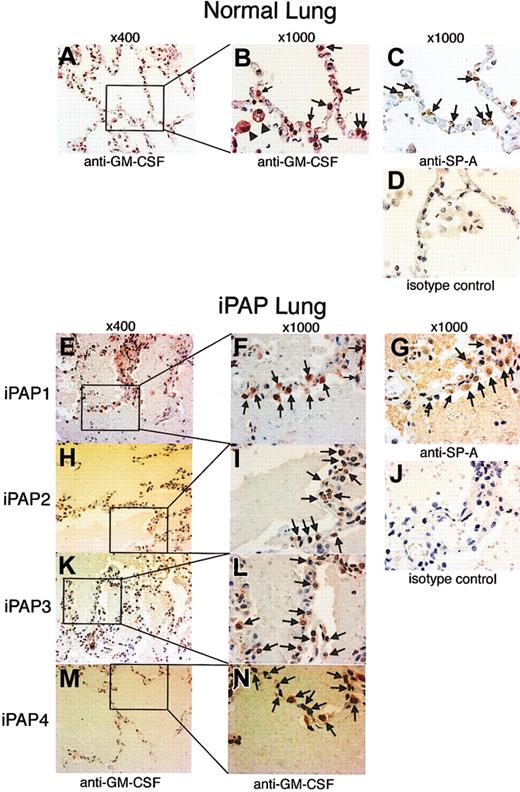
Functional GM-CSF activity appeared to be absent in BALF from iPAP patients, but it could be restored with the addition of large amounts of GM-CSF to medium containing iPAP BALF. Therefore, we evaluated GM-CSF levels in lung tissues from 4 iPAP patients and 4 controls using an immunohistochemical approach. Epithelial cells projecting into the alveolar lumen were strongly stained with anti-GM-CSF antibody in iPAP and control lungs (Figure 2). These cells were also stained with anti-SP-A antibody, establishing that they are alveolar type 2 epithelial cells. The proportion of alveolar epithelial cells showing GM-CSF expression was evaluated by immunohistochemistry, with similar percentages found for iPAP patients (n = 4; 50.5% ± 8.4%) and controls (n = 4; 40.8% ± 5.0%) (P = .08). Thus, GM-CSF protein production in the lungs of iPAP patients does not appear to be reduced compared with normal lungs.
Immunohistochemical detection of GM-CSF in the lungs of patients with iPAP. Lung tissue biopsy specimens from 4 patients with iPAP or 4 healthy controls were assessed for the presence of GM-CSF and SP-A by immunohistochemical staining. (A-D) Sections of normal lung. AM (B, arrowheads) and alveolar epithelial cells (B, arrows) were stained red by anti-GM-CSF antibody (A-B). Alveolar epithelial cells stained by anti-GM-CSF antibodies were stained brown by anti-SP-A antibodies on the serial section (C, arrows). Therefore, they were likely to be alveolar type 2 cells. (E-N) Sections of iPAP lung. Alveolar epithelial cells (F, I, L, N, arrows) stained red. Similar cells were stained brown on the serial section (G, arrows). Original magnifications are indicated above each column.
Immunohistochemical detection of GM-CSF in the lungs of patients with iPAP. Lung tissue biopsy specimens from 4 patients with iPAP or 4 healthy controls were assessed for the presence of GM-CSF and SP-A by immunohistochemical staining. (A-D) Sections of normal lung. AM (B, arrowheads) and alveolar epithelial cells (B, arrows) were stained red by anti-GM-CSF antibody (A-B). Alveolar epithelial cells stained by anti-GM-CSF antibodies were stained brown by anti-SP-A antibodies on the serial section (C, arrows). Therefore, they were likely to be alveolar type 2 cells. (E-N) Sections of iPAP lung. Alveolar epithelial cells (F, I, L, N, arrows) stained red. Similar cells were stained brown on the serial section (G, arrows). Original magnifications are indicated above each column.
GM-CSF bioactivity is reduced in the lungs of iPAP
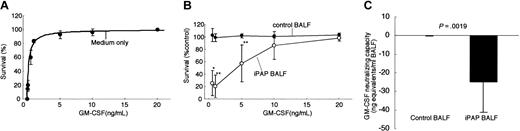
Discordance between the physical presence and functional activity of GM-CSF was apparent in the BALF of iPAP patients but not in controls. Therefore, we measured the bioactivity of GM-CSF in BALF using a bioassay based on GM-CSF-dependent growth stimulation of TF-1 cells.22,30 Increasing concentrations of GM-CSF stimulated an increasing proliferation of TF-1 cells, reaching a maximum at very low doses on the order of 2 to 3 ng/mL (Figure 3A). Adding BALF from healthy controls (n = 6; 20% vol/vol) had no effect on the survival of TF-1 cells at equivalent doses of exogenous GM-CSF, suggesting that neither large amounts of GM-CSF nor GM-CSF inhibitory activity was present (Figure 3B). In contrast, adding BALF from iPAP patients (n = 8; 20% vol/vol) shifted the viability curve significantly to the right, demonstrating the presence of a potent GM-CSF-inhibitory activity (Figure 3B). Importantly, the GM-CSF-inhibitory activity was reversible as maximum survival was achieved with high doses of exogenous GM-CSF (20 ng/mL). GM-CSF bioactivity in BALF was -24.9 ± 16.4 ng/mL (median, -23.3; range, -57.5 to -4.3) for iPAP and 0.017 ± 0.387 ng/mL (median, -0.126; range, -0.34 to 0.63) for healthy controls (Figure 3C). We also determined GM-CSF levels in control BALF to be 1.07 ± 0.16 pg/mL (range, 0.88-1.28) using an ELISA system (AN'ALYSA; R&D Systems), so GM-CSF-neutralizing capacity in iPAP-BALF was present in vast excess over the levels of GM-CSF normally present within the lung.
GM-CSF bioactivity is severely reduced in the lungs of iPAP patients. (A) Bioassay used to quantify GM-CSF bioactivity in BALF. TF-1 cells survived and proliferated in a dose-dependent fashion in the presence of GM-CSF added to the culture medium. Each point represents the mean of 6 determinations. (B) Quantification of inhibition of GM-CSF bioactivity by BALF of iPAP patients. Addition of 20% (vol/vol) BALF from healthy control subjects (closed symbols, n = 6 per point) or iPAP patients (open circles, n = 8 per point). Compared with cells cultured with medium without BALF (as in panel A), adding BALF from healthy controls had no measurable effect on the GM-CSF-dependent survival of TF-1 cells. In contrast, iPAP-BALF significantly inhibited GM-CSF-dependent survival of TF-1 cells (**P < .002 or *P < .05). This inhibitory effect was overcome by the addition of large amounts of GM-CSF to the medium. (C) Deficit of pulmonary GM-CSF bioactivity in iPAP. The bioactivity of GM-CSF in BALF from healthy controls or iPAP patients was calculated as described in “Patients, materials, and methods” and was shown as aggregate data in panel B. The deficit in GM-CSF bioactivity is expressed in nanogram of GM-CSF per milliliter of BALF. Error bars indicate SD.
GM-CSF bioactivity is severely reduced in the lungs of iPAP patients. (A) Bioassay used to quantify GM-CSF bioactivity in BALF. TF-1 cells survived and proliferated in a dose-dependent fashion in the presence of GM-CSF added to the culture medium. Each point represents the mean of 6 determinations. (B) Quantification of inhibition of GM-CSF bioactivity by BALF of iPAP patients. Addition of 20% (vol/vol) BALF from healthy control subjects (closed symbols, n = 6 per point) or iPAP patients (open circles, n = 8 per point). Compared with cells cultured with medium without BALF (as in panel A), adding BALF from healthy controls had no measurable effect on the GM-CSF-dependent survival of TF-1 cells. In contrast, iPAP-BALF significantly inhibited GM-CSF-dependent survival of TF-1 cells (**P < .002 or *P < .05). This inhibitory effect was overcome by the addition of large amounts of GM-CSF to the medium. (C) Deficit of pulmonary GM-CSF bioactivity in iPAP. The bioactivity of GM-CSF in BALF from healthy controls or iPAP patients was calculated as described in “Patients, materials, and methods” and was shown as aggregate data in panel B. The deficit in GM-CSF bioactivity is expressed in nanogram of GM-CSF per milliliter of BALF. Error bars indicate SD.
Autoantibodies occur at high levels in patients with iPAP
We sought to extend and quantify our observation regarding the presence of autoantibodies (anti-GM-CSF autoantibodies) in iPAP in a large cohort. Therefore, the levels of autoantibodies were quantified by ELISA in the BALF and sera of patients with iPAP or other lung disorders and in healthy controls. BALF and sera of iPAP patients contained high concentrations of autoantibodies (median, 1.15; range, 0.09-5.40 μg/mL in BALF [n = 34]; median, 88.6; range, 16.6-470 μg/mL in sera [n = 107], respectively) in contrast to healthy controls and patients with other lung diseases that were all under the detection limit (0.05 μg/mL for BALF and 3 μg/mL for serum) (Table 2). Thus, autoantibody levels are high in the lungs and sera of patients with iPAP and are specific for this disorder.
High levels of anti-GM-CSF autoantibodies in iPAP patients
Sample . | iPAP median, μg/mL . | Healthy control*median, μg/mL . | Other lung disorders*median, μg/mL . |
|---|---|---|---|
| BALF | 1.15 (0.094-5.40), n = 34 | < 0.050, n = 18 | < 0.050, n = 14 |
| Serum | 88.6 (16.6-470), n = 107 | < 3.00, n = 19 | < 3.00, n = 10 |
Sample . | iPAP median, μg/mL . | Healthy control*median, μg/mL . | Other lung disorders*median, μg/mL . |
|---|---|---|---|
| BALF | 1.15 (0.094-5.40), n = 34 | < 0.050, n = 18 | < 0.050, n = 14 |
| Serum | 88.6 (16.6-470), n = 107 | < 3.00, n = 19 | < 3.00, n = 10 |
Numbers in parentheses are ranges.
Concentrations of autoantibodies in BALF and serum were all under the detection limit (0.05 μg/mL in BALF; 3 μg/mL in serum).
Localization of free autoantibodies in the lung of iPAP patients
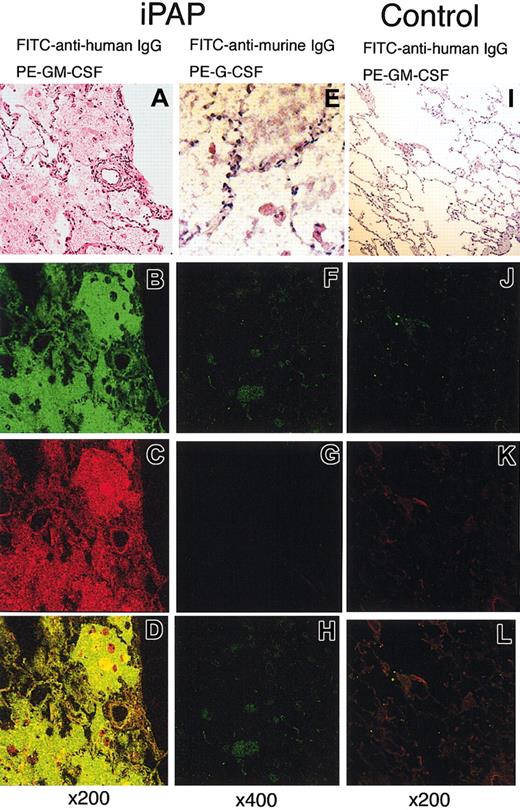
To demonstrate that GM-CSF-binding activity colocalized with immunoglobulin molecules in the lungs of iPAP patients, immunohistochemical staining was carried out with PE-conjugated GM-CSF and FITC-conjugated anti-IgG. By confocal microscopy, GM-CSF-binding activity and IgG were readily detected and demonstrated strong colocalization in the iPAP lung (Figure 4A-D). Nonspecific binding of PE- or FITC-conjugated proteins on the intra-alveolar material was ruled out by staining of the serial sections with FITC-conjugated antimurine-IgG and PE-conjugated human G-CSF (Figure 4E-H). The lungs of healthy controls had detectable, but vastly reduced, amounts of IgG, minimal or no GM-CSF-binding activity, and no colocalization (Figure 4I-L).
Confocal microscopic images of immunohistochemical double staining for the autoantibodies against GM-CSF. (A-H) Lung tissue from a patient with iPAP. (I-L) Histologically normal lung tissue from a patient with lung cancer. These tissues were stained with hematoxylin-eosin (A, E, I), FITC-conjugated antihuman IgG (green; B, J), FITC-conjugated antimurine IgG (green; F), PE-conjugated GM-CSF (red; C, K), and PE-conjugated G-CSF (red; G). A combination of both channels (B-C, F-G, J-K) clearly demonstrates the occurrence of the autoantibodies in the alveolar filling materials of the iPAP lung (D) but not in normal lung (L).
Confocal microscopic images of immunohistochemical double staining for the autoantibodies against GM-CSF. (A-H) Lung tissue from a patient with iPAP. (I-L) Histologically normal lung tissue from a patient with lung cancer. These tissues were stained with hematoxylin-eosin (A, E, I), FITC-conjugated antihuman IgG (green; B, J), FITC-conjugated antimurine IgG (green; F), PE-conjugated GM-CSF (red; C, K), and PE-conjugated G-CSF (red; G). A combination of both channels (B-C, F-G, J-K) clearly demonstrates the occurrence of the autoantibodies in the alveolar filling materials of the iPAP lung (D) but not in normal lung (L).
Autoantibodies form immune complexes in the lungs of iPAP patients
We next sought evidence of immune complexes containing GM-CSF by immunoprecipitation from BALF using protein-A Sepharose beads followed by Western blotting to detect GM-CSF. GM-CSF was detected in iPAP-BALF in the protein-A bound, but not unbound, fraction, consistent with the presence of GM-CSF bound to IgG (Figure 5A). GM-CSF was not detected in either fraction in BALF from healthy controls. When the BALF protein was evaluated by native gel electrophoresis and Western blotting, GM-CSF was also detected as a broad high-molecular-weight smear that coincided with a similar smear detected by blotting for IgG (Figure 5B). Using yet another approach, a “supershift” of GM-CSF was seen after incubation of [125I]-GM-CSF with iPAP-BALF but not with control-BALF (Figure 5C). Using several distinct experimental approaches, these data strongly support the notion that GM-CSF exists in the form of immune complexes in the lungs of iPAP patients.
Formation of GM-CSF-autoantibody immune complexes in iPAP patients. (A) Detection of GM-CSF containing immune complexes in the BALF in iPAP by immunoprecipitation. Protein A Sepharose beads were incubated with BALF from a healthy control (lanes 1-2), iPAP patient (lanes 3-4), or, as a control, purified yeast-derived rhGM-CSF (lane 5). Bound and unbound fractions (indicated) were separated and subjected to SDS-PAGE under reducing conditions and Western blotting using a rabbit anti-GM-CSF antibody. Under these conditions, the rhGM-CSF standard consists of 3 molecular species migrating at 19.5, 16.8, and 15.5 kDa. (B) Detection of GM-CSF and IgG in BALF by Western blotting. BALF from a healthy control (lanes 2, 4), iPAP patient (lanes 3, 5), or rhGM-CSF (lane 1) was subjected to native PAGE and then Western blotting using a rabbit anti-GM-CSF antibody (lanes 1-3) or anti-IgG antibody (lanes 4-5). (C) Supershift of [125I]-GM-CSF with iPAP-BALF. BALF from healthy controls (lanes 2-4) or iPAP patients (lanes 5-7) was incubated with [125I]-GM-CSF and then subjected to native PAGE and autoradiography. As a control, [125I]-GM-CSF alone was also included on the gel (lane 1).
Formation of GM-CSF-autoantibody immune complexes in iPAP patients. (A) Detection of GM-CSF containing immune complexes in the BALF in iPAP by immunoprecipitation. Protein A Sepharose beads were incubated with BALF from a healthy control (lanes 1-2), iPAP patient (lanes 3-4), or, as a control, purified yeast-derived rhGM-CSF (lane 5). Bound and unbound fractions (indicated) were separated and subjected to SDS-PAGE under reducing conditions and Western blotting using a rabbit anti-GM-CSF antibody. Under these conditions, the rhGM-CSF standard consists of 3 molecular species migrating at 19.5, 16.8, and 15.5 kDa. (B) Detection of GM-CSF and IgG in BALF by Western blotting. BALF from a healthy control (lanes 2, 4), iPAP patient (lanes 3, 5), or rhGM-CSF (lane 1) was subjected to native PAGE and then Western blotting using a rabbit anti-GM-CSF antibody (lanes 1-3) or anti-IgG antibody (lanes 4-5). (C) Supershift of [125I]-GM-CSF with iPAP-BALF. BALF from healthy controls (lanes 2-4) or iPAP patients (lanes 5-7) was incubated with [125I]-GM-CSF and then subjected to native PAGE and autoradiography. As a control, [125I]-GM-CSF alone was also included on the gel (lane 1).
Autoantibodies bind GM-CSF with high avidity and specificity
The absence of detectable GM-CSF bioactivity in BALF from iPAP patients suggested that the binding affinity of autoantibodies for GM-CSF might be sufficient to effectively compete for the binding of GM-CSF to its receptor. Thus, autoantibody binding avidity and capacity was determined by saturation-binding analysis using purified autoantibodies and [125I]-GM-CSF (Figure 6A). Autoantibodies purified from 11 iPAP patients had a similar, high avidity (19.96 ± 7.54 pM) in contrast to that of a commercial goat polyclonal antibody (275.4 pM) (Table 3). The binding capacity of autoantibodies from a range of iPAP patients was similar, with 0.24 ± 0.13 sites/mol IgG, indicating that 1.8 to 7.8 autoantibody molecules bound each molecule of GM-CSF (Table 3). Consistent with these avidity data, the autoantibodies exhibited strong neutralizing capacity. The mean neutralizing capacity of autoantibodies was 10- to 500-fold greater than those of the commercial neutralizing polyclonal or monoclonal antibodies tested, respectively (Table 3).
Binding affinity, capacity, and specificity of purified anti-GM-CSF autoantibodies in iPAP patients. (A) Saturation binding plot of [125I]-GM-CSF binding to purified anti-GM-CSF autoantibody. Vertical axis represents the molar fractional binding of GM-CSF to autoantibody. Binding capacity (maximal binding) was determined from the asymptote of the curve, and binding affinity (KAV) was determined from the concentration of GM-CSF at 50% of maximum binding. (B) Binding of autoantibodies to various forms of human GM-CSF. Reactivity of autoantibodies was measured by antigen capture and compared with binding to recombinant human GM-CSF produced in E coli, which was taken as 100%. Various forms were tested, including recombinant human GM-CSF produced in CHO cells; the large trypsin fragment that contained residues 31-58, 86-107, and 112-127, connected by disulfide bonds; and carboxymethylated GM-CSF, which had an intact primary amino acid sequence but lost the second and tertiary structure because of the loss of the 2 disulfide bonds. NS indicates not significant. (C) Xenospecificity of autoantibody binding. Reactivity of autoantibodies to murine and bovine GM-CSF was measured as above. (D) Specificity of autoantibody binding with respect to other human cytokines. Reactivity of autoantibodies to various human cytokines was measured as above. Error bars indicate SD.
Binding affinity, capacity, and specificity of purified anti-GM-CSF autoantibodies in iPAP patients. (A) Saturation binding plot of [125I]-GM-CSF binding to purified anti-GM-CSF autoantibody. Vertical axis represents the molar fractional binding of GM-CSF to autoantibody. Binding capacity (maximal binding) was determined from the asymptote of the curve, and binding affinity (KAV) was determined from the concentration of GM-CSF at 50% of maximum binding. (B) Binding of autoantibodies to various forms of human GM-CSF. Reactivity of autoantibodies was measured by antigen capture and compared with binding to recombinant human GM-CSF produced in E coli, which was taken as 100%. Various forms were tested, including recombinant human GM-CSF produced in CHO cells; the large trypsin fragment that contained residues 31-58, 86-107, and 112-127, connected by disulfide bonds; and carboxymethylated GM-CSF, which had an intact primary amino acid sequence but lost the second and tertiary structure because of the loss of the 2 disulfide bonds. NS indicates not significant. (C) Xenospecificity of autoantibody binding. Reactivity of autoantibodies to murine and bovine GM-CSF was measured as above. (D) Specificity of autoantibody binding with respect to other human cytokines. Reactivity of autoantibodies to various human cytokines was measured as above. Error bars indicate SD.
KAV, Bmax, and IC50of autoantibodies from patients with iPAP
Patient . | KAVavidity, pM . | Bmaxcapacity, sites/mol IgG . | IC50, mol/mol rhGM-CSF . |
|---|---|---|---|
| 1 | 20.1 | 0.129 | 5.84 |
| 2 | 13.3 | 0.153 | 4.43 |
| 3 | 16.7 | 0.168 | 3.83 |
| 4 | 24.9 | 0.253 | 6.65 |
| 5 | 11.1 | 0.134 | 8.66 |
| 6 | 24.8 | 0.332 | 6.65 |
| 7 | 12.1 | 0.194 | 2.42 |
| 8 | 15 | 0.184 | 11.88 |
| 9 | 22.3 | 0.565 | 2.42 |
| 10 | 37 | 0.273 | 12.08 |
| 11 | 22.3 | 0.281 | 12.69 |
| Average ± SD | 19.96 ± 7.54 | 0.24 ± 0.13 | 7.05 ± 3.81 |
| Commercialized goat anti—GM-CSF* | 275.4 | 0.402 | 74.91 |
| Commercialized murine anti—GM-CSF* | ND | ND | > 4000 |
Patient . | KAVavidity, pM . | Bmaxcapacity, sites/mol IgG . | IC50, mol/mol rhGM-CSF . |
|---|---|---|---|
| 1 | 20.1 | 0.129 | 5.84 |
| 2 | 13.3 | 0.153 | 4.43 |
| 3 | 16.7 | 0.168 | 3.83 |
| 4 | 24.9 | 0.253 | 6.65 |
| 5 | 11.1 | 0.134 | 8.66 |
| 6 | 24.8 | 0.332 | 6.65 |
| 7 | 12.1 | 0.194 | 2.42 |
| 8 | 15 | 0.184 | 11.88 |
| 9 | 22.3 | 0.565 | 2.42 |
| 10 | 37 | 0.273 | 12.08 |
| 11 | 22.3 | 0.281 | 12.69 |
| Average ± SD | 19.96 ± 7.54 | 0.24 ± 0.13 | 7.05 ± 3.81 |
| Commercialized goat anti—GM-CSF* | 275.4 | 0.402 | 74.91 |
| Commercialized murine anti—GM-CSF* | ND | ND | > 4000 |
ND indicates not determined.
Neutralizing antibodies.
The binding of autoantibodies to GM-CSF was also specific. Purified autoantibodies from 12 iPAP patients showed similar binding to rhGM-CSF prepared in E coli (nonglycosylated) and CHO cells (glycosylated), indicating the carbohydrate moiety was not involved in binding (Figure 6B). Autoantibodies did not bind GM-CSF that had been carboxymethylated (which disrupts disulfide bonds), suggesting that a secondary or tertiary structure may be recognized. Autoantibodies did not bind GM-CSF after trypsin cleavage, which retains the 2 disulfide bonds but not peptides 1 to 30, 59 to 85, and 108 to 111, suggesting that these peptides may contain critical binding epitopes (Figure 6B). Autoantibodies bound bovine GM-CSF, which has 68% amino acid homology with human GM-CSF, but not murine GM-CSF, which has only 53% homology (Figure 6C). None of a large variety of cytokines tested was bound by autoantibodies (Figure 6D). These findings demonstrate that autoantibodies consistently bind GM-CSF with high avidity and specificity and likely recognize a secondary or tertiary, nonglycosylated portion of the molecule.
Autoantibodies target a functional domain of GM-CSF
The specific epitopes on GM-CSF recognized by autoantibodies were identified using a competitive binding assay with [125I]-GM-CSF and 4 different epitope-specific murine monoclonal antibodies (Figure 7A). Monoclonal antibodies recognizing GM-CSF amino acids 1 to 11, 40 to 77, and 110 to 127 exhibited variable fractional inhibition by autoantibodies from separate patients (Figure 7B). In contrast, the monoclonal antibody recognizing GM-CSF residues 78 to 94 was consistently and strongly inhibited by autoantibodies from all patients, with a mean percentage inhibition of 78.97% ± 11.71% (Figure 7B). This epitope has been reported to be an important functional domain of GM-CSF.27,28,36,37 Thus, though autoantibodies can recognize various regions of the GM-CSF molecule, the consistent, strong targeting of an epitope known to be important for GM-CSF function strongly supports the notion that autoantibodies specifically neutralize GM-CSF in patients with iPAP.
Epitope targeting of the autoantibodies on the GM-CSF molecule. (A) Three-dimensional diagram of GM-CSF showing the 4 regions covered by the murine monoclonal antibodies used for epitope mapping of autoantibodies isolated from iPAP patients. Murine antibodies and the epitopes on human GM-CSF they recognize included 4117 (residues 1-11), 1089 (residues 40-77), 3092 (residues 78-94), and 1022 (residues 110-127), respectively. (B) Competitive GM-CSF binding assay of the autoantibodies with epitope-specific murine monoclonal antibodies. Each bar indicates the percentage of inhibition of [125I]-GM-CSF binding to each monoclonal antibody by the autoantibodies from each patient with iPAP. Percentage of inhibition for the reactions without the autoantibodies and without monoclonal antibody was defined as 0% and 100% inhibition, respectively. Control human IgG and rabbit anti-GM-CSF peptide 54-73 polyclonal antibodies were used for negative and positive controls, respectively. Distribution of the percentage of inhibition was variable among the patients, but the autoantibodies consistently and strongly inhibited GM-CSF binding to the monoclonal antibody 3092.
Epitope targeting of the autoantibodies on the GM-CSF molecule. (A) Three-dimensional diagram of GM-CSF showing the 4 regions covered by the murine monoclonal antibodies used for epitope mapping of autoantibodies isolated from iPAP patients. Murine antibodies and the epitopes on human GM-CSF they recognize included 4117 (residues 1-11), 1089 (residues 40-77), 3092 (residues 78-94), and 1022 (residues 110-127), respectively. (B) Competitive GM-CSF binding assay of the autoantibodies with epitope-specific murine monoclonal antibodies. Each bar indicates the percentage of inhibition of [125I]-GM-CSF binding to each monoclonal antibody by the autoantibodies from each patient with iPAP. Percentage of inhibition for the reactions without the autoantibodies and without monoclonal antibody was defined as 0% and 100% inhibition, respectively. Control human IgG and rabbit anti-GM-CSF peptide 54-73 polyclonal antibodies were used for negative and positive controls, respectively. Distribution of the percentage of inhibition was variable among the patients, but the autoantibodies consistently and strongly inhibited GM-CSF binding to the monoclonal antibody 3092.
Discussion
The present study was designed to determine the functional significance of autoantibodies in patients with iPAP and to characterize their specific molecular interactions with GM-CSF. Results demonstrate that BALF from iPAP patients strongly inhibited AM growth and development in contrast to BALF from healthy controls. This effect was caused by IgG in iPAP patients that bound GM-CSF with high avidity. GM-CSF was readily detected by immunohistochemistry in the lungs of iPAP patients. However, neutralizing capacity by the autoantibodies was in nanogram per milliliter order, which far exceeds the concentration of GM-CSF in BALF from healthy lung (ie, picogram per milliliter order). This “deficit” of GM-CSF could be overcome by the addition of excess GM-CSF, demonstrating that the neutralizing capacity was saturable. Autoantibodies colocalized with GM-CSF-binding activity in the lungs of iPAP patients. GM-CSF-autoantibody complexes were also demonstrated in BALF from iPAP patients by immunoprecipitation and Western blotting. Finally, epitope mapping demonstrated that most patients with iPAP had autoantibodies with epitopes targeting a region of GM-CSF within amino acids 78 to 94, an important functional domain. Together these data demonstrate that the neutralizing anti-GM-CSF autoantibodies in iPAP patients are functionally significant and severely impair pulmonary GM-CSF bioactivity, likely impairing the development of AM terminal maturation.
Characterization of neutralizing anti-GM-CSF autoantibodies in iPAP
Autoantibody levels were surprisingly high in most iPAP patients. Although the reasons for this are unclear, the use of a highly purified autoantibody as a standard for ELISA ensured the accuracy of these findings. One possible explanation is that an “immunologic stimulus” is continuously present in the lung or elsewhere. Improving pulmonary functions after therapeutic whole lung lavage may support this idea because it may remove such stimulus.2 Autoantibodies bound GM-CSF with high avidity, which was far higher than that of commercially produced anti-GM-CSF antibodies. Interestingly, the binding avidity was similar to that of anti-GM-CSF antibodies in commercial preparations of pooled IgG concentrates from healthy donors (approximately 4-11 pM).38 The significance of the latter is unclear, but their presence is intriguing. Autoantibody avidity was also similar to that of autoantibodies directed at other cytokines including IL-1α (5.5-11 pM),39 IL-5 (15 pM),38 IL-10 (80-351 pM),38 and IFN-α (30 pM).40 The similarity of ligand binding affinity for these autoantibodies and the natural cytokine receptors35,38-41 suggests that the autoantibodies probably are functionally important in vivo, where they would be expected to effectively compete for ligand binding, thus reducing cytokine receptor occupancy and cytokine signaling. In the case of GM-CSF, this may have pathologic significance with respect to AM function. For example, GM-CSF initially binds with low affinity (5-10 nM) to the GM-CSF receptor α chain.42 Subsequently, this complex associates with the GM-CSF receptor β chain, which does not actually bind GM-CSF but converts the binding of the α chain to a high affinity bond (25-100 pM). The affinity of the autoantibodies (approximately 20 pM) for GM-CSF is stronger than that of the α chain. Thus, it is likely that the autoantibodies may inhibit GM-CSF signaling by blocking the initial binding to the low-affinity receptor. The presence of immune complexes consisting of autoantibodies and GM-CSF provides further strong evidence for a functional role in vivo.
The present data that autoantibodies similarly bound glycosylated and nonglycosylated human GM-CSF excludes the carbohydrate moiety as a component of any autoantibody epitopes. The observation that carboxymethylation completely abolished autoantibody binding suggests that autoantibodies may recognize epitopes that are composed of noncontiguous regions of the primary structure—that is, a secondary, tertiary, or quaternary structure of GM-CSF. It is possible that autoantibody binding may alter GM-CSF conformation, thus altering recognition by the monoclonal antibodies used for epitope mapping. Thus, our studies do not precisely define the target epitopes of the autoantibodies. Crystallographic analysis will likely be useful to determine the nature of the specific molecular interactions of GM-CSF and autoantibodies.
The observation that autoantibodies in most iPAP patients consistently targeted an epitope in the region of residues 78 to 94 of the mature GM-CSF molecule is of particular interest for 2 reasons. First, this region of GM-CSF is known to be functionally important.27,28,36,37 Second, identifying an epitope that is consistently targeted by autoantibodies in iPAP patients suggests that disease severity may relate to epitope targeting by autoantibodies in a given iPAP patient rather than simply autoantibody levels. Alternatively, residues 78 to 94 may simply represent a hot spot, the targeting of which is necessary for neutralizing GM-CSF and thus for physiological function of the autoantibodies. Such functional domains of GM-CSF have been defined.27,28,36,37 Further clinicopathologic correlations in longitudinal studies will be required to answer these questions.
Implications of impaired pulmonary GM-CSF bioactivity for AM function
The presence of abundant, high-affinity autoantibodies capable of neutralizing pulmonary GM-CSF bioactivity has important implications for the pathogenesis of iPAP. The present study demonstrates that these autoantibodies effectively eliminate GM-CSF bioactivity from the lung. The true role of GM-CSF on human AMs has not been fully elucidated. However, in mice, pulmonary GM-CSF is required to stimulate surfactant catabolism by AMs and a number of other functions strongly supporting the concept that GM-CSF is the principal factor regulating AM terminal differentiation.12,15,16 In mice, the absence of GM-CSF or of its receptor causes PAP.4-7 A number of similarities in the molecular, cellular, and pathophysiologic features of murine PAP in GM-/- mice and human iPAP suggest that the pathogenesis of iPAP also involves the disruption of pulmonary GM-CSF signaling.1,3-7,14,16,18-21 The similarities in murine and human PAP have been, and continue to be, important for several reasons. First, the murine findings continue to identify markers of AM function that are potentially useful as surrogate markers of disease activity to test therapeutic interventions such as the administration of GM-CSF.8,12,15 Second, and equally important, they continue to provide molecular clues regarding the potentially critical role of GM-CSF in regulating human AM functions, surfactant homeostasis, and the pathogenesis of iPAP. Our observation that autoantibodies from iPAP patients strongly inhibited the growth and development of AMs is consistent with the concept that GM-CSF may be required for terminal differentiation of human AMs
Implications of anti—GM-CSF autoantibodies for the diagnosis, management, and pathogenesis of iPAP
Our results demonstrate, in a large cohort, that autoantibodies directed at GM-CSF occur at high levels in the sera and lungs of patients with iPAP but not in those with other lung diseases or in healthy controls. This is consistent with our earlier findings22,23 and with a subsequent report from another group.43 These results also demonstrate in this cohort that high autoantibody levels are sensitive and specific for the diagnosis of iPAP. Thus, detecting autoantibodies by ELISA has good predictive value for iPAP and suggests the potential clinical usefulness of this assay for the diagnosis of iPAP. These results also agree with results of a latex agglutination assay recently developed by our laboratory as a screening tool for iPAP.23 Either or both of these assays could be useful in the management of patients with iPAP to follow changes in autoantibody levels in response to therapy. However, in one report, the autoantibody titer crudely expressed in terms of total autoantibody protein concentration did not correlate well with other measures of disease severity.32 Thus, additional studies are needed to assess the potential usefulness of autoantibody levels in the management of iPAP therapy.
Notwithstanding the potential diagnostic implications of the autoantibodies, their etiology remains unclear. Similarly, the initial or principal site of their production is unknown. The higher prevalence of iPAP in smokers suggests that cigarette smoke may be an important factor.2 However, no mechanism has been suggested; again, further studies are needed. Future investigations should also evaluate the relationship of autoantibody titer, neutralizing capacity, and epitope mapping to the severity, duration, and remittance of the disease. Although many questions remain, the results of this study strongly support the concept that iPAP is an autoimmune disorder mediated by antibodies directed at GM-CSF.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1565.
Supported by a Grant-in-Aid for Translational Research (no. H14-trans-014) from the Japanese Ministry of Labor, Health, and Welfare.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank to Dr Ian Clark-Lewis (Biomedical Research Center and Department of Biochemistry and Molecular Biology, University of British Columbia), Dr Kenneth Kaushansky (Division of Hematology, University of Washington), and Dr Roberto P. Revoltella (Institute of Mutagenesis and Differentiation, C.N.R.) for their helpful technical suggestions.

![Figure 1. Morphology and in vitro growth of adherent AMs from a patient with iPAP. (A) Adherent AMs from a patient with iPAP and a healthy subject. iPAP AMs showed a small, monocyte-like appearance with basophilic cytoplasm (phase-contrast micrograph [i], original magnification × 200; and Wright-Giemsa staining [ii], original magnification × 1000), whereas those from control-BALF had large eosinophilic cytoplasm (phase-contrast micrograph [iii], original magnification × 200; and Wright-Giemsa staining [iv], original magnification × 1000). (B) After incubation with control-BALF for 14 days, iPAP-AM size increased markedly (ii) (original magnification × 200) compared with before incubation (Ai) or incubation with medium alone (i), and adding 20 ng/mL GM-CSF further promoted growth (iii). After incubation with iPAP-BALF, AMs remained small (iv), but adding 20 ng/mL GM-CSF restored their growth (v).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-05-1565/6/m_zh80030456190001.jpeg?Expires=1767779928&Signature=ZEpG04m6skGSLtfI-fbVzFxNlMLqIGhwn~oO26K9JGMSz5EMMpxdwE6ZRnfO4~Bl7PcWREDb94OtAnbkC7tTrKNz~jH5MicMAAr3p92yOwgaCFONMdfcHSvKwRnKDt~rRYi0EkveOf~EpDq09z-MflIHu5yXlhV7PO2qQh8abAC8gPeG7UaI0dM9RjauME39~zhq2boOw4tzbPPh7HSYpuH0Vnr2dZPZw-VZNPX6if7P2uFp8iBRGSO6SvN6kKcINXHEsmZzi~9hXUPewNoWouo1TnHu-gpJmNpcLbRumqyYvcfW-~QriZdZkOpNQLAcyjdKVvHnrFkDTHbk7yI71g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Formation of GM-CSF-autoantibody immune complexes in iPAP patients. (A) Detection of GM-CSF containing immune complexes in the BALF in iPAP by immunoprecipitation. Protein A Sepharose beads were incubated with BALF from a healthy control (lanes 1-2), iPAP patient (lanes 3-4), or, as a control, purified yeast-derived rhGM-CSF (lane 5). Bound and unbound fractions (indicated) were separated and subjected to SDS-PAGE under reducing conditions and Western blotting using a rabbit anti-GM-CSF antibody. Under these conditions, the rhGM-CSF standard consists of 3 molecular species migrating at 19.5, 16.8, and 15.5 kDa. (B) Detection of GM-CSF and IgG in BALF by Western blotting. BALF from a healthy control (lanes 2, 4), iPAP patient (lanes 3, 5), or rhGM-CSF (lane 1) was subjected to native PAGE and then Western blotting using a rabbit anti-GM-CSF antibody (lanes 1-3) or anti-IgG antibody (lanes 4-5). (C) Supershift of [125I]-GM-CSF with iPAP-BALF. BALF from healthy controls (lanes 2-4) or iPAP patients (lanes 5-7) was incubated with [125I]-GM-CSF and then subjected to native PAGE and autoradiography. As a control, [125I]-GM-CSF alone was also included on the gel (lane 1).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-05-1565/6/m_zh80030456190005.jpeg?Expires=1767779928&Signature=ftY42obHFm18~j9TuYMPSmMsQWQySGzqBUAdY0nUZ5Se0vFmTHRtEbbADErLPod9EkFudSar2sGhDPMW5ZxZhUqJ-x0S0FDnVQtdbiYuKyTXJhhLndiGfXHVkKkSjwb8TXvCP-nGtxjCYuFDUMp36GYI9RV4JbBZtBiGz4QQfurpnxUcYV3vpHz4I7rZjlyCkRuHfOzwLtj8ESIDyjAq13r6u7xpW~VHLsawWiyYAmkE0NMWQTRRcUNyloFgoxN~5vWnEW3rJOjbMKRtGqIpYIcn0N6ZzcUr351Gl9Ox~HGO1ZqxF26hiCauMyoWK2pJ~66~ygjQtWH~aRXkE7Ti8g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Binding affinity, capacity, and specificity of purified anti-GM-CSF autoantibodies in iPAP patients. (A) Saturation binding plot of [125I]-GM-CSF binding to purified anti-GM-CSF autoantibody. Vertical axis represents the molar fractional binding of GM-CSF to autoantibody. Binding capacity (maximal binding) was determined from the asymptote of the curve, and binding affinity (KAV) was determined from the concentration of GM-CSF at 50% of maximum binding. (B) Binding of autoantibodies to various forms of human GM-CSF. Reactivity of autoantibodies was measured by antigen capture and compared with binding to recombinant human GM-CSF produced in E coli, which was taken as 100%. Various forms were tested, including recombinant human GM-CSF produced in CHO cells; the large trypsin fragment that contained residues 31-58, 86-107, and 112-127, connected by disulfide bonds; and carboxymethylated GM-CSF, which had an intact primary amino acid sequence but lost the second and tertiary structure because of the loss of the 2 disulfide bonds. NS indicates not significant. (C) Xenospecificity of autoantibody binding. Reactivity of autoantibodies to murine and bovine GM-CSF was measured as above. (D) Specificity of autoantibody binding with respect to other human cytokines. Reactivity of autoantibodies to various human cytokines was measured as above. Error bars indicate SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-05-1565/6/m_zh80030456190006.jpeg?Expires=1767779928&Signature=CE646ZdG8C8n2u9BBK0~ygc567kusarnsX9bSYTQWAs3FSDvxUr9XymT~KPkRs7XlrxuQxm3I87cK~PHA~Oe7BvTv3lT1mGG8xfWElHUHv7oeIoYffZom-1bf8FpDWgFtCu6ud8K5bX9O2xWvFYm~~-jMDpLdBorFp1TBBfAJv3mmAKEpvAAB6urLwnTTWjmdKfNYZsGAz2EZ8cYRzb30tI8yln63FFxI1YCCcMlAOsOicKotnOLac8sY1SV~EpIaJE-aVwumsM~-5NXkrMaAZzMSOX4v86DlaEJjxQm~TT1CFr23FjmF34AO-qcKzwC5jf57UDFupehxOf2uG-wng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Epitope targeting of the autoantibodies on the GM-CSF molecule. (A) Three-dimensional diagram of GM-CSF showing the 4 regions covered by the murine monoclonal antibodies used for epitope mapping of autoantibodies isolated from iPAP patients. Murine antibodies and the epitopes on human GM-CSF they recognize included 4117 (residues 1-11), 1089 (residues 40-77), 3092 (residues 78-94), and 1022 (residues 110-127), respectively. (B) Competitive GM-CSF binding assay of the autoantibodies with epitope-specific murine monoclonal antibodies. Each bar indicates the percentage of inhibition of [125I]-GM-CSF binding to each monoclonal antibody by the autoantibodies from each patient with iPAP. Percentage of inhibition for the reactions without the autoantibodies and without monoclonal antibody was defined as 0% and 100% inhibition, respectively. Control human IgG and rabbit anti-GM-CSF peptide 54-73 polyclonal antibodies were used for negative and positive controls, respectively. Distribution of the percentage of inhibition was variable among the patients, but the autoantibodies consistently and strongly inhibited GM-CSF binding to the monoclonal antibody 3092.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-05-1565/6/m_zh80030456190007.jpeg?Expires=1767779928&Signature=3PHs3vvSm5gpLCSk7VPszz0zNEW3JnPsNtKIxcgdvxhvmHSSKTcUGKo1kcgappl3raGYcqHlVEWuXzOOLjsxJRT085A8aMWWpLU~8o2JDMiBmL7fLaaEfUP964C-xrMaEocCHf5DM2XuDT0EdSjBNxsjzp1yuKqjVrlCVLT~NKn6f5LlQtYOT6VOs06VcC53CSFLMryV9oQ4ZXGe1LoByFkDs9xP6LbyANjOmr8-rEEHoT2zqMwAQMp4TVqNs0TVTUMoZANKmG13xHueahS8LVgiyqdplE-8bUUIZpbn-DaZkixV6IzoXN1MxjtIcK7dVTCeB~f9Soxj3aUf5jRY8w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal